Abstract
Cabraleadiol monoacetate, atranorin, methyl-β.-orcinolcarboxylate, zeorin, 4-O.-methylcryptochlorophaeic acid, and lichexanthone were isolated from the lichen Pyxine consocians Vainio... Heterodermia leucomelos (L)... Poelt contained a new triterpenoid, 6α-hydroxyhop-21β.H-22(29)-en, along with atranorin, zeorin, glyceryl trilinolate, and 3,6-dimethyl-2-hydroxy-4-methoxybenzoic acid. Cabraleadiol monoacetate, 4-O.-methylcryptochlorophaeic acid, lichexanthone, and 3,6-dimethyl-2-hydroxy-4-methoxybenzoic acid showed mosquito larvicidal activity against the second instar larvae of Aedes aegypti.. Lichexanthone exhibited human sperm motility enhancing activity.
Introduction
Lichens are distributed worldwide and grow on rocks and poorly developed soils or as epiphytes on trees and shrubs. They produce large concentrations of lichen substances, which are bioactive (Huneck, Citation1999). In our continuing search for biologically active compounds from tropical lichens, we have investigated Pyxine consocians. Vainio and Heterodermia leucomelos (L)... Poelt, which are foliose type lichens belonging to the family Physciaceae. In this paper, we report the isolation and structural elucidation of several metabolites, including a new triterpenoid 6α-hydroxyhop-21β.H-22(29)-en (7). A number of these constituents have been evaluated for their mosquito larvicidal and spermicidal/sperm motility enhancing activity.
Materials and Methods
General experimental procedures
Melting points (uncorrected) were determined by using a Kofler hot-stage apparatus (UK). UV absorptions were measured with a Shimadzu 1601 UV spectrophotometer (Japan). 1H and 13C NMR, correlation spectroscopy (COSY), distortionless enhanced polarization transfer (DEPT), heteronuclear correlation spectroscopy (HETCOR), heteronuclear multiple quantum correlation (HMQC), and heteronuclear multiple bond correlation (HMBC) spectra were recorded on a VARIAN (1H 300 and 13C 75.45 MHz) in CDCl3 with TMS as the internal standard (USA). Low- and high-resolution electron impact mass spectra were recorded on a Kratos/AEI MS-902 spectrometer (USA). Silica gel used was Merck Kieselgel (230–400 mesh ASTM) (Germany).
Lichen collection
P. consocians. was collected from the campus of the University of Peradeniya growing on the stem bark of the palm tree Roystonia regia., and H. leucomelos. was collected from the mossy rocks in the Horton Plains, Central Province, Sri Lanka. Specimens were identified by Pat Wolseley of the British Natural History Museum, and voucher specimens have been deposited at the Royal Botanic Gardens, Peradeniya, Sri Lanka.
Extraction and isolation
Cleaned and air-dried P. consocians. lichen (100 g) was ground into a powder and sequentially extracted into hexane, CH2Cl2, and MeOH (1 l each) in a Soxhlet extractor for 2 h. The solvent was removed under reduced pressure (<40°C) using a rotavapor. Cleaned and air-dried H. leucomelos. lichen (40 g) was ground into a powder.
Pyxine consocians
The hexane extract of P. consocians. was subjected to MPLC (eluent: hexane to ethyl acetate to MeOH) yielded atranorin (2) (13 mg) (crystallized CH2Cl2). Further separation by MPLC (eluent: ethyl acetate:hexane (1:19) to ethyl acetate) yielded methyl-β.-orcinolcarboxylate (3) (5.4 mg) and zeorin (4) (24.6 mg). Further separation by MPLC [eluent: CH2Cl2: hexane (1:1) to MeOH: CH2Cl2 (1:39)] to give cabraleadiol monoacetate (1) (50 mg) and 4-O.-methylcryptochlorophaeic acid (5) (12 mg). MPLC of the methanol extract (eluent: hexane to CH2Cl2 to MeOH) yielded lichexanthone (6) (51.2 mg).
