Abstract
Context
Tanzania has rich medicinal plant (MP) resources, and most rural inhabitants rely on traditional healing practices for their primary healthcare needs. However, available research evidence on antimalarial MPs is highly fragmented in the country.
Objective
This systematic review compiles ethnomedicinal research evidence on MPs used by Tanzanians as antimalarials.
Materials and methods
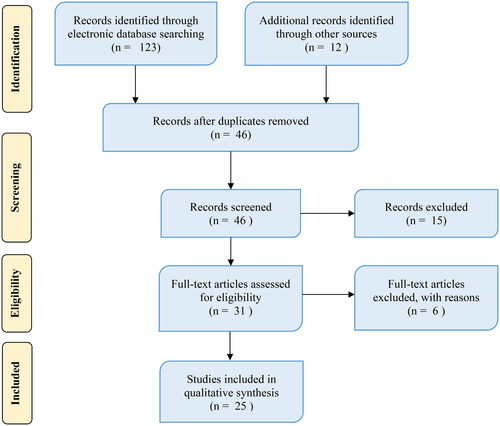
A systematic web search was conducted using various electronic databases and grey materials to gather relevant information on antimalarial MPs utilized by Tanzanians. The review was per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The data were collected from 25 articles, and MS Excel software was used to analyse relevant ethnobotanical information using descriptive statistics.
Results
A total of 227 MPs belonging to 67 botanical families and 180 genera were identified. Fabaceae (15.9%) is the most frequently utilized family. The ethnobotanical recipes analysis indicated leaves (40%) and trees (44%) are the preferred MPs part and life form, respectively. Decoctions (67%) are the dominant preparation method of remedies. Of the recorded MPs, 25.9% have been scientifically investigated for antimalarial activities with positive results. However, 74.1% of MPs have no scientific records on antimalarial activities, but they could be potential sources of remedies.
Conclusions
The study discloses a wealth of antimalarial MPs possessed by Tanzanians and suggests a need for research to authenticate the healing potential of antimalarial compounds from the unstudied MPs. Additionally, it indicates that some of the presented MPs are potential sources for developing safe, effective and affordable antimalarial drugs.
Introduction
Malaria is one of the life-threatening diseases in malaria-endemic regions of the world (Dao et al. Citation2021). The disease caused about 627,000 deaths in 2020. Most deaths (99%) were due to a parasite, Plasmodium falciparum Welch (Plasmodiidae), transmitted by the Anopheles mosquito. About 90% of malaria cases and deaths occur in Africa, and most (93%) deaths occur in sub-Saharan Africa, where 95% of the global burden of malaria is concentrated. Children under five years of age are the most affected group in the region (World Health Organization, WHO 2021). In Tanzania, nearly 88.5% of the population lives in expanses considered malarious (Rumisha et al. Citation2019), and the country stands among the top 10 countries with high malaria transmission in the sub-Saharan region (WHO Citation2021). Regardless of recent developments in control strategies, malaria remains a significant public health problem and a prime cause of morbidity and mortality, particularly in children under five years (Dao et al. Citation2021). Regarding statistics, the disease ranks number one among outpatients and inpatients, and malaria's socio-economic impact is so high that it leads to poverty and underdevelopment (Makundi et al. Citation2007; Rumisha et al. Citation2019).
Recently, drug-resistant Plasmodium species have been reported in various studies (Wicht et al. Citation2020; Hodoameda et al. Citation2022). Explicitly, P. falciparum has established resistance to almost all presently available antimalarial drugs (White Citation2004; Thu et al. Citation2017; Rasmussen et al. Citation2022). Subsequently, research on antimalarial alternative medicines has intensified over the past decades (Alebie et al. Citation2017; Omara Citation2020; Tabuti et al. Citation2023). Generally, MPs have been the focus of many researchers to discover alternative antimalarial remedies (Ntie-Kang et al. Citation2014), leading to the discovery of numerous active antimalarial compounds with significant structural varieties, such as coumarins, quinones, triterpenes, quassinoids, sesquiterpenoids, limonoids, steroids, lignans, saponins and alkaloids (Phillipson and Wright Citation1991; Laryea and Borquaye Citation2019; Tiko et al. Citation2020).
Nearly, 80% of people in Africa, including Tanzania, still depend on MPs for treating numerous diseases, including malaria (WHO Citation2014; Okaiyeto and Oguntibeju Citation2021). This is well associated with the availability and accessibility challenges of modern health facilities and the cultural acceptability of traditional remedies. Likewise, the prevalent use of MPs is attributed to their presence on the ground and affordability. For instance, the ratio of orthodox health practitioners to the people in Africa is 1:500, whereas the proportion of modern medical practitioners to the people is 1:40,000 (WHO Citation2014). Thus, for most rural inhabitants, traditional healers remain their health providers (Frumence et al. Citation2013; Tabuti et al. Citation2023). Due to high dependency on MPs, communities from different settings in Tanzania have developed their medical plant collections and knowledge of their utilization, management and conservation (Kayombo et al. Citation2012; Mbinile et al. Citation2020). Many MPs are used as traditional malaria remedies in diverse areas of the country (Gessler et al. Citation1995; Begum et al. Citation2020).
Proper documentation of MPs used in managing malaria and its associated traditional knowledge establishes a critical task in preserving valuable indigenous knowledge, biological diversity and improving community admittance to and stakes in enhancing malaria control interventions. It is also essential for thought-provoking forthcoming research on the safety and efficacy of MPs and recognizing active chemical entities, which can be advanced into new homogenous phytomedicines. In contrast, ethnobotanical and ethnopharmacological investigation on aboriginal antimalarial MPs is at an elementary stage in most African countries, including Tanzania (Kidane et al. Citation2014; Begum et al. Citation2020; Manuel et al. Citation2020). Furthermore, research evidence on indigenous antimalarial MPs is highly fragmented, thus underscoring the solemn need for systematic documentation. Therefore, this systematic review attempted to collect and document ethnomedicinal information on antimalarial MPs and their utilization in Tanzania.
