Abstract
Context: Berberine (BBR) can regulate enteric glial cells (EGCs) and the gut vascular barrier (GVB).
Objective: To explore whether BBR regulates GVB permeability via the S100B pathway.
Materials and methods: GVB hyperpermeability in C57BL/6J mice was induced by burns or S100B enema. BBR (25 or 50 mg/kg/d, 3 d) was gavaged preburn. S100B monoclonal antibody (S100BmAb) was i.v. injected postburn. Mouse intestinal microvascular endothelial cells (MIMECs) were treated with S100B, S100B plus BBR, or Z-IETD-FMK. GVB permeability was assayed by FITC-dextran, S100B by ELISA, caspase-8, β-catenin, occludin and PV-1 by immunoblot.
Results: Burns elevated S100B in serum and in colonic mucosa to a peak (147.00 ± 4.95 ng/mL and 160.30 ± 8.50 ng/mg, respectively) at 36 h postburn, but BBR decreased burns-induced S100B in serum (126.20 ± 6.30 or 90.60 ± 3.78 ng/mL) and in mucosa (125.80 ± 12.40 or 91.20 ± 8.54 ng/mg). Burns raised GVB permeability (serum FITC-dextran 111.40 ± 8.56 pg/mL) at 48 h postburn, but BBR reduced GVB permeability (serum FITC-dextran 89.20 ± 6.98 or 68.60 ± 5.50 ng/mL). S100B enema (1 μM) aggravated burns-raised GVB permeability (142.80 ± 8.07 pg/mL) and PV-1, but the effect of S100B was antagonized by BBR. Z-IETD-FMK (5 μM) increased S100B-induced permeability to FITC-dextran (205.80 ± 9.70 to 263.80 ± 11.04 AUs) while reducing β-catenin in MIMECs. BBR (5 μM) reduced S100B-induced permeability (104.20 ± 9.65 AUs) and increased caspase-8, β-catenin and occludin.
Discussion and conclusion: BBR decreases burns-induced GVB hyperpermeability via modulating S100B/caspase-8/β-catenin pathway and may involve EGCs.
Introduction
Like gut epithelial barrier, GVB is involved in controlling the systemic dissemination of gut bacteria (Spadoni et al. Citation2015). EGCs are a large population in the enteric nervous system and serve as a component of gut vascular unit (Spadoni et al. Citation2016). The phenotypes of EGCs can be classified into the activated, reactive and gliopathy phenotypes (Seguella and Gulbransen Citation2021). The activated phenotype is defined as the response to physiological stimuli while the reactive phenotype is the response to pathological stimuli with a perturbation of any severity. Reactive EGCs not only secrete neurotrophins such as glial-derived S-nitrosoglutathione (GSNO), nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and glial-derived neurotrophic factor (GDNF) (Costantini et al. Citation2010; von Boyen et al. Citation2011), but also substantially produce pro-inflammatory molecules such as S100 and TNF-α at the expense of homeostatic functions (Seguella and Gulbransen Citation2021).
S100B protein is not only a small and easily diffusible neurotrophic factor, but also a cytokine. S100B exerts either a trophic or detrimental effect depending on its extracellular level. In the brain, it has a proliferative and neurogenic effect on the astroglia and neurons at nanomolar concentrations, while a neurodegenerative function at micromolar concentrations. Studies have suggested the involvement of S100B in intestinal homeostasis and inflammatory processes. S100B protein in gut is specifically and physiologically expressed and released by EGCs (Rühl Citation2005; Cirillo et al. Citation2011). In a pathological condition, EGCs can substantially generate S100B and high level of S100B often leads to dysbiosis (Kimono et al. Citation2019; Costa et al. Citation2021), epithelial barrier disruption (Cirillo et al. Citation2011; Kimono et al. Citation2019). It is noteworthy that many similarities exist between the blood-brain barrier (BBB) and GVB (Spadoni et al. Citation2015, Citation2016). High level of S100B impairs BBB integrity and function (Koh and Lee Citation2014; Zhang et al. Citation2021). However, it is not clear whether high concentrations of S100B in the gut can compromise GVB.
Berberine (BBR) is an isoquinoline alkaloid presenting in numerous medicinal plants of Coptis Salisb (Ranunculaceae) and Phellodendron Rupr. (Rutaceae), initially well-known for the significant effects on gastroenteritis and bacillary dysentery in Chinese and Ayurvedic medicinal systems. BBR can inhibit gut inflammation and bacteria dissemination (Feng et al. Citation2012; Xu et al. Citation2017) via modulation of gut microbial (Xu et al. Citation2017), epithelial barrier (Feng et al. Citation2012; Gu et al. Citation2013) and vascular barrier (He et al. Citation2018; Li et al. Citation2020). Recently, this alkaloid has also been reported to be effective to attenuate colitis and nonsteroidal anti-inflammatory drugs (NSAIDs)- induced gut mucosal injury via regulation of the function of EGCs (Chao et al. Citation2020; Li et al. Citation2020).
Gut-derived sepsis is an important cause of death in severely burned patients. Studies have indicated that severe burns may elicit a remarkable increase in the intestinal permeability in patients and rat/mouse models with ≥30% total body surface area (TBSA) full-thickness burns (Ziegler et al. Citation1988; Costantini et al. Citation2009, Citation2022; Earley et al. Citation2015; Feng et al. Citation2019), leading to bacterial translocation and sepsis. In addition, severe burns may affect the activity and function of EGCs (Costantini et al. Citation2010). In this mouse model of burns, we explored whether BBR could modulate EGCs- derived S100B production to affect GVB permeability. Our results revealed that BBR pretreatment inhibited S100B generation to reduce GVB permeability via modulation of caspase-8/β-catenin signalling.
Materials and methods
Experimental animals
Male C57BL/6J mice (6-week-old, 22–26 g) were purchased from the Laboratory Animal Centre of Southern Medical University, China. Mice were housed in a 21–22 °C temperature and humidity environment with a 12 h light/dark cycle and free access to chow diet and water. This experiment protocol was approved by the Animal Care and Ethics Committee of Guangdong Medical University (GDY2202033). All researchers complied with ARRIVE Guidelines 2.0.