Heterodermia leucomelos
The finely powdered lichen of H. leucomelos. was ground together with the silica gel (40 g) and subjected to MPLC [eluent: CH2Cl2:hex (1:9) to MeOH] to yield glyceryl trilinolate (8) (4 mg) and atranorin (2). Further separation by MPLC [eluent: CH2Cl2:hexane (3:2) to CH2Cl2] gave 3,6-dimethyl-2-hydroxy-4-methoxybenzoic acid, 9 (1.6 mg), the 6α-hydroxyhop-21β.H-22(29)-en, 7 (4.2 mg), and zeorin, 4 (130 mg).
Cabraleadiol monoacetate (.1)..: Pale yellow needles (CH2Cl2); m.p. 158–160°C (lit. m.p. 148–149°C) (Qui et al., 2001); Cabraleadiol (.1′)..: Pale yellow needles (CH2Cl2); m.p. 180–182°C (lit. m.p. 176–177°C) (Qui et al., 2001); [α]D25.7 + 13.3° (CH2Cl2, C 0.3); lit. [α]D + 18.0° (C 1.0) (Rao et al., Citation1975). Atranorin (.2)..: Colorless needles (CH2Cl2) (Brassy et al., Citation1982). Methyl-β.-orcinolcarboxylate (.3)..: Colorless small needles (CH2Cl2) (Caccamese et al., Citation1986). Zeorin (.4).:. Colorless needles (CH2Cl2) (Yosioka et al., Citation1972). 4-O-Methylcryptochlorophaeic acid (.5)..: Colorless needles (CH2Cl2) (Shibuya et al., Citation1983). Lichexanthone (.6).:. Straw yellow needles (CH2Cl2) (Hay & Harris, Citation1972).
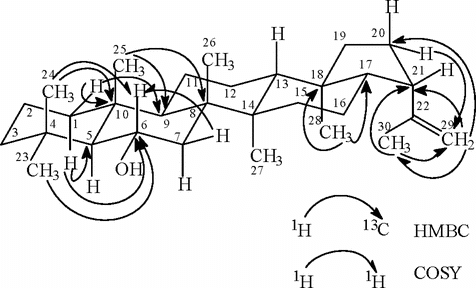
6α-Hydroxyhop-21β.H-22(29)-en (.7)..: Colorless powder; m.p. 153–155°C; 1H NMR (500 MHz, CDCl3): δ 0.60 (3H, s, Me-28), 0.75 (3H, s, Me-25), 0.85 (3H, s, Me-24), 0.88 (1H, s, H-1β.), 0.91 (3H, s, Me-27), 0.98 (3H, s, Me-26), 1.03 (3H, s, Me-23), 1.37 (2H, m, H-19), 1.47 (1H, s, H-7), 1.58 (1H, s, H-1α), 1.78 (3H, s, Me-30), 2.20 (1H, m, H-21), 2.70 (2H, m, H-20), 3.98 (1H, dt, J. = 16.8, 3.9 Hz, H-6), 4.80 (2H, bs, =CH2, H-29) and 1.10–2.00 (16H); 13C NMR (125 MHz, CDCl3): C-1: 40.5, C-2, C-26: 18.7, C-3, C-19: 44.0, C-4, C-15: 33.8, C-5: 61.3, C-6: 69.5, C-7: 45.7, C-8, C-14, C-18: 42.0, C-9: 49.2, C-10: 39.5, C-11: 21.2, C-12: 24.0, C-13: 50.0, C-16: 21.8, C-17: 55.1, C-20: 25.2, C-21: 46.6, C-22: 148.8, C-23: 36.9, C-24: 22.3, C-25: 17.3, C-27: 17.0, C-28: 16.2, C-29: 110.3, C-30: 27.5; EIMS m/z (rel. int. %) 409, 205, 189, 154, 135, 121, 107 (100); HREIMS (M-H)+, 425.3756; calcd. for C30H49O, 425.3783.
Glyceryl trilinolate (.8).:. Yellow oil (Yueh–Hsiung Kuo & Pei–Hungchu, 2002). 3,6-Dimethyl-2-hydroxy-4-methoxybenzoic acid (.9)..: Pale yellow needles; m.p. 195–196°C (lit. m.p. 196°C) (Tsuda et al., Citation1980).