Methodology
This review has collected information on antimalarial MPs used in Tanzania. The review was performed as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Liberati et al. Citation2009) (). A web-based literature search was conducted using various electronic databases, including Web of Science, Google Scholar, Science Direct, PubMed, Scopus, African Journals Online, Wiley online library and grey literature to access relevant works dealing with antimalarial plants. The keywords used during the investigation included antimalarial plants, malaria, traditional medicine, ethnobotany, ethnopharmacology, alternative medicine, antiplasmodial activity, antimalarial activity, chloroquine, quinine, medicinal plants (MPs) and Tanzania. The search was independently performed in each database, and published articles, theses or dissertations containing the above terms were considered and screened. Lastly, Tanzanian traditional MPs exclusively utilized for malaria were selected. This review did not consider any study that did not include botanic names of MPs and plant parts used to treat malaria. Studies that possessed required information, such as family name, scientific name, local name, growth habits, method of preparation (if available) and route of administration (if available), were extracted and considered. The accuracy of the MPs' scientific names was confirmed through the Tropicos (https://www.tropicos.org) botanical databases.
Ethnobotanical data analysis
The collected ethnobotanical data were entered into the Microsoft Excel software and analysed for information for descriptive statistics (percentages and frequencies) of the reported antimalarial botanical families, MPs, parts used, growth forms, modes of preparation, routes of administration and related indigenous knowledge. Also, from various literature, all recorded MPs were checked for their antiplasmodial/antimalarial activity, active phytochemicals and toxicity. The findings were subsequently presented as tables and figures.
Ethical approval statement
The statement was not required as the review did not involve human and animal participants.
Results and discussion
Medicinal plants diversity
In total, 25 ethnobotanical studies acknowledged 227 MPs belonging to 67 botanical families and 180 genera used as antimalarial from different parts of Tanzania (). Fabaceae (15.9%), Asteraceae (7.9%), Rubiaceae (6.6%), Euphorbiaceae (5.3%) and Lamiaceae (5.3%) were the most common families (). The number of antimalarial MPs recorded in this review is higher than the one recorded in Ethiopia (200 species) (Alebie et al. Citation2017) but less than that of Kenya (289 species) (Omara Citation2020). The most frequently encountered MPs were Azadirachta indica A. Juss (Meliaceae) (six times), Vernonia amygdalina Del. (Asteraceae), Bridelia micrantha (Hochst.) Baill. (Euphorbiaceae), Cassia abbreviata Oliv. (Fabaceae), Psidium guajava L. (Myrtaceae), Zanthoxylum chalybeum Engl. (Rutaceae) (five times each), Vangueria infausta Burch. (Rubiaceae), Leonotis nepetifolia (L.) R. Br. (Lamiaceae), Hoslundia opposita Vahl. (Lamiaceae) (four times each), Mangifera indica L. (Anacardiaceae), Ozoroa insignis Del. (Anacardiaceae), Pseudospondias microcarpa (A. Rich) Engl. (Anacardiaceae), Kigelia africana (Lamarck) Benth. (Bignoniaceae), Canarium schweinfurthii Engl. (Burseraceae), Momordica foetida Schum (Cucurbitaceae) and Abrus precatorius L. (Fabaceae) (three times each). The recurrent citation of particular MPs or families designates potentially higher bioactive antimalarial ingredients. Such evidence is vital for prioritizing pharmacological studies.
Table 1. List of antimalarial plants of Tanzania, life form, parts used, mode of preparation (MoP), and their route of application (RoA).
Table 2. Top 15 botanical families (with >3 species) from which antimalarial remedies are obtained in Tanzania.
Some MPs presented in this review have also been reported in other ethnobotanical studies conducted elsewhere as antimalarial. For instance, Senna occidentalis (Fabaceae), A. precatorius, V. amygdalina, M. indica and A. indica are reported to be the most frequently used MPs for treating Malaria in Uganda (Tabuti et al. Citation2023). Cyathula cylindrica Moq. (Amaranthaceae), Rhus vulgaris Meikle (Anacardiaceae), Artemisia afra Jacq. (Asteraceae), Bidens pilosa L. (Asteraceae) and K. africana have been reported in Kenya (Omara Citation2020), M. foetida in Uganda (Okello and Kang Citation2019), Zimbabwe (Ngarivhume et al. Citation2015) and Cameroon (Pierre et al. Citation2011), A. afra, M. indica, and Datura stramonium L. (Solanaceae) in Ethiopia (Mesfin et al. Citation2009), and Flacourtia indica (Burm. l.) Merr. (Salicaceae), and Tamarindus indica L. (Fabaceae) in Ethiopia (Nigussie and Wale Citation2022) and Indonesia (Taek et al. Citation2018), and A. indica in Nigeria (Odugbemi et al. Citation2006), and Kenya (Gathirwa et al. Citation2011; Nguta and Mbaria Citation2013), and Senna siamea (Lam.) H.S. Irwin & Barneby (Fabaceae) in Togo (Agbodeka et al. Citation2016). The wide acceptance of MPs' usage signifies their potential for treating malaria. Thus, using modern science and technology, the MPs can be used to improve the local remedies or develop new affordable contemporary drugs.
In this review, few MPs are reported to be used for treating malaria simultaneously in different regions. This could be attributed to the prolific distribution of the referent active ingredients among species, especially those belonging to dominant families, such as Fabaceae, Asteraceae, Rubiaceae, Euphorbiaceae and Lamiaceae. The variances in geographical and climatic conditions may also influence the availability of flora in a given locality. However, some MPs have a broader distribution and are commonly used by most communities.