Mouse model of scald injury
The protocols producing full-thickness cutaneous burns covering 40% total body surface area (TBSA) in the dorsal skin of mice were previously described (Zang et al. Citation2010). In brief, mice were anesthetized with 3% isoflurane. Scald injury was generated by applying brass probes (2 × 3 cm with 3 mm thickness) that were preheated to 100 °C in boiling water to the animal’s sides and back for 5 s. Sham animals were subjected to a room temperature brass probe. All mice were resuscitated with 2 mL saline and the complications were monitored. All mice were euthanized by cervical dislocation at the end of this experiment.
S100BmAb, recombinant S100B and BBR treatment
Mouse S100BmAb (InvitrogenTM, MA1-25005), dissolved in saline, was i.v. injected into the mice at the dose of 5 (S100BmAb5), 10 (S100BmAb10), or 15 (S100BmAb15) μg/kg immediately after burn injury. Mouse recombinant S100B (Absin®, abs04491) protein was diluted by saline to two different concentrations (1 or 5 μM). S100B solution (200 μL) was given via colorectum enema following burn injury. BBR (MedChemExpress, CAS:633-65-8), dissolved in saline by heating, was given via oral gavage at the dose of 25 (BBR25) or 50 mg/kg/d (BBR50) for consecutive 3 days prior to burn procedure.
Gut vascular barrier permeability assessment
A laparotomy was performed on the anesthetized mice to expose ilealcecum. Two ligatures were placed in caecum to make a loop to assess GVB permeability to FITC-dextran 70 kDa (Spadoni et al. Citation2015). 100 μL FITC-dextran 70 kDa (1 mg/mL) was injected into the gut loop and blood was collected by a cardiac puncture at indicated time-points. Blood samples were centrifuged (8000 rpm, 10 min, 4 °C) to obtain serum samples. FITC-dextran 70 kDa level in serum was assayed by a spectrophotometer (Infinite M200 PRO, Tecan) at 480 nm excitation and 520 nm emission.
S100B content in serum and colonic mucosa
The preparation of gut mucosal scrapings was performed as previously described (Feng et al. Citation2011). In brief, the caecum of each mouse was removed and rinsed with 0.9% physiological saline. About 2 cm gut segments were prepared for immunoblot experiment. The gut mucosal scrapings were scraped with a glass slide. S100B content in colonic mucosal scrapings and in serum was detected by using ELISA kits according to manufacturer’s instructions. The levels of serum TNF-α and IL-1β were also determined by using TNF-α and IL-1β ELISA kits.
Cell culture and treatment
Cell line MIMECs (Cat#: C1476) were purchased from ChenXue BioTech Co. Guangzhou, China. MIMECs were cultured in DMEM containing 15% fetal bovine serum (FBS), 100 U/mL of penicillin and streptomycin and 0.584 g/L of glutamine in a 37 °C incubator under 5% CO2. Cells were treated with 0.25% trypsin and 0.02% EDTA and then passaged twice. The subcultured cells were allowed to grow to 70%–80% confluence before experiments.
Transendothelial permeability assays
Transwell inserts (6.5 mm) were used for endothelial permeability experiments. MIMECS were treated with different concentrations of mouse recombinant S100B or Z-IETD-FMK (caspase-8 inhibitor, ab141382, Abcam). HBSS (100 μL) containing 0.5 mg/mL FITC-dextran 70 kDa was added into confluent MIMECs; 2 h later, FITC-dextran clearance across the filter to the lower chamber was assayed by a spectrophotometer (Infinite M200 PRO, Tecan) at λem/ex = 520/480 nm. Data were expressed as arbitrary units (AUs) (Dudek et al. Citation2011).
Western blot
Total protein from gut mucosa and MIMECs was extracted in RIPA lysis buffer, followed by ultrasonication, and centrifugation at 12,000 rpm for 10 m at 4 °C. Protein concentration in the supernatant was determined with a bicinchoninic acid (BCA) assay (Thermo Fisher Scientific, Waltham, USA). Proteins were separated on 10% SDS-PAGE gels and then transferred to PVDF membranes. Membrane was blocked with 5% BSA/TBST for 1 h at room temperature and then incubated with primary antibodies overnight at 4 °C: occludin (1:500, ab222691, Abcam), PV-1 (1:500, LS-C803663, LSBio), β-catenin (1:500, ab264261, Abcam) and β-actin (1:1000, ab8227, Abcam) antibodies, followed by incubation with corresponding secondary antibody (1:3000; Santa Cruz) for 1 h at room temperature. Immune complexes were revealed by using the enhanced chemiluminescence detection reagents. Immunoblots were analyzed by an ImageQuant LAS 4000 Scanner (GE Healthcare Life Sciences).
Statistical analysis
All statistical analyses were performed by using the SPSS software (version 19.0; SPSS Inc., Chicago, IL, USA). The results from tests of normality showed data were normally distributed. Data were expressed as mean ± standard deviation (SD) or error (SEM). Significance among groups was tested by the analysis of Student’s t-test or one-way variance (ANOVA) followed by Bonferroni post hoc test. p < 0.05 was considered statistically significant.
Results
BBR pretreatment lowered serum TNF-α, IL-1β, and S100B concentration in burned mice
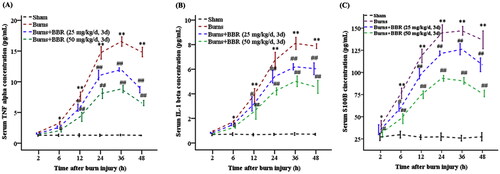
Severe burns may elicit the production and release of proinflammatory molecules (Zhang et al. Citation2008; Ruan et al. Citation2014). We assayed TNF-α (A), IL-β (B), and S100B (C) in serum. Data showed the concentrations of these cytokines were low in sham mice (TNF-α: 1.30 ± 0.15, IL-β: 0.71 ± 0.09, and S100B: 27.40 ± 3.71 ng/mL at 48 h postburn), but increased in burned individuals. The levels of TNF-α, IL-1β and S100B were 1.70 ± 0.15, 0.94 ± 0.12, 39.20 ± 6.53 ng/mL, respectively, at 2 h postburn, and rose up to 16.54 ± 0.78, 8.10 ± 0.50, 147.00 ± 4.95 ng/mL, respectively, at 36 h postburn, and reduced to 14.84 ± 1.02, 7.88 ± 0.28, 136.80 ± 10.12 ng/mL, respectively, at 48 h postburn (p < 0.01). BBR treatment lowered serum S100B, TNF-α and IL-1β levels in burned mice. For instance, serum TNF-α, IL-1β, and S100B levels were 11.98 ± 0.43, 6.20 ± 0.36, 126.20 ± 6.30 ng/mL, respectively, in BBR25 subgroup, and 9.22 ± 0.46, 5.02 ± 0.43, 90.60 ± 3.78 ng/mL, respectively, in BBR50 subgroup at 36 h postburn (p < 0.01) ().
Figure 1. BBR pretreatment lowered serum TNF-α, IL-1β and S100B levels in burned mice. Mice were assigned into sham, burns, burns plus BBR (25 mg/kg/d, 3 d), or burns plus BBR (50 mg/kg/d, 3 d) group. At indicated timepoints (2, 6, 12, 24, 36, 48 h postburn) 4 mice were euthanized. Blood samples were centrifuged and serum TNF-α (A), IL-1β (B) and S100B (C) levels were assayed by ELISA. Data were expressed as mean ± SD. *p < 0.05, **p < 0.01 vs. Sham; #p < 0.05, ##p < 0.01 vs. Burns.
BBR pretreatment reduced S100B generation in colonic mucosa of burned mice
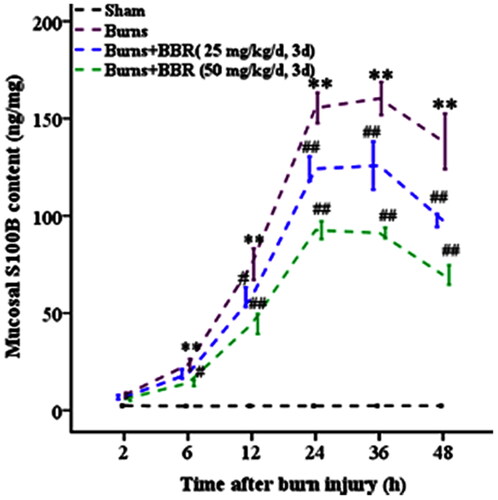
EGCs can be activated by burns to produce neurotrophins and cytokines. Data showed that in sham mice S100B content in colonic mucosa was low (2.29 ± 0.40 ng/mg). In burned mice S100B content slowly increased in 6 h postburn (23.48 ± 3.00 ng/mg at 6 h postburn), and rapidly elevated in 6–24 h postburn (155.43 ± 7.83 ng/mg at 24 h postburn), and kept a steady state in 24–36 h postburn (160.30 ± 8.50 ng/mg at 36 h postburn), and then declined in 36–48 h postburn (138.28 ± 14.20 ng/mg at 48 h postburn) (p < 0.01). BBR pretreatment significantly decreased burn-elicited S100B generation in colonic mucosa (125.80 ±12.40 ng/mg in BBR25 and 91.20 ± 8.54 ng/mg in BBR50 subgroup at 36 h postburn) (p < 0.01) ().
Figure 2. BBR pretreatment reduced S100B generation in colonic mucosa in burned mice. Mice were assigned into sham, burns, burns plus BBR (25 mg/kg/d, 3 d), or burns plus BBR (50 mg/kg/d, 3 d) groups. At every postburn timepoint 4 mice were euthanized. Gut mucosal tissues were scrapped and S100B content in mucosa was determined by ELISA. Data were expressed as mean ± SD. *p < 0.05, **p < 0.01 vs. Sham; #p < 0.05, ##p < 0.01 vs. Burns.
BBR and S100BmAb treatment lowered GVB permeability in burned mice
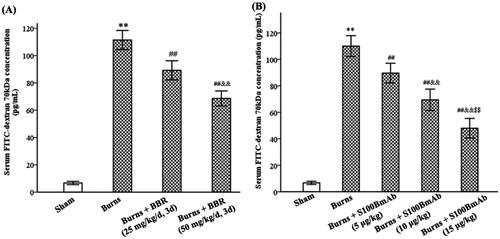
The effect of S100B in gut mucosa on GVB permeability was assessed. As shown in , burns significantly increased GVB permeability to FITC-dextran. The levels of serum FITC-dextran were 6.70 ± 1.21 and 111.40 ± 8.56 pg/mL in sham and burned mice at 48 h postburn, respectively (p < 0.01). Subsequently, the effect of BBR and S100BmAb on GVB permeability was assessed. The results showed BBR and S100BmAb lowered burn-induced GVB permeability. FITC-dextran levels were 89.20 ± 6.98 and 68.60 ± 5.50 ng/mL, respectively, in BBR25 and BBR50 subgroups, as well as 89.60 ± 7.40, 69.40 ± 8.11 and 48.00 ± 7.38 ng/mL, respectively, in S100BmAb5, S100BmAb10, and S100BmAb15 subgroups at 48 h postburn (p < 0.01).
Figure 3. BBR and S100BmAb treatment reduced GVB permeability in burned mice. FITC-dextran 70 kDa was injected into colonic loops of mice treated with sham, burns, burns plus BBR (25 or 50 mg/kg/d, 3 d) (A), or buns plus S100BmAb (5, 10 or 15 μg/kg) (B). Serum FITC-dextran 70 kDa was measured at 48 h postburn. Data were expressed as mean ± SD. **p < 0.01 vs. Sham; ##p < 0.01 vs. Burns; &&p < 0.01 vs. Burns plus BBR (25 mg/kg/d, 3 d) or Burns plus S100BmAb (5 μg/kg); $$p < 0.01 vs. Burns plus S100BmAb (10 μg/kg).
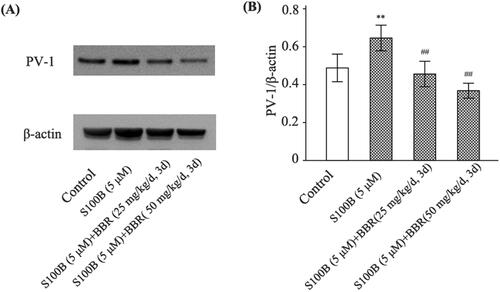
BBR pretreatment lowered S100B-increased GVB permeability and PV-1 expression
GVB permeability was also evaluated in mice treated with recombinant S100B. As shown in , S100B enema not only increased GVB permeability in control mice (FITC-dextran levels were 4.40 ± 1.14, 18.60 ± 2.41, and 80.20 ± 4.92 pg/mL in saline, S100B25, and S100B50 subgroups, respectively, p < 0.01), but also raised GVB permeability in burned mice (FITC-dextran levels were 4.66 ± 1.17, 93.80 ± 5.51, and 142.80 ± 8.07 pg/mL in sham, burns, and burns plus S100B groups, respectively, p < 0.01). BBR pretreatment abolished the effect of S100B enema on GVB permeability in both control and burned mice. In addition, immunoblot analysis revealed S100B enema upregulated the expression of PV-1, a marker of endothelial leakage (Spadoni et al. Citation2015), but this effect of S100B enema was abrogated by BBR. The density ratio of PV-1 to β-actin was 0.48 ± 0.08 and 0.66 ± 0.07, respectively, in control and S100B group (p < 0.01), as well as 0.46 ± 0.06 and 0.37 ± 0.04, respectively, in BBR subgroups (p < 0.01) (), indicating BBR exerted an inhibition on PV-1 expression.
Figure 4. S100B enema increased while berberine pretreatment reduced GVB permeability. Control mice were treated with saline enema, S100B enema (1 or 5 μM), or S100B enema plus BBR (25 or 50 mg/kg/d, 3 d) (A). Burned mice were treated with S100B enema (1 μM), BBR (25 or 50 mg/kg/d, 3 d), or S100B enema plus BBR (B). Serum FITC-dextran 70 kDa was assayed 12 h after S100B enema. Data were expressed as mean ± SD. **p < 0.01 vs. saline or sham; ##p < 0.01 vs. S100B (5 μM) or Burns; &&p < 0.01 vs. S100B (5 μM) plus BBR (25 mg/kg/d, 3 d) or Burns plus BBR (25 mg/kg/d, 3 d); δδp < 0.01 vs. Burns plus S100B (1 μM).
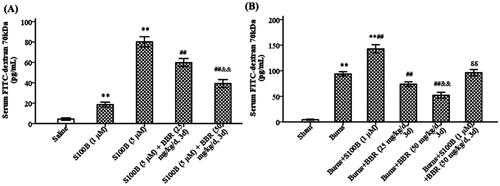
Figure 5. S100B enema increased while BBR pretreatment decreased PV-1 expression in mice. Control mice were treated with saline enema, S100B enema (5 μM), or S100B enema plus BBR (25 or 50 mg/kg/d, 3 d). PV-1 expression was assayed by western blot. Representative gels for PV-1 (A). The relative density of PV-1 was calculated relative to that of β-actin (B). Results shown are means ± SD. **p < 0.01 vs. Control; ##p < 0.01 vs. S100B (5 μM).
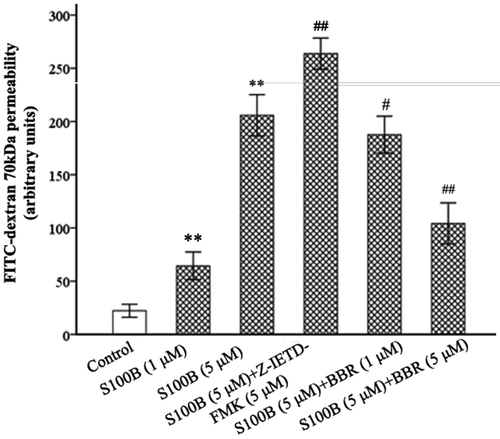
Berberine treatment lowered S100B-increased endothelial permeability of MIMECs
The effect of recombinant S100B on the endothelial permeability of MIMECs was evaluated. As demonstrated in , after 2 h S100B (1 and 5 μM) incubation the permeability of MIMECs to FITC-dextran markedly increased (64.40 ± 6.50 and 205.80 ± 10.70 AUs, respectively, p < 0.01), S100B augmented the leakage of the endothelial barrier. However, S100B-raised permeability of MIMECs to FITC-dextran was decreased by 1 μΜ BBR (187.60 ± 8.68 AUs) (p = 0.018) and 5 μΜ BBR (104.20 ± 9.65 AUs) (p < 0.01).
Figure 6. S100B and Z-IETD-FMK increased while BBR decreased MIMECs permeability. MIMECs were treated with saline (Control), S100B (1 or 5 μM), S100B plus Z-IETD-FMK (5 μM), or S100B plus BBR (1 or 5 μM). FITC-dextran 70 kDa was used to assess the endothelial permeability. The experiments were performed in triplicate. Data were expressed as means ± SEM. **p < 0.01 vs. Control; #p < 0.05, ##p < 0.01 vs. S100B (5 μM).
Berberine treatment increased occludin expression in S100B-treated MIMECs
The endothelial barriers are characterized by the presence of junctional complexes including tight junction (TJ). Occludin, a member of TJ protein family, plays a role in the maintenance of GVB integrity (Spadoni et al. Citation2015; Xiao et al. Citation2022). Data showed that recombinant S100B reduced occludin generation (the density ratio of occludin to β-actin was 0.83 ± 0.05 and 0.29 ± 0.03, respectively, in control and S100B groups, p < 0.01). However, the expression of occludin in S100B-treated MIMECs was up-regulated by BBR. The density ratio was 0.39 ± 0.04 (p = 0.032) and 0.73 ± 0.05 (p < 0.01) in BBR subgroups, respectively ().
Figure 7. S100B reduced while BBR increased occludin and caspase-8 expression in MIMECs. MIMECs were treated with saline (Control), S100B (5 μM), or S100B plus BBR (1 or 5 μM). Occludin and caspase-8 was assayed by Western blot. Representative gels were shown in the left panel (A) and statistical results in the right panel (B). The experiments were performed in triplicate. Data were expressed as means ± SEM. **p < 0.01 vs. Control; #p < 0.05, ##p < 0.01 vs. S100B (5 μM).
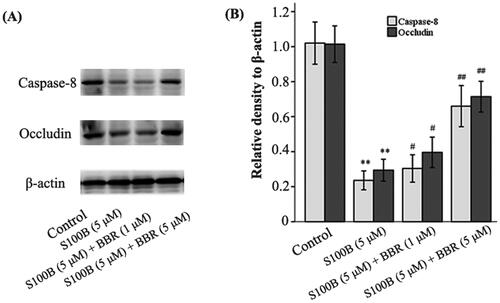
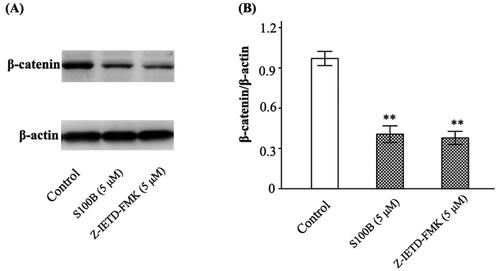
Berberine treatment increased caspase-8 and β-catenin in S100B-treated MIMECs
Caspase-8 and β-catenin contribute to the maintenance of GVB (Spadoni et al. Citation2015; Tisch et al. Citation2022). Data showed that recombinant S100B (5 μΜ) decreased caspase-8 (the density ratio to β-actin was 0.90 ± 0.06 and 0.24 ± 0.03 in control and S100B group, respectively, p < 0.01) and β-catenin (the density ratio to β-actin was 0.97 ± 0.07 and 0.40 ± 0.06 in control and S100B group, respectively, p < 0.01) in MIMECs. This effect of 5 μΜ S100B was antagonized by 1 μΜ BBR (0.29 ± 0.03, p = 0.037) and 5 μΜ BBR (0.67 ± 0.08, p < 0.01) ( and ). The inhibition of caspase-8 by Z-IETD-FMK not only raised the permeability of S100B-treated MIMECs (263.80 ± 11.04 AUs, p < 0.01) (), but also decreased β-catenin expression (the density ratio to β-actin was 0.38 ± 0.05, p < 0.01) ().
Figure 8. S100B and Z-IETD-FMK decreased β-catenin expression in MIMECs. MIMECs were treated with saline (Control), S100B (5 μM), or Z-IETD-FMK (5 μM). β-catenin was determined by Western blot. Representative gels were shown in the left panel (A) and the statistical results in the right panel (B). The experiments were performed in triplicate. Data were expressed as means ± SEM. **p < 0.01 vs. Control.
Discussion
GVB is an anatomical barrier in intestinal mucosa in human and mice (Spadoni et al. Citation2015). This specific barrier controls the trafficking of antigens and microbiota into blood. The disruption and hyperpermeability of GVB is related to diseases such as sepsis, liver cirrhosis, spondylitis, colorectal cancer metastasis and steatohepatitis (Ciccia et al. Citation2017; Mouries et al. Citation2019; Sorribas et al. Citation2019; Grander et al. Citation2020; Bertocchi et al. Citation2021). Studies have suggested that severe burns can induce gut mucosal damage, high permeability, and systemic dissemination of bacteria in patients and in animal models. In this mouse model, we observed that severe burns not only induced the generation and release of proinflammatory cytokines (TNF-α, IL-1β, and S100B) into blood, but also damaged GVB integrity resulting in the increase in GVB permeability and PV-1 expression in intestinal mucosa. Importantly, we also confirmed that BBR pretreatment exerted a protective action to GVB as previously described (He et al. Citation2018), with the evidence that it prevented against curtail burn-elicited GVB hyperpermeability and PV-1 protein overexpression.
BBR is an isoquinoline alkaloid that is poorly absorbed in the gastrointestinal tract so that it can adequately contact with the gut mucosal barrier. Studies have suggested that BBR possesses multiple pharmacological properties containing antibacterial, antifungal, and anti-inflammatory actions, as well as blocking bacterial dissemination. The protective roles of BBR on gut epithelial and vascular barriers have also been reported in previous literature (Feng et al. Citation2011; Gu et al. Citation2013; He et al. Citation2018), but the mechanisms are not fully clear. However, BBR has been reported to affect the enteric nervous system including the neuron and glial cells (Chen et al. Citation2013; Yu et al. Citation2019) and reduces barrier permeability via modulation of EGCs activity (Chao et al. Citation2020; Li et al. Citation2020). Therefore, in this mouse model of severe burns we explored whether the protection of BBR on GVB was associated with its regulating EGCs activity and function.
The significant role of EGCs in modulation of GVB and systemic dissemination of bacteria (Spadoni et al. Citation2015) drew our attention. In the enteric nervous system, EGCs regulate not only the function of enteric neurons and muscle contraction, but also the gut microbial, immune and epithelial barriers. According to the criteria (Seguella and Gulbransen Citation2021), EGCs in response to the thermal stimulus are classified as a reactive phenotype, which describes the status of EGCs responding to a pathophysiological perturbation of any severity. EGCs exert different biological effects depending on its release of neurotrophins (e.g., GDNF, BDNF) and cytokines (e.g., TNF-α, IL-1β). For example, during the early postburn stage EGCs may protect gut barrier via release of GSNO (Costantini et al. Citation2010). However, reactive EGCs in most pathological conditions are harmful due to the substantial release of inflammatory cytokines.
S100B protein is not only a neurotrophins, but also a proinflammatory cytokine. S100B in the gut is produced by EGCs (Cirillo et al. Citation2011). Many studies have suggested that high levels of S100B can amplify/sustain inflammation (Cirillo et al. Citation2011; Esposito et al. Citation2014; Seguella and Gulbransen Citation2021) and impair gut epithelial barrier (Cirillo et al. Citation2011). Severe trauma is a strong inducer for S100B generation. High serum S100B level usually present in severely burned patients (Anderson et al. Citation2001). In this mouse model, we observed S100B, as well as TNF-α and IL-1β, in serum elevated in first 0–36 h and then descended in 36–48 h postburn. We also found that S100B content in colonic mucosa were low in 0–6 h postburn, rapidly increased in 6–24 h postburn, and then kept a peak during 24–36 postburn, and then slowly declined in 36–48 h postburn, suggesting S100B might exert different biological effects with time after severe burns. BBR pretreatment lowered S100B in serum and mucosa, suggesting BBR could regulate S100B generation.
There are similarities between BBB and GVB. High levels of S100B is pro-inflammatory and toxic leading to the increase in BBB permeability (Li et al. Citation2014; Zou et al. Citation2022). We thus suspected high S100B could impair GVB and augment GVB permeability. In order to confirm this hypothesis, we used S100BmAb to block S100B signalling. We assessed GVB permeability via measurement of serum FITC-dextran 70 kDa as previously described (Spadoni et al. Citation2015). The results demonstrated that burns increased serum FITC-dextran level with time. S100BmAb dose- dependently reduced burns-induced GVB permeability. In another experiment, we treated mice with recombinant S100B via enema. As expected, S100B enema increased GVB permeability to FITC-dextran in both control and burned mice. PV-1 is a reliable marker of vascular endothelial leakage (Spadoni et al. Citation2015). We demonstrated that S100B enema increased PV-1 expression in colonic mucosa. Recently, one study reports that BBR reduces sepsis-induced GVB permeability (Li et al. Citation2020). In this study we showed that BBR lowered burn- and S100B-elicited GVB hyperpermeability and reduced PV-1 protein expression. In vitro experiments, S100B at μM concentrations increased the permeability of MIMECs, but the effect of it was counteracted by BBR. Accordingly, BBR could reduce GVB permeability via regulation of S100B pathway.
The increase in permeability of GVB in burned mice and S100B-treated MIMECs indicated the disruption of the endothelial barrier. The endothelial barriers are characterized by the presence of junctional complexes that include tight junctional complexes, which control the paracellular trafficking of solutes and fluids. Occludin protein is usually used to evaluate tight junction and GVB integrity (Spadoni et al. Citation2015; Li et al. Citation2020; Liu et al. Citation2020). In this study, the expression of occludin protein in MIMECs treated by recombinant S100B was measured. The results showed that S100B decreased occludin expression in MIMECs. Inversely, BBR up-regulated occludin in S100B-treated MIMECs, suggesting this alkaloid regulated the tight junction of intestinal micro-vascular endothelial barrier through modulation of S100B pathway.
Caspase-8 is a member of caspase family with a dual role in cell death and survival (Xia et al. Citation2021). It activates caspase-3 to induce apoptosis (Kang et al. Citation2004; Chandirasegaran et al. Citation2017). However, caspase-8 is also a pro-survival factor for gut epithelial cells (Günther et al. Citation2011) and mature endothelial cells (Tisch et al. Citation2022). Importantly, caspase-8 is required to maintain vascular homeostasis in the intestine. The loss of caspse-8 in the gut endothelial cells leads to haemorrhages, inflammation, and GVB hyperpermeability (Tisch et al. Citation2022). In this study, Z-IETD-FMK was used to interrupt caspase-8 signalling. Our results showed that Z-IETD-FMK not only aggravated S100B-mediated endothelial hyperpermeability, but also decreased the expression of occludin in MIMECs, suggesting that caspase-8 affected GVB integrity via S100B pathway. Additionally, we confirmed that S100B reduced caspase-8 in MIMECs, while this effect was abolished by BBR, in other words, BBR was capable of regulating S100B/caspase-8 signalling pathway.
Wnt/β-catenin signalling plays a key role in the maintenance of GVB integrity (Spadoni et al. Citation2015). In this experiment, we confirmed that recombinant S100B interfered with the activation of β-catenin pathway, as shown by the reduction in β-catenin expression in MIMECs. We also confirmed that Z-IETD-FMK not only increased the permeability of S100B-treated MIMECs to FITC-dextran, but also decreased β-catenin expression in S100B-treated MIMECs, indicating that caspase-8 regulated β-catenin generation. Finally, we confirmed that BBR upregulated caspase-8 in S100B-treated MIMECs. Accordingly, BBR was a regulator of caspase-8/β-catenin signalling.
Conclusions
BBR pretreatment curtailed S100B production in mucosa to lower GVB permeability in burned mice. Mechanistically, BBR protected against burns-induced GVB injury in part via modulation of S100B/caspase-8/β-catenin signalling and may involve enteric glial cells.
Disclosure statement
The authors declare that they have no conflicts of interest.
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon request.
Additional information
Funding
References
- Anderson RE, Hansson LO, Nilsson O, Dijlai-Merzoug R, Settergren G. 2001. High serum S100B levels for trauma patients without head injuries. Neurosurgery. 48(6):1255–1260. discussion 1258-1260. doi: 10.1227/00006123-200106000-00012.
- Bertocchi A, Carloni S, Ravenda PS, Bertalot G, Spadoni I, Lo Cascio A, Gandini S, Lizier M, Braga D, Asnicar F, et al. 2021. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell. 39(5):708–724 e711. doi: 10.1016/j.ccell.2021.03.004.
- Chandirasegaran G, Elanchezhiyan C, Ghosh K, Sethupathy S. 2017. Berberine chloride ameliorates oxidative stress, inflammation and apoptosis in the pancreas of Streptozotocin induced diabetic rats. Biomed Pharmacother. 95:175–185. doi: 10.1016/j.biopha.2017.08.040.
- Chao G, Ye F, Yuan Y, Zhang S. 2020. Berberine ameliorates non-steroidal anti-inflammatory drugs-induced intestinal injury by the repair of enteric nervous system. Fundam Clin Pharmacol. 34(2):238–248. doi: 10.1111/fcp.12509.
- Chen DP, Xiong YJ, Lv BC, Liu FF, Wang L, Tang ZY, Lin Y. 2013. Effects of berberine on rat jejunal motility. J Pharm Pharmacol. 65(5):734–744. doi: 10.1111/jphp.12026.
- Ciccia F, Guggino G, Rizzo A, Alessandro R, Luchetti MM, Milling S, Saieva L, Cypers H, Stampone T, Di Benedetto P, et al. 2017. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann Rheum Dis. 76(6):1123–1132. doi: 10.1136/annrheumdis-2016-210000.
- Cirillo C, Sarnelli G, Esposito G, Turco F, Steardo L, Cuomo R. 2011. S100B protein in the gut: the evidence for enteroglial-sustained intestinal inflammation. World J Gastroenterol. 17(10):1261–1266. doi: 10.3748/wjg.v17.i10.1261.
- Costa DVS, Moura-Neto V, Bolick DT, Guerrant RL, Fawad JA, Shin JH, Medeiros P, Ledwaba SE, Kolling GL, Martins CS, et al. 2021. S100B inhibition attenuates intestinal damage and diarrhea severity during Clostridioides difficile infection by modulating inflammatory response. Front Cell Infect Microbiol. 11:739874. doi: 10.3389/fcimb.2021.739874.
- Costantini TW, Bansal V, Krzyzaniak M, Putnam JG, Peterson CY, Loomis WH, Wolf P, Baird A, Eliceiri BP, Coimbra R. 2010. Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am J Physiol Gastrointest Liver Physiol. 299(6):G1308–1318. doi: 10.1152/ajpgi.00156.2010.
- Costantini TW, Coimbra R, Weaver JL, Eliceiri BP. 2022. Precision targeting of the vagal anti-inflammatory pathway attenuates the systemic inflammatory response to burn injury. J Trauma Acute Care Surg. 92(2):323–329. doi: 10.1097/TA.0000000000003470.
- Costantini TW, Loomis WH, Putnam JG, Kroll L, Eliceiri BP, Baird A, Bansal V, Coimbra R. 2009. Pentoxifylline modulates intestinal tight junction signalling after burn injury: effects on myosin light chain kinase. J Trauma. 66(1):17–24. discussion 24-15. doi: 10.1097/TA.0b013e318191bb1f.
- Dudek SM, Muñoz NM, Desai A, Osan CM, Meliton AY, Leff AR. 2011. Group V phospholipase A2 mediates barrier disruption of human pulmonary endothelial cells caused by LPS in vitro. Am J Respir Cell Mol Biol. 44(3):361–368. doi: 10.1165/rcmb.2009-0446OC.
- Earley ZM, Akhtar S, Green SJ, Naqib A, Khan O, Cannon AR, Hammer AM, Morris NL, Li X, Eberhardt JM, et al. 2015. Burn injury alters the intestinal microbiome and increases gut permeability and bacterial translocation. PLOS One. 10(7):e0129996. doi: 10.1371/journal.pone.0129996.
- Esposito G, Capoccia E, Turco F, Palumbo I, Lu J, Steardo A, Cuomo R, Sarnelli G, Steardo L. 2014. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-alpha activation. Gut. 63(8):1300–1312. doi: 10.1136/gutjnl-2013-305005.
- Feng AW, Gao W, Zhou GR, Yu R, Li N, Huang XL, Li QR, Li JS. 2012. Berberine ameliorates COX-2 expression in rat small intestinal mucosa partially through PPARgamma pathway during acute endotoxemia. Int Immunopharmacol. 12(1):182–188. doi: 10.1016/j.intimp.2011.11.009.
- Feng AW, Yu C, Mao Q, Li N, Li QR, Li JS. 2011. Berberine hydrochloride attenuates cyclooxygenase-2 expression in rat small intestinal mucosa during acute endotoxemia. Fitoterapia. 82(7):976–982. doi: 10.1016/j.fitote.2011.05.013.
- Feng Y, Huang Y, Wang Y, Wang P, Wang F. 2019. Severe burn injury alters intestinal microbiota composition and impairs intestinal barrier in mice. Burns Trauma. 7(20):1–14.
- Grander C, Grabherr F, Spadoni I, Enrich B, Oberhuber G, Rescigno M, Tilg H. 2020. The role of gut vascular barrier in experimental alcoholic liver disease and A. muciniphila supplementation. Gut Microbes. 12(1):1851986. doi: 10.1080/19490976.2020.1851986.
- Gu L, Li N, Yu W, Gong J, Li Q, Zhu W, Li J. 2013. Berberine reduces rat intestinal tight junction injury induced by ischemia-reperfusion associated with the suppression of inducible nitric oxide synthesis. Am J Chin Med. 41(6):1297–1312. doi: 10.1142/S0192415X13500870.
- Günther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF, et al. 2011. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 477(7364):335–339. doi: 10.1038/nature10400.
- He Y, Yuan X, Zuo H, Sun Y, Feng A. 2018. Berberine exerts a protective effect on gut-vascular barrier via the modulation of the Wnt/beta-catenin signalling pathway during sepsis. Cell Physiol Biochem. 49(4):1342–1351. doi: 10.1159/000493412.
- Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A, Waisman A, Brenner O, Haffner R, Gustafsson E, et al. 2004. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 173(5):2976–2984. doi: 10.4049/jimmunol.173.5.2976.
- Kimono D, Sarkar S, Albadrani M, Seth R, Bose D, Mondal A, Li Y, Kar AN, Nagarkatti M, Nagarkatti P, et al. 2019. Dysbiosis-associated enteric glial cell immune-activation and redox imbalance modulate tight junction protein expression in Gulf War illness pathology. Front Physiol. 10:1229. doi: 10.3389/fphys.2019.01229.
- Koh SX, Lee JK. 2014. S100B as a marker for brain damage and blood-brain barrier disruption following exercise. Sports Med. 44(3):369–385. doi: 10.1007/s40279-013-0119-9.
- Li H, Fan C, Lu H, Feng C, He P, Yang X, Xiang C, Zuo J, Tang W. 2020. Protective role of berberine on ulcerative colitis through modulating enteric glial cells-intestinal epithelial cells-immune cells interactions. Acta Pharm Sin B. 10(3):447–461. doi: 10.1016/j.apsb.2019.08.006.
- Li X, Wilder-Smith CH, Kan ME, Lu J, Cao Y, Wong RK. 2014. Combat-training stress in soldiers increases S100B, a marker of increased blood-brain-barrier permeability, and induces immune activation. Neuro Endocrinol Lett. 35(1):58–63.
- Li Y, Zhou J, Qiu J, Huang Z, Wang W, Wu P, Feng A. 2020. Berberine reduces gut-vascular barrier permeability via modulation of ApoM/S1P pathway in a model of polymicrobial sepsis. Life Sci. 261:118460. doi: 10.1016/j.lfs.2020.118460.
- Liu P, Bian Y, Fan Y, Zhong J, Liu Z. 2020. Protective effect of naringin on in vitro gut-vascular barrier disruption of intestinal microvascular endothelial cells induced by TNF-alpha. J Agric Food Chem. 68(1):168–175. doi: 10.1021/acs.jafc.9b06347.
- Mouries J, Brescia P, Silvestri A, Spadoni I, Sorribas M, Wiest R, Mileti E, Galbiati M, Invernizzi P, Adorini L, et al. 2019. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol. 71(6):1216–1228. doi: 10.1016/j.jhep.2019.08.005.
- Ruan Q, Zhao C, Ye Z, Zhang W, Xie Q, Xie W. 2014. Role of phosphoinositide 3 kinase/protein kinase B signal pathway in monocyte-endothelial adhesion induced by serum of rats with electrical burn. Zhonghua Shao Shang Za Zhi. 30:237–242.
- Rühl A. 2005. Glial cells in the gut. Neurogastroenterology Motil. 17(6):777–790. doi: 10.1111/j.1365-2982.2005.00687.x.
- Seguella L, Gulbransen BD. 2021. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat Rev Gastroenterol Hepatol. 18(8):571–587. doi: 10.1038/s41575-021-00423-7.
- Sorribas M, Jakob MO, Yilmaz B, Li H, Stutz D, Noser Y, de Gottardi A, Moghadamrad S, Hassan M, Albillos A, et al. 2019. FXR modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis. J Hepatol. 71(6):1126–1140. doi: 10.1016/j.jhep.2019.06.017.
- Spadoni I, Pietrelli A, Pesole G, Rescigno M. 2016. Gene expression profile of endothelial cells during perturbation of the gut vascular barrier. Gut Microbes. 7(6):540–548. doi: 10.1080/19490976.2016.1239681.
- Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, Caprioli F, Bottiglieri L, Oldani A, Viale G, et al. 2015. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 350(6262):830–834. doi: 10.1126/science.aad0135.
- Tisch N, Mogler C, Stojanovic A, Luck R, Korhonen EA, Ellerkmann A, Adler H, Singhal M, Schermann G, Erkert L, et al. 2022. Caspase-8 in endothelial cells maintains gut homeostasis and prevents small bowel inflammation in mice. EMBO Mol Med. 14(6):e14121. doi: 10.15252/emmm.202114121.
- von Boyen GBT, Schulte N, Pflüger C, Spaniol U, Hartmann C, Steinkamp M. 2011. Distribution of enteric glia and GDNF during gut inflammation. BMC Gastroenterol. 11(1):3. doi: 10.1186/1471-230X-11-3.
- Xia J, Zhang J, Wang L, Liu H, Wang J, Liu J, Liu Z, Zhu Y, Xu Y, Yang W, et al. 2021. Non-apoptotic function of caspase-8 confers prostate cancer enzalutamide resistance via NF-kappaB activation. Cell Death Dis. 12(9):833. doi: 10.1038/s41419-021-04126-4.
- Xiao L, Hu L, Chu H, Chen L, Yan J, Wang W, Yang X, Zhu Q, Du F, Song Y, et al. 2022. Retrorsine cooperates with gut microbiota to promote hepatic sinusoidal obstruction syndrome by disrupting the gut barrier. J Clin Transl Hepatol. 10(6):1086–1098. doi: 10.14218/JCTH.2021.00398.
- Xu JH, Liu XZ, Pan W, Zou DJ. 2017. Berberine protects against diet-induced obesity through regulating metabolic endotoxemia and gut hormone levels. Mol Med Rep. 15(5):2765–2787. doi: 10.3892/mmr.2017.6321.
- Yu ZC, Cen YX, Wu BH, Wei C, Xiong F, Li DF, Liu TT, Luo MH, Guo LL, Li YX, et al. 2019. Berberine prevents stress-induced gut inflammation and visceral hypersensitivity and reduces intestinal motility in rats. World J Gastroenterol. 25(29):3956–3971. doi: 10.3748/wjg.v25.i29.3956.
- Zang QS, Maass DL, Wigginton JG, Barber RC, Martinez B, Idris AH, Horton JW, Nwariaku FE. 2010. Burn serum causes a CD14-dependent mitochondrial damage in primary cardiomyocytes. Am J Physiol Heart Circ Physiol. 298(6):H1951–1958. doi: 10.1152/ajpheart.00927.2009.
- Zhang JP, Ying X, Chen Y, Yang ZC, Huang YS. 2008. Inhibition of p38 MAP kinase improves survival of cardiac myocytes with hypoxia and burn serum challenge. Burns. 34(2):220–227. doi: 10.1016/j.burns.2007.03.009.
- Zhang L, Jiang Y, Deng S, Mo Y, Huang Y, Li W, Ge C, Ren X, Zhang H, Zhang X, et al. 2021. S100B/RAGE/Ceramide signalling pathway is involved in sepsis-associated encephalopathy. Life Sci. 277:119490. doi: 10.1016/j.lfs.2021.119490.
- Ziegler TR, Smith RJ, O’Dwyer ST, Demling RH, Wilmore DW. 1988. Increased intestinal permeability associated with infection in burn patients. Arch Surg. 123(11):1313–1319. doi: 10.1001/archsurg.1988.01400350027003.
- Zou Z, Li L, Li Q, Zhao P, Zhang K, Liu C, Cai D, Maegele M, Gu Z, Huang Q. 2022. The role of S100B/RAGE-enhanced ADAM17 activation in endothelial glycocalyx shedding after traumatic brain injury. J Neuroinflammation. 19(1):46. doi: 10.1186/s12974-022-02412-2.