Bioassays
The mosquito larvicidal assay was carried out on cabraleadiol monoacetate (1), atranorin (2), methyl-β.-orcinolcarboxylate (3), zeorin (4), 4-O-. methylcryptochlorophaeic acid (5), lichexanthone (6), and 3,6-dimethyl-2-hydroxy-4-methoxybenzoic acid (9) against the second instar larvae of Aedes aegypti. (Bandara et al., Citation2000).
Human sperm motility stimulating activity was measured against ejaculated human spermatozoa (Belsy et al., 1980) at 10, 100, 1000 µg/ml of test compound following 5, 15, 30 and 60 min of incubation. Evaluation was carried out on atranorin (2), zeorin (4), and lichexanthone (6).
Results and Discussion
The hexane extract of P. consocians. yielded cabraleadiol monoacetate (1). Treatment of compound (1) in 6% MeOH/KOH resulted in the loss of the acetyl moiety, yielding the corresponding alcohol (1′). Spectral and physical data of the hydrolyzed product were identical to those reported for cabraleadiol (1′) reported from the plants Dysoxylum malabaricum. (stem bark) (Hisham et al., Citation1996), Cabralea eichleriana. (wood) (Rao et al., Citation1975), Cabralea polytricha. (fruits) (Cascon & Brown, Citation1972), Aglaia lawaii. (Qui et al., Citation2001), and Nothofagus dombeyi. (Thoison et al., Citation2004). That our study constitutes the first report of a triterpenoid of the cabraleadiol family from a lichen is of chemotaxonomic significance.
The lichen powder of H. leucomelos. furnished compound 7 whose high resolution electron ionization mass spectrometry (HREIMS) showed a self-deprotonated molecular ion at 425.3756 suggesting an elemental composition of C30H50O. The 13C NMR spectrum showed 26 signals of which some were overlapping. The 1H/13C NMR spectra of compound 7 exhibited seven quarternary methyl singlets. HMBC and COSY data () readily identified the A–E ring system of a triterpenoid with an α-hydroxyl functionality at C-6 of ring B (δ 3.98, dt, J. = 16.8, 3.9 Hz, H-6) and an α-isopropylidene moiety attached to C-21 of ring E. A comparison of the 1H NMR chemical shift values for H-21 in zeorin (4) (δ 2.28, dt, J. = 14.7, 3.3 Hz), acetyl zeorin (δ 2.19, dt, J. = 12.4, 3.9 Hz), zeorinone (δ 2.19, dt, J. = 12.3, 1.8 Hz) with the triterpenoid 7 (δ 2.20, m), lent credence to the position and stereochemistry of the isopropylidine group. Furthermore, the NMR spectral data of compound 7 differed from those of lupeol (isopropylidene group at C-19) [compound 7: H-21 (1H δ 2.20, m; 13C δ 44.0); lupeol: H-19 (1H δ 2.38, dt, J. = 16.5, 5.7 Hz; 13C δ 48.3)]. To the best of our knowledge, the triterpenoid 7 is a new natural product.
The following compounds showed significant mosquito larvicidal activity against the second instar larvae of Aedes aegypti. at 10 ppm: Cabraleadiol monoacetate (1) (90% moribund after 24 h), 4-O.-methylcryptochlorophaeic acid (5) (60% dead after 24 h), lichexanthone (6) (80% moribund after 24 h), and 3,6-dimethyl-2-hydroxy-4-methoxybenzoic acid (9) (90% moribund after 24 h). 4-O.-Methylcryptochlorophaeic acid (5) is a known inhibitor of prostaglandin synthetase (Shibuya et al., Citation1983). Lichexanthone (6) exhibited sperm motility enhancing activity when incubated with human spermatozoa; the increment in motility was significant from the fifth min and lasted throughout the incubation period of 60 min. Currently there are only a few known sperm motility stimulants, including caffeine (Aitken et al., Citation1983), and there is a need and demand for new sperm stimulants to be used in asthenozoospermia and in some assisted reproductive program.
Acknowledgments
The authors thank the NSF and NRC, Sri Lanka, the International Foundation for Science, Stockholm, Sweden, and the Organisation for the Prohibition of Chemical Weapons (OPCW), The Hague, The Netherlands, through a grant to Dr. Veranja Karunaratne.
References
- Aitken RJ, Best F, Richardson DW, Schats R, Simm G (1983): Influence of Caffeine on movement characteristics, fertilizing capacity and ability to penetrate cervical mucus of human spermatozoa. J Reprod Fert 67: 19–27, [CSA]
- Bandara KANP, Kumar V, Jacobsson U, Molleyres P (2000): An insecticidal piperidine alkaloid from Microcos paniculata. stem bark. Phytochemistry 54: 29–32, [INFOTRIEVE], [CROSSREF], [CSA]
- Belsey MA, Eliasson R, Gallegos AJ, Moghissi KS, Paulsen KS, Prasad MRN (1980): WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction. Singapore, Press Concern, pp. 1–23.
- Brassy C, Bachet B, Bodo B, Molho D (1982): Structure de atranorine. Acta Crystallogr B 38: 3126–3128, [CROSSREF], [CSA]
- Caccamese S, Compagnini A, Toscano R. M, Cascio O (1986): Methyl-β.-orcinolcarboxylate and atranol from the lichen Stereocaulon vesuvianum.. J Nat Prod 49: 1159–1160, [CROSSREF], [CSA]
- Cascon SC, Brown KS, J (1972): Biogenetically significant triterpenes in a species of Meliaceae: Cabralea polytricha.. A. JUSS. Tetrahedron 28: 315–323, [CROSSREF], [CSA]
- Hay JV, Harris TM (1972): Biogenetic type syntheses of heptaketide natural products: Alternariol and lichexanthone. Chem Commun 16: 953–955, [CSA]
- Hisham A, Ajitha Bai MD, Fujimoto Y, Hara N, Shimada, H (1996): Complete 1H and 13C NMR spectral assignment of cabraleadiol, a dammarane triterpene from Dysoxylum malabaricum. Bedd. Magn Reson Chem 34: 146–150, [CROSSREF], [CSA]
- Huneck S (1999): The significance of lichens and their metabolites. Naturwissenschaften 86: 559–570, [INFOTRIEVE], [CROSSREF], [CSA]
- Kuo Y-H, Chu P-H (2002): Studies on the constituents from the bark of Bauhinia purpurea.. J. Chin Chem Soc 49: 269–274, [CSA]
- Qui S-X, van Hung N, Xuan LT, Gu J-Q, Lobkovsky E, Khanh, TC, Soejarto, DD, Clardy J, Pezzuto JM, Dong Y, Tri MV, Huong, LM, Fong HHS (2001): A pregnane steroid from Aglaia lawaii. and structure confirmation of caraleadiol monoacetate by X-ray crystallography. Phytochemistry 56: 775–780, [CROSSREF], [CSA]
- Rao MM, Meshulam H, Zelnik R, Lavie D (1975): Cabralea eichleriana. DC. (Meliaceae)—Structure and stereochemistry of wood extractives. Tetrahedron 31: 333–339, [CROSSREF], [CSA]
- Shibuya M, Ebizuka Y, Noguchi H, Iitaka Y, Sankawa U (1983): Inhibition of prostaglandin biosynthesis by 4-O.-methylcryptochlorophaeic acid; Synthesis of monomeric arylcarboxylic acids for inhibitory activity testing and X-ray analysis of 4-O.-methylcryptochlorophaeic acid. Chem Pharm Bull 31: 407–413, [INFOTRIEVE], [CSA]
- Thoison O, Sevenet T, Niemeyer HM, Russel GB (2004): Insect antifeedant compounds from Nothofagus dombeyi. and N. pumilio.. Phytochemistry 65: 73–76, [CROSSREF], [CSA]
- Tsuda Y, Nakajima S, Udayawa S-I, Uzawa J (1980): The isolation from Myrothecium. species and.long-range selective proton decoupling 13C NMR of rhizonic acid. J Nat Prod 43: 467–471, [CROSSREF], [CSA]
- Yosioka I, Nakanishi T, Yamauchi H, Kitagawa I (1972): Lichen triterpenoids. III. The final conclusion on the stereostructure of zeorin and its correlation with leucotylin. Chem Pharm Bull 20: 147–156, [CSA]