Life forms
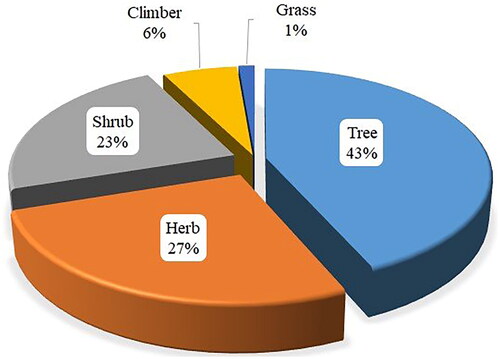
Antimalarial MPs used in Tanzania are dominated by trees (43%), followed by herbs (27%), shrubs (23%), climbers (6%) and grasses (1%) (). The predominant use of trees in herbal remedies formulation is also reported in other ethnobotanical studies (Omara et al. Citation2020; Mogha et al. Citation2022). The local traditional healers favour using a particular life form because of their accessibility and familiarity (Shukla et al. Citation2018). Furthermore, cultural influence plays a substantial role in selecting MPs' life forms for preparations of herbal remedies.
Parts used
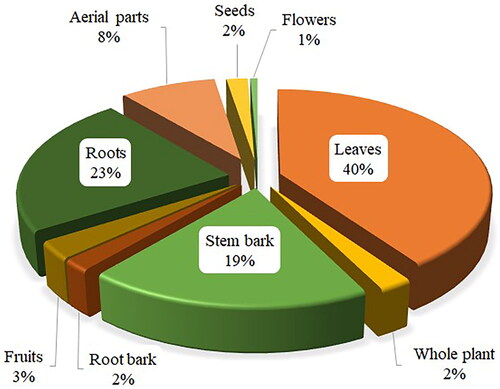
The most commonly used MP parts for the formulation of antimalarial remedies are leaves (40%), followed by roots (23%), and stem bark (19%) (). The MPs parts, such as seeds, fruits, flowers, buds and bulbs, known for accruing phytochemicals, are seldom used, comparable to ethnobotanical studies conducted elsewhere (Ngarivhume et al. Citation2015; Okello and Kang Citation2019). Similar to present findings, leaves of trees, shrubs and herbs are reported as the dominant plant part used for the preparation of antimalarial decoctions in some countries such as Kenya (Omara Citation2020), Ethiopia (Alebie et al. Citation2017), Nigeria (Oyeyemi et al. Citation2019) and Democratic Republic of Congo (Katemo et al. Citation2012). Plant leaves are well known for synthesizing the majority of plant secondary metabolites; hence, they are rich sources of bioactive chemical constituents. Systematic harvest of leaves poses no or little threat to the survival of MPs, encouraging frequent and safe utilization of leaves for herbal remedies preparations. Plant roots and associated structures, such as rhizomes and tubers, are rich sources of potent bioactive chemical compounds (Alebie et al. Citation2017). However, their persistent usage in herbal preparations can still be risky to the survival of a plant species. Therefore, proper harvesting approaches and conservation strategies are indispensable for safeguarding the sustainable utilization of MPs resources.
The majority (75%) of herbal remedies used as antimalarial are prepared from fresh MP materials, followed by dry form (18%) and both fresh and dry materials (7%). The predominant use of fresh materials mirrors an endeavour to capture potent volatile substances that determine the therapeutic efficacy of herbal remedies (Addis et al. Citation2021). The use of dry materials is favoured when the MPs are poorly accessible. Similar to other ethnobotanical reviews, some traditional practitioners collect MP materials far away from their places and practice long-term preservation (Alebie et al. Citation2017; Omara Citation2020; Ajao et al. Citation2022).
Preparation and administration of remedies
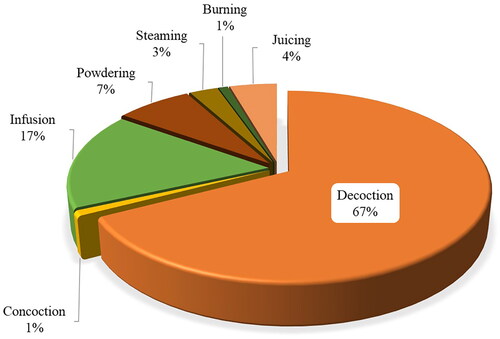
Antimalarial remedies are mainly prepared through decoctions (67%), followed by infusion (17%) and rarely prepared through powdering (7%), juicing (4%), steaming (3%), burning (1%), and concoction (1%) (). Most (78%) of the herbal preparations are orally administered, as is the case for contemporary medicine, and seldom administered through the intranasal route. Only liquid from squeezed fresh leaves of Aspilia natalensis (Sond.) Wild. (Asteraceae) is reported to be applied through the nasal passage. Moreover, fresh fruits of Syzygium cumini (L.) Skeels (Myrtaceae), Eriobotrya japonica (Thunb.) Lindl. (Rosaceae), Tricalysia coriacea (Benth.) Hiern. (Rubiaceae), Vangueria infausta Burch. (Rubiaceae), roots and aerial parts of Costus afer Ker-Gawl. (Zingiberaceae) and fresh leaves of Adansonia digitata L. (Bignoniaceae) are eaten directly upon collection or after initial processing through pounding or crushing. Some MPs materials are burnt or steamed and inhaled. These findings agree with observations in other ethnobotanical studies conducted in other countries, such as Nigeria (Adekunle Citation2008), Ethiopia (Alebie et al. Citation2017), Zimbabwe (Maroyi Citation2013) and Kenya (Omara et al. Citation2020). However, a few reports (19.6%) failed to point out modes of administration of some herbal remedies.
Malaria is an acute feverish illness mainly caused by plasmodium parasites, and its management requires adequate circulating concentrations of relevant antiprotozoal components. The oral route is an appropriate and non-offensive method of systemic therapy as it permits relatively hasty absorption and distribution of bioactive ingredients from herbal remedies, allowing sufficient therapeutic power (Teklay et al. Citation2013). The potential risk of enzymatic breakdown and microbial fermentation of functional chemical entities may necessitate alternative routes of herbal remedy administration.
Similarly, this review noted that a few MPs (5.2%) are used to treat malaria in combination with other plants. For instance, the infusion of dried powdered aerials parts of Justicia matammensis Oliv. (Acanthaceae) are combined with those of Cassia gracilior (Ghesq.) Steyaert (Fabaceae), Antherotoma naudinii Hook.f. (Melastomataceae) or Indigofera asparagoides Taub. ssp. (Fabaceae) or Ephemera spp. Gillett (Ephemeridae) for managing cerebral malaria. The decoctions of V. amygdalina Del. leaves and roots are combined with the stem bark of Sapium ellipticum (Krauss) Pax (Euphorbiaceae), and the leaves of Dalbergia nitidula Baker (Fabaceae), Desmodium salicifolium (Poir) DC (Fabaceae), Eriosema psoraleoides Lam. G.Don. (Fabaceae). The stem bark and roots of Garcinia buchananii Bak. (Clusiaceae) are combined with Tragia furialis Prain (Euphorbiaceae) and administered orally. Other ethnobotanical studies conducted elsewhere also reported similar observations in treating various diseases, including malaria (Maroyi Citation2013; Alebie et al. Citation2017; Omara Citation2020).
Other ethnomedicinal uses of antimalarial plants
Most antimalarial MPs listed in this review are used for the traditional management of other ailments in different parts of the country and elsewhere globally. For instance, in Tanzania, A. precatorius, T. indica, Z. chalybeum, C. abbreviata and Flueggea virosa (Roxb. ex Willd.) Royle, are reported to be used by Nyamwezi people to manage erectile dysfunction (Kacholi and Amir Citation2023), and Moringa oleifera Lam, Z. chalybeum and P. guajava are used as anti-haemorrhoids (Kacholi and Amir Citation2022). In Southern India, A. precatorius is reported to have multiple applications like neuromuscular effects, neuro-protective, abortifacient, antiepileptic, antiviral, antifertility, nephroprotective, immune-modulator, immune-stimulatory properties, anti-inflammatory activity and antidiabetic effect (Garaniya and Bapodra Citation2014). T. indica leaves and fruits are used for managing foot pain and constipation in Tanzania (Hilonga et al. Citation2019), while the leaves of this species are used for treating smallpox and abdominal upsets and fruits for managing constipation in Uganda (Ebifa-Othieno et al. Citation2017). Other plants, such as V. amygdalina, and B. pilosa are used in Tanzania (Kingo and Maregesi Citation2020), India (Pattanayak et al. Citation2017), and Trinidad and Tobago (Lans Citation2006) for curing urinary transmitted infections.
Antimalarial, antiplasmodial and toxicity studies
Of the reported antimalarial MPs used in Tanzania in this review, 59 (25.9%) have demonstrated therapeutic potential in pre-clinical and clinical investigations (). Antimalarial compounds with antiplasmodial activities have been identified and isolated from these MPs species (Gakunju et al. Citation1995; Oketch-Rabah et al. Citation1999; Clarkson et al. Citation2004; Koch et al. Citation2005). Regardless of the accomplishment of traditional medicinal practices and the wealth of MPs (), most antimalarial MPs studies in Tanzania are limited to ethnobotanical investigations, with extensions to the assessment of crude extracts from MPs against Plasmodium species (Weenen et al. Citation1990; Kweyamba et al. Citation2019). The gap is evident regarding studies geared towards identifying and isolating plant bioactive compounds, establishing the efficacy and safety of MPs through in vitro assays using human Plasmodium parasites and in vivo assays involving higher animal models and randomized clinical trials (Gessler et al. Citation1995; Malebo et al. Citation2015). In examining antiplasmodial activities, whether due to a specific or general toxicity effect, the experimental selectivity index (SI) must be calculated. However, in Tanzania, few studies have attempted this (Malebo et al. Citation2015; Kassimu et al. Citation2022). The discrepancy in toxicity depends on the sensitivity of the experimental animals, tissue or cells used, type of extract, nature of tested substance, dose, and mode of administration (Parasuraman Citation2011; Turner et al. Citation2011).
Table 3. Literature on antimalarial and antiplasmodial activities and toxicity of some recorded medicinal plants.
In this review, 47.5% of the cited MPs in the literature (28 species) had no reports of their toxicities, while 52.5% (29 species) were evaluated for their toxicities (). Of the evaluated MPs (), 16.9% showed no toxicity to humans, while the rest showed toxicity effect when applied. This indicates that many MPs extracts with good antimalarial activity are potentially toxic too. Notable examples are the methanol extract of Toddalia asiatica (L.) Lam (Rutaceae) (Orwa et al. Citation2013; Muthaura et al. Citation2015) and the extract of dimethyl sulphoxide or ethanol of S. occidentalis (Loomis and Hayes Citation2014; Murugan et al. Citation2016) revealed to have low to moderate toxicity. In contrast, petroleum ether leaf extract of V. amygdalina was potent as antimalarial but with potent toxicity (Adia et al. Citation2016; Kiraithe et al. Citation2016; Obbo et al. Citation2019). As reported earlier in , few scientific trials have been conducted on antimalarial MPs. This can be due to regulatory requirements for clinical studies and the required financial input. Therefore, this study suggests that further pharmacological and phytochemical studies for the unstudied MPs are needed to establish their antimalarial activities, efficacy, toxicity and safety.
Conclusions
This review presents, for the first time, all known MPs used by Tanzanians to treat malaria. The work has shown that indigenous knowledge of MPs in Tanzania is a good resource for malaria management. However, experimental research and advanced analysis are required to isolate, identify and validate the bioactive compounds from promising MPs, which can be used to standardize plant materials to install a reproducible herbal medicine practice and formulation of contemporary drugs. Safety and toxicological studies are paramount as some MPs are used as admixtures in managing malaria. Additionally, sustainable development and harvest of indigenous MPs' resources require collaborative engagement between researchers, traditional health practitioners, government bodies and commercial investors.
Author contributions
Conceptualization, data collection, data analysis, manuscript writing and manuscript revision: DSK.
Acknowledgements
The author is indebted to the Dar es Salaam University College of Education (DUCE) for providing space and time for this work. Moreover, the author commends all previous authors for their contribution to the knowledge of medicinal plants in Tanzania.
Disclosure statement
The author declares no competing interest in the publication of this work.
Additional information
Funding
References
- Addis T, Mekonnen Y, Ayenew Z, Fentaw S, Biazin H. 2021. Bacterial uropathogens and burden of antimicrobial resistance pattern in urine specimens referred to Ethiopian Public Health Institute. PLOS One. 16(11):e0259602. doi: 10.1371/journal.pone.0259602.
- Adekunle M. 2008. Indigenous uses of plant leaves to treat malaria fever at Omo Forest Reserve (OFR) Ogun state, Nigeria. Ethiop J Environ Stud Manag. 1(1):31–35.
- Adia MM, Emami SN, Byamukama R, Faye I, Borg-Karlson A-K. 2016. Antiplasmodial activity and phytochemical analysis of extracts from selected Ugandan medicinal plants. J Ethnopharmacol. 186:14–19. doi: 10.1016/j.jep.2016.03.047.
- Agbodeka K, Gbekley HE, Karou SD, Anani K, Agbonon A, Tchacondo T, Batawila K, Simpore J, Gbeassor M. 2016. Ethnobotanical study of medicinal plants used for the treatment of malaria in the Plateau region, Togo. Pharmacogn Res. 8(Suppl. 1):S12–S18. doi: 10.4103/0974-8490.178646.
- Ajaiyeoba E, Ashidi J, Abiodun O, Okpako L, Ogbole O, Akinboye D, Falade C, Bolaji O, Gbotosho G, Falade M, et al. 2005. Antimalarial ethnobotany: in vitro antiplasmodial activity of seven plants identified in the Nigerian middle belt. Pharm Biol. 42(8):588–591. doi: 10.1080/13880200490902455.
- Ajao AA, Mukaila YO, Sabiu S. 2022. Wandering through southwestern Nigeria: an inventory of Yoruba useful angiosperm plants. Heliyon. 8(1):e08668. doi: 10.1016/j.heliyon.2021.e08668.
- Akah PA, Nwafor SV. 1999. Studies on anti-ulcer properties of Cissampelos mucronata leaf extract. Indian J Exp Biol. 37(9):936–938.
- Alebie G, Urga B, Worku A. 2017. Systematic review on traditional medicinal plants used for the treatment of malaria in Ethiopia: trends and perspectives. Malar J. 16(1):307. doi: 10.1186/s12936-017-1953-2.
- Alli LA, Adesokan AA, Salawu AO. 2016. Antimalarial activity of fractions of aqueous extract of Acacia nilotica root. J Intercult Ethnopharmacol. 5(2):180–185. doi: 10.5455/jice.20160331064817.
- Alozieuwa UB, Mann A, Kabiru AY, Ogbadoyi EO. 2022. In vivo antimalarial efficacy of Psidium guajava leaf crude extract and fractions in Plasmodium berghei infected mice. AROC Nat Prod Res. 2(1):28–37. doi: 10.53858/arocnpr02012837.
- Alshawsh MA, Mothana RA, Al-Shamahy HA, Alsllami SF, Lindequist U. 2009. Assessment of antimalarial activity against Plasmodium falciparum and phytochemical screening of some Yemeni medicinal plants. Evid Based Complement Alternat Med. 6(4):453–456. doi: 10.1093/ecam/nem148.
- Amoa Onguéné P, Ntie-Kang F, Lifongo LL, Ndom JC, Sippl W, Mbaze LM. 2013. The potential of anti-malarial compounds derived from African medicinal plants. Part I: a pharmacological evaluation of alkaloids and terpenoids. Malar J. 12(1):449. doi: 10.1186/1475-2875-12-449.
- Amri E, Kisangau DP. 2012. Ethnomedicinal study of plants used in villages around Kimboza forest reserve in Morogoro, Tanzania. J Ethnobiol Ethnomed. 8(1):1.
- Arome D, Chinedu E, Ameh S, Sunday A. 2016. Comparative antiplasmodial evaluation of Cymbopogon citratus extracts in Plasmodium berghei-infected mice. J Curr Res Sci Med. 2(1):29–35. doi: 10.4103/2455-3069.184126.
- Asanga EE, Okoroiwu H, Edet UO, Amaechi D, Nelson PE, Uchenwa M, Eseyin OA, Samuel G, Ettah LA, Obongha OA. 2023. Antimalarial activity of Mangifera indica aqueous extract in Plasmodium berghei's apicoplast. Trop J Pharm Res. 22(5):1007–1015. doi: 10.4314/tjpr.v22i5.11.
- Asase A, Akwetey GA, Achel DG. 2010. Ethnopharmacological use of herbal remedies for the treatment of malaria in the Dangme West District of Ghana. J Ethnopharmacol. 129(3):367–376. doi: 10.1016/j.jep.2010.04.001.
- Asres K, Bucar F, Knauder E, Yardley V, Kendrick H, Croft S. 2001. In vitro antiprotozoal activity of extract and compounds from the stem bark of Combretum molle. Phytother Res. 15(7):613–617. doi: 10.1002/ptr.897.
- Augustino S, Gillah PR. 2005. Medicinal plants in urban districts of Tanzania: plants, gender roles and sustainable use. Int Forest Rev. 7(1):44–58. doi: 10.1505/ifor.7.1.44.64157.
- Augustino S, Hall JB, Makonda FBS, Ishengoma RC. 2011. Medicinal resources of the Miombo woodlands of Urumwa, Tanzania: plants and its uses. J Med Plants Res. 5:6352–6372.
- Ayalew M, Atnafie SA, Bekele A. 2022. Antimalarial activity of solvent fractions of a leaf of Eucalyptus globulus Labill against Plasmodium berghei infected mice. BMC Complement Med Ther. 22(1):221. doi: 10.1186/s12906-022-03702-1.
- Begum S, Munissi JJE, Buriyo AS, Makangara JJ, Lucantoni L, Avery VM, Erdelyi M, Nyandoro SS. 2020. Antiplasmodial, antimicrobial and cytotoxic activities of extracts from selected medicinal plants growing in Tanzania. J Biol Act Prod Nat. 10(2):165–176. doi: 10.1080/22311866.2020.1770125.
- Bero J, Frédérich M, Quetin-Leclercq J. 2009. Antimalarial compounds isolated from plants used in traditional medicine. J Pharm Pharmacol. 61(11):1401–1433. doi: 10.1211/jpp/61.11.0001.
- Bray DH, Warhurst DC, Connolly JD, O'Neill MJ, Phillipson JD. 1990. Plants as sources of antimalarial drugs. Part 7. Activity of some species of Meliaceae plants and their constituent limonoids. Phytother Res. 4(1):29–35. doi: 10.1002/ptr.2650040108.
- Burkill HM. 2000. The useful plants of West Tropical Africa. Vol. 5: Families S-Z, addenda, corrigenda, cryptogamata. 2nd ed. Kew: Royal Botanic Gardens; p. 686.
- Challand S, Willcox M. 2009. A clinical trial of the traditional medicine Vernonia amygdalina in the treatment of uncomplicated malaria. J Altern Complement Med. 15(11):1231–1237. doi: 10.1089/acm.2009.0098.
- Chhabra SC, Mahunnah BLA, Mshiu EN. 1987. Plants used in traditional medicine in eastern Tanzania. I. Pteridophytes and angiosperms (Acanthaceae to Canellaceae). J Ethnopharmacol. 21(3):253–277. doi: 10.1016/0378-8741(87)90103-6.
- Chhabra SC, Mahunnah RLA, Mshiu EN. 1989. Plants used in traditional medicine in Eastern Tanzania. II. Angiosperms (Capparidaceae to Ebenaceae). J Ethnopharmacol. 25(3):339–359. doi: 10.1016/0378-8741(89)90038-x.
- Chhabra SC, Mahunnah RLA, Mshiu EN. 1990a. Plants used in traditional medicine in Eastern Tanzania. III. Angiosperms (Euphorbiaceae to Menispermaceae). J Ethnopharmacol. 28(3):255–283. doi: 10.1016/0378-8741(90)90078-8.
- Chhabra SC, Mahunnah RLA, Mshiu EN. 1990b. Plants used in traditional medicine in Eastern Tanzania. IV. Angiosperms (Mimosaceae to Papilionaceae). J Ethnopharmacol. 29(3):295–323. doi: 10.1016/0378-8741(90)90041-q.
- Chhabra SC, Mahunnah RLA, Mshiu EN. 1991. Plants used in traditional medicine in Eastern Tanzania. V. Angiosperms (Passifloraceae to Sapindaceae). J Ethnopharmacol. 33(1–2):143–157. doi: 10.1016/0378-8741(91)90173-b.
- Chhabra SC, Mahunnah RLA, Mshiu EN. 1993. Plants used in traditional medicine in Eastern Tanzania. VI. Angiosperms (Sapotaceae to Zingiberaceae). J Ethnopharmacol. 39(2):83–103. doi: 10.1016/0378-8741(93)90024-y.
- Chhabra SC, Mahunnah RLA. 1994. Plants used in traditional medicine by Hayas of the Kagera region, Tanzania. Econ Bot. 48(2):121–129. doi: 10.1007/BF02908198.
- Clarkson C, Maharaj VJ, Crouch NR, Grace OM, Pillay P, Matsabisa MG, Bhagwandin N, Smith PJ, Folb PI. 2004. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J Ethnopharmacol. 92(2–3):177–191. doi: 10.1016/j.jep.2004.02.011.
- Dao F, Djonor SK, Ayin CT-M, Adu GA, Sarfo B, Nortey P, Akuffo KO, Danso-Appiah A. 2021. Burden of malaria in children under five and caregivers' health-seeking behaviour for malaria-related symptoms in artisanal mining communities in Ghana. Parasit Vect. 14:418.
- Ebifa-Othieno E, Mugisha A, Nyeko P, Kabasa JD. 2017. Knowledge, attitudes and practices in tamarind (Tamarindus indica L.) use and conservation in Eastern Uganda. J Ethnobiol Ethnomed. 13:5.
- Fandohan P, Gnonlonfin B, Laleye A, Gbenou JD, Darboux R, Moudachirou M. 2008. Toxicity and gastric tolerance of essential oils from Cymbopogon citratus, Ocimum gratissimum and Ocimum basilicum in Wistar rats. Food Chem Toxicol. 46(7):2493–2497. doi: 10.1016/j.fct.2008.04.006.
- Froelich S, Onegi B, Kakooko A, Siems K, Schubert C, Jenett-Siems K. 2007. Plants traditionally used against malaria: phytochemical and pharmacological investigation of Momordica foetida. Rev Bras Farmacogn. 17(1):1–17. doi: 10.1590/S0102-695X2007000100002.
- Frumence G, Nyamhanga T, Mwangu M, Hurtig A-K. 2013. Challenges to the implementation of health sector decentralization in Tanzania: experiences from Kongwa District Council. Glob Health Action. 6(1):20983. doi: 10.3402/gha.v6i0.20983.
- Gakunju DM, Mberu EK, Dossaji SF, Gray AI, Waigh RD, Waterman PG, Watkins WM. 1995. Potent antimalarial activity of the alkaloid nitidine, isolated from a Kenyan herbal remedy. Antimicrob Agents Chemother. 39(12):2606–2609. doi: 10.1128/AAC.39.12.2606.
- Garaniya N, Bapodra A. 2014. Ethno botanical and phytopharmacological potential of Abrus precatorius L.: a review. Asian Pac J Trop Biomed. 4(Suppl. 1):S27–S34. doi: 10.12980/APJTB.4.2014C1069.
- Gathirwa JW, Rukunga GM, Mwitari PG, Mwikwabe NM, Kimani CW, Muthaura CN, Kiboi DM, Nyangacha RM, Omar SA. 2011. Traditional herbal antimalarial therapy in Kilifi district, Kenya. J Ethnopharmacol. 134(2):434–442. doi: 10.1016/j.jep.2010.12.043.
- Gessler MC, Msuya DE, Nkunya MHH, Mwasumbi LB, Schär A, Heinrich M, Tanner M. 1995. Traditional healers in Tanzania: the treatment of malaria with plant remedies. J Ethnopharmacol. 48(3):131–144. doi: 10.1016/0378-8741(95)01293-m.
- Hedberg I, Hedberg O, Madat PJ, Mshigeni KE, Mshiu EN, Samuelsson G. 1983. Inventory of plants used in traditional medicine in Tanzania. II. Plants of the families Dilleniaceae—Opiliaceae. J Ethnopharmacol. 9(1):105–127. doi: 10.1016/0378-8741(83)90030-2.
- Hilonga S, Otieno JN, Ghorbani A, Pereus D, Kocyan A, de Boer H. 2019. Trade of wild-harvested medicinal plant species in local markets of Tanzania and its implications for conservation. S Afr J Bot. 122:214–224. doi: 10.1016/j.sajb.2018.08.012.
- Hodoameda P, Duah-Quashie NO, Quashie NB. 2022. Assessing the roles of molecular markers of antimalarial drug resistance and the host pharmacogenetics in drug-resistant malaria. J Trop Med. 2022:3492696–3492699. doi: 10.1155/2022/3492696.
- Iwalewa E, Suleiman MM, Mdee L, Eloff J. 2009. Antifungal and antibacterial activities of different extracts of Harungana madagascariensis stem bark. Pharm Biol. 47(9):878–885. doi: 10.1080/13880200903029316.
- Iwalewa EO, Omisore NO, Adewunmi CO, Gbolade AA, Ademowo OG, Nneji C, Agboola OI, Daniyan OM. 2008. Anti-protozoan activities of Harungana madagascariensis stem bark extract on trichomonads and malaria. J Ethnopharmacol. 117(3):507–511. doi: 10.1016/j.jep.2008.02.019.
- Joseph CC, Magadula JJ, Nkunya MHH. 2007. A novel antiplasmodial 3′,5′-diformylchalcone and other constituents of Friesodielsia obovata. Nat Prod Res. 21(11):1009–1015. doi: 10.1080/14786410701194310.
- Kacholi DS, Amir HM. 2022. Herbal remedies used by traditional healers to treat haemorrhoids in Tabora region, Tanzania. Pharm Biol. 60(1):2182–2188. doi: 10.1080/13880209.2022.2136204.
- Kacholi DS, Amir HM. 2023. Ethnobotanical study of medicinal plants traditionally used against erectile dysfunction in Tabora region, Tanzania. Ethnobot Res Appl. 25:1–12. doi: 10.32859/era.30.5.1-12.
- Kacholi DS. 2014. Indigenous tree uses, use-values and impact of human population on forest size, structure and species richness in Uluguru, Morogoro, Tanzania. Tanz J Sci. 40(1):34–50.
- Kacholi DS. 2020. Density and aboriginal uses of wild tree species in Milawilila Forest Reserve in Morogoro region, Tanzania. Tanz J Sci. 46(1):85–100.
- Kamau PK, Nga'ng'a Z, Njeruh FM, Thuita J. 2020. In vitro antiplasmodial, cytotoxicity assay and partial chemical characterization of Kenyan Physalis peruviana L. (Solanaceae family) extracts. J Med Plants Res. 14:73–80.
- Kaou AM, Mahiou-Leddet V, Hutter S, Aïnouddine S, Hassani S, Yahaya I, Azas N, Ollivier E. 2008. Antimalarial activity of crude extracts from nine African medicinal plants. J Ethnopharmacol. 116(1):74–83. doi: 10.1016/j.jep.2007.11.001.
- Karthivashan G, Tangestani Fard M, Arulselvan P, Abas F, Fakurazi S. 2013. Identification of bioactive candidate compounds responsible for oxidative challenge from hydro-ethanolic extract of Moringa oleifera leaves. J Food Sci. 78(9):C1368–C1375.
- Kassimu K, Milando F, Omolo J, Mdemu A, Nyaulingo G, Mbarak H, Mohamed L, Rashid R, Ahmed S, Rashid M, et al. 2022. Safety and tolerability of an antimalarial herbal remedy in healthy volunteers: an open-label, single-arm, dose-escalation study on Maytenus senegalensis in Tanzania. Trop Med Infect Dis. 7(12):396. doi: 10.3390/tropicalmed7120396.
- Katemo M, Mpiana PT, Mbala BM, Mihigo SO, Ngbolua KN, Tshibangu DST, Koyange PR. 2012. Ethnopharmacological survey of plants used against diabetes in Kisangani City (DR Congo). J Ethnopharmacol. 144(1):39–43. doi: 10.1016/j.jep.2012.08.022.
- Katuura E, Waako P, Ogwal-Okeng J, Bukenya-Ziraba R. 2007. Traditional treatment of malaria in Mbarara District, Western Uganda. Afr J Ecol. 45(S1):48–51. doi: 10.1111/j.1365-2028.2007.00737.x.
- Kayembe JS, Taba KM, Ntumba K, Tshiongo MTC, Kazadi TK. 2010. In vitro anti-malarial activity of 20 quinones isolated from four plants used by traditional healers in the Democratic Republic of Congo. J Med Plants Res. 4:991–994.
- Kayombo EJ, Uiso FC, Mahunnah RL. 2012. Experience on healthcare utilization in seven administrative regions of Tanzania. J Ethnobiol Ethnomed. 8:5.
- Kidane B, Van Andel T, Van Der Maesen LJG, Asfaw Z. 2014. Use and management of traditional medicinal plants by Maale and Ari ethnic communities in southern Ethiopia. J Ethnobiol Ethnomed. 10:46.
- Kingo RM, Maregesi SM. 2020. Ethnopharmacological study on some medicinal plants used in Ujiji, Kigoma, Tanzania. J Phytopharmacol. 9(2):102–109. doi: 10.31254/phyto.2020.9205.
- Kiraithe MN, Nguta JM, Mbaria JM, Kiama SG. 2016. Evaluation of the use of Ocimum suave Willd. (Lamiaceae), Plectranthus barbatus Andrews (Lamiaceae) and Zanthoxylum chalybeum Engl. (Rutaceae) as antimalarial remedies in Kenyan folk medicine. J Ethnopharmacol. 178:266–271. doi: 10.1016/j.jep.2015.12.013.
- Kirira PG, Rukunga GM, Wanyonyi AW, Muregi FM, Gathirwa JW, Muthaura CN, Omar SA, Tolo F, Mungai GM, Ndiege IO. 2006. Anti-plasmodial activity and toxicity of extracts of plants used in traditional malaria therapy in Meru and Kilifi Districts of Kenya. J Ethnopharmacol. 106(3):403–407. doi: 10.1016/j.jep.2006.01.017.
- Koch A, Tamez P, Pezzuto J, Soejarto D. 2005. Evaluation of plants used for antimalarial treatment by the Maasai of Kenya. J Ethnopharmacol. 101(1–3):95–99. doi: 10.1016/j.jep.2005.03.011.
- Kuria K, Coster D, Muriuki G, Masengo W, Kibwage I, Hoogmartens J, Laekeman G. 2001. Antimalarial activity of Ajuga remota Benth (Labiatae) and Caesalpinia volkensii Harms (Caesalpiniaceae): in vitro confirmation of ethnopharmacological use. J Ethnopharmacol. 74(2):141–148. doi: 10.1016/s0378-8741(00)00367-6.
- Kweyamba PA, Zofou D, Efange N, Assob J-CN, Kitau J, Nyindo M. 2019. In vitro and in vivo studies on anti-malarial activity of Commiphora africana and Dichrostachys cinerea used by the Maasai in Arusha region, Tanzania. Malar J. 18(1):119. doi: 10.1186/s12936-019-2752-8.
- Lacroix D, Prado S, Kamoga D, Kasenene J, Namukobe J, Krief S, Dumontet V, Mouray E, Bodo B, Brunois F. 2011. Antiplasmodial and cytotoxic activities of medicinal plants traditionally used in the village of Kiohima, Uganda. J Ethnopharmacol. 133(2):850–855. doi: 10.1016/j.jep.2010.11.013.
- Lai B-Y, Chen T-Y, Huang S-H, Kuo T-F, Chang T-H, Chiang C-K, Yang M-T, Chang CL-T. 2015. Bidens pilosa formulation improves blood homeostasis and β-cell function in men: a pilot study. Evid Based Complement Alternat Med. 2015:832314–832315. doi: 10.1155/2015/832314.
- Lans CA. 2006. Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitus. J Ethnobiol Ethnomed. 2:45.
- Laryea MK, Borquaye LS. 2019. Antimalarial efficacy and toxicological assessment of extracts of some Ghanaian medicinal plants. J Parasitol Res. 2019:1630405–1630409. doi: 10.1155/2019/1630405.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. PLoS Med. 6(7):e1000100. doi: 10.1371/journal.pmed.1000100.
- Limmatvapirat C, Sirisopanaporn S, Kittakoop P. 2004. Antitubercular and antiplasmodial constituents of Abrus precatorius. Planta Med. 70(3):276–278. doi: 10.1055/s-2004-818924.
- Liu NQ, Van der Kooy F, Verpoorte R. 2009. Artemisia afra: a potential flagship for African medicinal plants? S Afr J Bot. 75(2):185–195. doi: 10.1016/j.sajb.2008.11.001.
- Loomis TA, Hayes AW. 2014. Loomis's essentials of toxicology. 4th ed. San Diego: Academic Press; p. 282.
- Makundi EA, Mboera LEG, Malebo HM, Kitua AY. 2007. Priority setting on malaria interventions in Tanzania: strategies and challenges to mitigate against the intolerable burden. Am J Trop Med Hyg. 77(6_Suppl.):106–111. doi: 10.4269/ajtmh.2007.77.106.
- Malebo HM, Wiketye V, Katani SJ, Kitufe NA, Nyigo VA, Imeda CP, Ogondiek JW, Sunguruma R, Mhame PP, Massaga JJ, et al. 2015. In vivo antiplasmodial and toxicological effect of Maytenus senegalensis traditionally used in the treatment of malaria in Tanzania. Malar J. 14(1):79. doi: 10.1186/s12936-014-0525-y.
- Malik A, Akbar E, Afza N, Hai S. 2002. Flavone glycosides and bergenin derivatives from Tridax procumbens. Heterocycles. 57(4):733–739. doi: 10.3987/COM-02-9432.
- Manuel L, Bechel A, Noormahomed EV, Hlashwayo DF, Madureira MdC. 2020. Ethnobotanical study of plants used by the traditional healers to treat malaria in Mogovolas district, northern Mozambique. Heliyon. 6(12):e05746. doi: 10.1016/j.heliyon.2020.e05746.
- Maregesi SM, