Abstract
This study was designed to evaluate and determine the free radical scavenging activity and total flavonoid content of the ethanol extract of six Iranian Achillea. species (A. micrantha. Willd Asteraceae, A. filipendula. Lam., A. millefolium. L., A. tenuifolia. Lam., A. vermicularis. Trin and A. wilhelmsii. C. Koch.) (Asteraceae) and investigate the possible composition-activity relationship. The free radical scavenging activity of the extracts was characterized by the DPPH scavenging test. The total flavonoid content of the extracts was determined using the colorimetric technique by AlCl3 reagent. Two of six extracts [A. micrantha. (IC50 = 32.92 µg/ml) and A. millefolium. (IC50 = 49.43 µg/ml)] revealed a suitable free radical scavenging activity. The strongest activity was found for A. micrantha. (IC50 = 32.92 µg/ml), whereas the activity of A. wilhelmsii. (IC50 = 118.90 µg/ml) was significantly lower than the others. On the other hand, the extracts of A. vermicularis. (58.17 µg/mg) and A. wilhelmsii. (55.96 µg/mg) had very high content of flavonoid, but the lowest content was determined in A. tenuifolia. (35.66 µg/mg). However, a favorable correlation was not found between the scavenging activity and the total flavonoid content of the extracts. The study suggests that A. micrantha. and A. millefolium. are the possible sources of natural radical scavengers.
Introduction
Free radicals play an important role in tissue damage and pathological events. They are considered to be the cause of several health problems and chronic diseases such as cancer, cardiovascular diseases, arthritis, neurodegenerative disorders like Parkinson and Alzheimer diseases, inflammation, etc. (Mensor et al., Citation2001; Parejo et al., Citation2002; Hou et al., Citation2003; Orhan et al., Citation2003; Tepe et al., Citation2005). Free radicals can also affect food quality, reducing its nutritional content and promoting the development of food deterioration (Vichi et al., Citation2001; Melo et al., Citation2005; Anagnostopoulou et al., Citation2006). A large amount of research has been carried out to find the radical scavengers and antioxidative drugs that not only reduce damage to the human body but also prolong the shelf life of food products (Parejo et al., Citation2002; Orhan et al., Citation2003; Miliauskas et al., Citation2004). Synthetic antioxidants such as BHA (butylated hydroxyl anisole) and/or BHT (butylated hydroxyl toluene) are very effective and are used for industrial processing, but they may possess some side effects and toxic properties to human health (Hou et al., Citation2003; Wangensteen et al., Citation2004; Tepe et al., Citation2005; Anagnostopoulou et al., Citation2006). For this reason, there is an increasing interest in the investigation of naturally occurring antioxidants from plants (Vichi et al., Citation2001; Hou et al., Citation2003; Galvez et al., Citation2005). In recent years, the extracts of many plants have been prepared and screened for their antioxidant activities and some of them are well-known for their antioxidant properties (Bandoniene et al., Citation2002; Melo et al., Citation2005). Among the natural compounds participating against free radicals, phenoilc compounds, particularly flavonoids, constitute one of the major groups of herbal compounds acting as radical scavengers and antioxidants. Therefore, these substances have been proposed as health-promoting natural products (Formica & Regelson, Citation1995; Lee et al., Citation2003; Atoui et al., Citation2005; Capecka et al., Citation2005).
The genus Achillea. is one important member of the Asteraceae family. It comprises nearly 115 species throughout the world. In Iranian flora, the genus is represented by about 19 species (Mozaffarian, Citation1996). Achillea. species are well-known in Iranian traditional medicine as highly effective medicinal plants for various purposes such as the treatment of abdominal pain, inflammation, hemorrhage, rheumatic pain, menstrual disorders, etc. (Amin, Citation1991; Zargari, Citation1992). Some of the biological activities are related to antioxidant and free radical scavenging properties of the plants. Previous chemical studies on some Achillea. species have shown the presence of various compounds, especially flavonoids (Ivancheva & Tsvetkova, Citation2003) but, despite the richness of phenolic and flavonoid compounds in Achillea., most species have not been screened for the antioxidative activity before the work reported here.
The aim of the study was to (i) assess and compare the free radical scavenging activity of six Achillea. species (A. micrantha. Willd., A. filipendula. Lam., A. millefolium. L., A. tenuifolia. Lam., A. vermicularis. Trin., and A. wilhelmsii. C. Koch.) from Iran using the stable DPPH (1,1-diphenyl-2-picrylhydrazyl) radical, (ii) determine the total flavonoid content of each plant by AlCl3, and finally (iii) investigate the possible composition-activity relationship. To the best of our knowledge, there is no previous report on the free radical scavenging and/or antioxidant properties of A. micrantha., A. filipendula., A. tenuifolia., A. vermicularis., and A. wilhelmsii.. However, the antioxidative and radical scavenging activities of A. millefolium. have been reported elsewhere (McCune & Johns, Citation2002; Candan et al., Citation2003).
Materials and Methods
Plant material
The plants (A. micrantha., A. filipendula., A. millefolium., A. tenuifolia., A. vermicularis., and A. wilhelmsii.) were collected in Tehran province during the flowering period in summer 2003 and authenticated by M. Kamalinejad. A voucher specimen for each plant was deposited at the Herbarium of the Department of Pharmacognosy, Shaheed Beheshti University of Medical Sciences (Tehran, Iran). The plants were dried in the drying room with active ventilation at ambient temperature. Only the aerial parts of the plants were used for investigation.
Chemicals
All of the chemicals used in this work were purchased from Merck (Darmstadt, Germany), with the exception of DPPH, which was purchased from Sigma (St. Louis, MO, USA). The chemicals were of analytical grade.
Extraction
The dried and finely ground plants (100.0 g) were extracted with ethanol 90% (500.0 ml) at ambient temperature for 48 h. The extracts were filtered and concentrated under reduced pressure at approximately 40°C. The dried extracts were dissolved in ethanol 90% to a final concentration of 1000.0 µg/ml (sample stock solution).
DPPH radical scavenging activity
Sample stock solutions were diluted to final concentrations of 500.0, 250.0, 100.0, 50.0, 25.0, 10.0, 5.0 µg/ml in ethanol 90%. One milliliter of a 120.0 µg/ml DPPH ethanolic solution was added to 2.5 ml of sample solutions at different concentrations and allowed to react at room temperature. After 30 min, the absorbance values (A) were measured at 518 nm in a Shimadzu Multispect-1501 spectrophotometer (Kyoto, Japan) and converted into the percentage radical scavenging activity (RSA) by the following formula:
Ethanol 90% (1.0 ml) plus each sample solution (2.5 ml) was used as a blank. DPPH solution (1.0 ml) plus ethanol 90% (2.5 ml) was used as a negative control. Also, rutin solution (at the concentrations of 100.0, 50.0, 25.0, 10.0, 5.0, 2.5 µg/ml) was used as a positive control using the above procedure (Mensor et al., Citation2001).
The RSA% was plotted against the sample concentration, and a logarithmic regression curve was established in order to calculate the IC50 value (inhibitory concentration), which is the concentration of sample (µg/ml) necessary to decrease by 50% the absorbance of DPPH.
Determination of total flavonoid content
The amount of total flavonoid content (TFC) for each extract was determined according to the colorimetric assay using rutin as a reference compound. Rutin solution, 2.5 mL (at the concentrations of 75.0, 50.0, 37.5, 25.0 µg/ml) was mixed with 2.5 ml AlCl3 reagent in ethanol 90% (20.0 mg/ml). After 40 min, the absorbance at 415 nm was measured with a Shimadzu Multispect-1501 spectrophotometer. Ethanol 90% (2.5 ml) plus rutin solution (2.5 ml) was used as a blank (Miliauskas et al., Citation2004). Then, a linear calibration curve (absorbance versus concentration) was developed. The same procedure was carried out with 2.5 ml of each sample in ethanol 90% instead of rutin solution. The TFC for each extract [as µg rutin equivalents (RE)/mg of extract] was determined on the basis of the standard curve.
Statistical analysis
All of the experiments were carried out in triplicate. The IC50 values were estimated by non-linear curve-fitting and were presented by their respective 95% confidence limits. The TFCs (µg/mg) were shown as mean ± SEM. One-way analysis of variance (ANOVA) followed by Tukey's post test was used to assess significant differences (p < 0.05) between extracts. All of the statistical analyses were accomplished using the computer software GraphPad Prism 3.02 for Windows (GraphPad Software, San Diego, CA, USA).
Results and Discussion
The use of DPPH method provides an effective and easy way to evaluate the radical scavenging and/or antioxidant activities of plant extracts (Soler-Rivas et al., Citation2000; Argolo et al., Citation2004; Roginsky & Lissi, Citation2005). Therefore, in this study, the selected Achillea. extracts were screened for their possible radical scavenging activity by DPPH technique and their IC50 values were calculated for further comparisons. The IC50 value for each Achillea. species studied was obtained from a logarithmic regression curve, RSA% versus concentration (). shows the IC50 values for the extracts tested. They ranged from 118.90 to 32.92 µg/ml. As indicated in , some extracts demonstrated a suitable DPPH radical scavenging activity. When the IC50 values were compared for six extracts analyzed, A. micrantha. showed the highest scavenging activity with an IC50 value (with 95% confidence intervals) of 32.92 (29.56–36.66) µg/ml (the lowest IC50). The extract with the weakest activity (the highest IC50) was A. wilhelmsii. with an IC50 value of 118.90 (108.80–130.0) µg/ml, which had significantly lower activity than the other samples. A. vermicularis. and A. tenuifolia. showed a similar activity with no significant difference between them (p > 0.05). Therefore, the scavenging activity of the extracts in decreasing order was A. micrantha. > A. millefolium. > A. filipendula. > A. vermicularis., A. tenuifolia. > A. wilhelmsii.. The results showed that A. micrantha. is more active than the official species, A. millefolium. (p < 0.001).
Figure 1 Radical scavenging activity of the studied Achillea. species extracts using DPPH. Each point represents the mean of three experiments, and the vertical bars represent the SEM.
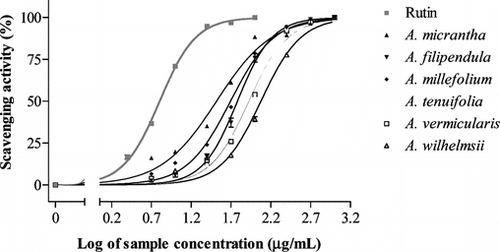
Table 1 IC50 values of DPPH scavenging activity and total flavonoid contents (TFCs) of the studied Achillea. extracts.
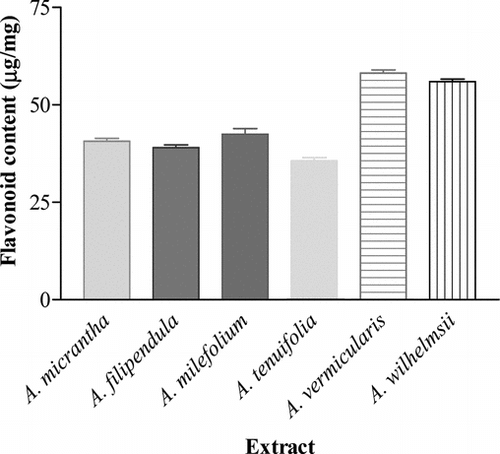
The results imply that the radical scavenging activity of the extracts may be attributed to their proton-donating abilities (Parejo et al., Citation2002; Galvez et al., Citation2005; Melo et al., Citation2005). Thus, the flavonoid content of the extracts, as one of the most important H-donating groups, was tested. The amount of TFC was determined using the calibration curve. The plot was found to be linear across the ranged assay (75.0–25.0 µg/mL, r2 > 0.99). presents the TFC for each extract. The TFC values ranged from 58.17 to 35.66 µg RE/mg of extract. When the TFC of each extract was compared with the others, it could be observed that A. vermicularis. (58.17 ± 0.82 µg/mg) and A. wilhelmsii. (55.96 ± 0.61 µg/mg) were the richest in flavonoid content, whereas A. tenuifolia. (35.66 ± 0.83 µg/mg) had the lowest content ().
Figure 2 Total flavonoid contents of the studied Achillea. extracts. Data are represented as mean ± SEM (n = 3). Vertical bars represent the SEM.
After the analyses of the RSA and TFC for each extract, the relationship between activity and TFC was studied. However, among the analyzed extracts of Achillea., a favorable correlation between the two parameters was not demonstrated by the linear regression analysis. This lack of relationship is in agreement with other literature (Heinonen et al., Citation1998; Matthaus, Citation2002). It is known that only flavonoids with a certain structure and particularly hydroxyl position in the molecule can act as proton-donating and show radical scavenging activity (Mensor et al., Citation2001; Hou et al., Citation2003). On the other hand, the extracts are very complex mixtures of many different compounds with distinct activities (Mensor et al., Citation2001; Hou et al., Citation2003; Galvez et al., Citation2005). Therefore, the high scavenging activity of some species tested could be due to the mixture in their composition of flavonoids and other compounds that may exert a synergistic effect.
The results of this study indicate that some Achillea. species, especially A. micrantha. and A. millefolium., can serve as natural sources to develop the free radical scavengers and might prevent radicals attack in biological and food systems. Hence, these species could possess therapeutic effects in different areas and they could be considered as useful sources of materials for human health and as antioxidant food preservatives.
Acknowledgment
We would like to thank Shaheed Beheshti University of Medical Sciences for financial support of this project.
References
- Amin G (1991): Popular Medicinal Plants of Iran, Vol. 1. Tehran, Research Deputy of Health Ministry, pp. 44–45.
- Anagnostopoulou MA, Kefalas P, Papageorgiou VP, Assimopoulou AN, Boskou D (2006): Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis.). Food Chem 94: 19–25, [CSA]
- Argolo ACC, Sant'Ana AEG, Pletsch M, Coelho LCBB (2004): Antioxidant activity of leaf extracts from Bauhinia monandra.. Bioresource Technol 95: 229–233, [CROSSREF], [CSA]
- Atoui AK, Mansouri A, Boskou G, Kefalas P (2005): Tea and herbal infusions: Their antioxidant activity and phenolic profile. Food Chem 89: 27–36, [CROSSREF], [CSA]
- Bandoniene D, Murkovic M, Pfannhauser W, Venskutonis PR, Gruzdiene D (2002): Detection and activity evaluation of radical scavenging compounds by using DPPH free radical and on-line HPLC-DPPH methods. Eur Food Res Technol 214: 143–147, [CROSSREF], [CSA]
- Candan F, Unlu M, Tepe B, Daferera D, Polissiou M, Sokmen A, Akpulat A (2003): Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium. subsp. millefolium. Afan. (Asteraceae). J Ethnopharmacol 87: 215–220, [INFOTRIEVE], [CROSSREF], [CSA]
- Capecka E, Mareczek A, Leja M (2005): Antioxidant activity of fresh and dry herbs of some Lamiaceae species. Food Chem 93: 223–226, [CROSSREF], [CSA]
- Formica JV, Regelson W (1995): Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol 33: 1061–1080, [INFOTRIEVE], [CROSSREF], [CSA]
- Galvez M, Martin-Cordero C, Houghton PJ, Ayuso MJ (2005): Antioxidant activity of methanol extracts obtained from Plantago. species. J Agric Food Chem 53: 1927–1933, [INFOTRIEVE], [CROSSREF], [CSA]
- Heinonen IM, Lehtonen PJ, Hopia AI (1998): Antioxidant activity of berry and fruit wines and liquors. J Agric Food Chem 46: 25–31, [INFOTRIEVE], [CROSSREF], [CSA]
- Hou WC, Lin RD, Cheng KT, Hung YT, Cho CH, Chen CH, Hwang SY, Lee MH (2003): Free radical scavenging activity of Taiwanese native plants. Phytomedicine 10: 170–175, [INFOTRIEVE], [CROSSREF], [CSA]
- Ivancheva S, Tsvetkova R (2003): Distribution of flavonoid aglycones in tribe Anthemideae (Asteraceae). In: Imperato F, ed., Advances in Phytochemistry. Trivandrum, Research Signpost, pp. 85–95.
- Lee KW, Kim YJ, Lee HJ, Lee CY (2003): Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J Agric Food Chem 51: 7292–7295, [INFOTRIEVE], [CROSSREF], [CSA]
- Matthaus B (2002): Antioxidant activity of extracts obtained from residues of different oil seeds. J Agric Food Chem 50: 3444–3452, [INFOTRIEVE], [CROSSREF], [CSA]
- McCune LM, Johns T (2002): Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the North American boreal forest. J Ethnopharmacol 82: 197–205, [INFOTRIEVE], [CROSSREF], [CSA]
- Melo EA, Filho JM, Guerra NB (2005): Characterization of antioxidant compounds in aqueous coriander extract (Coriander sativum. L.). Lebensm-Wiss Technol 38: 15–19, [CROSSREF], [CSA]
- Mensor LL, Menezes FS, Leitao GG, Reis AS, dos Santos TC, Coube CS, Leitao SG (2001): Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15: 127–130, [INFOTRIEVE], [CROSSREF], [CSA]
- Miliauskas G, Venskutonis PR, van Beek TA (2004): Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem 85: 231–237, [CROSSREF], [CSA]
- Mozaffarian V (1996): A Dictionary of Iranian Plant Names. Tehran, Farhang Moaser Publishers, pp. 11–12.
- Orhan I, Aydin A, Colkesen A, Sener B, Isimer AI (2003): Free radical scavenging activities of some edible fruit seeds. Pharm Biol 41: 163–165, [CSA]
- Parejo I, Viladomat F, Bastida J, Rosas-Romero A, Flerlage N, Burillo J, Codina C (2002): Comparison between the radical scavenging activities and antioxidant activity of six distilled and nondistilled mediterranean herbs and aromatic plants. J Agric Food Chem 50: 6882–6890, [INFOTRIEVE], [CROSSREF], [CSA]
- Roginsky V, Lissi EA (2005): Review of methods to determine chain-breaking antioxidant activity in food. Food Chem 92: 235–254, [CROSSREF], [CSA]
- Soler-Rivas C, Espin JC, Wichers HJ (2000): An easy and fast test to compare total free radical scavenger capacity of foodstuffs. Phytochem Analysis 11: 1–9, [CROSSREF], [CSA]
- Tepe B, Sokmen M, Akpulat HA, Sokmen A (2005): In vitro. antioxidant activities of the methanol extracts of four Helichrysum. species from Turkey. Food Chem 90: 685–689, [CROSSREF], [CSA]
- Vichi S, Zitterl-Eglseer K, Jugl M, Franz C (2001): Determination of the presence of antioxidants deriving from sage and oregano extracts added to animal fat by means of assessment of the radical scavenging capacity by photochemiluminescence analysis. Nahrung/Food 45: 101–104, [CROSSREF], [CSA]
- Wangensteen H, Samuelsen AB, Malterud KE (2004): Antioxidant activity in extracts from coriander. Food Chem 88: 293–297, [CROSSREF], [CSA]
- Zargari A (1992): Medicinal Plants, Vol. 3. Tehran, Tehran University Publications, pp. 106–117.
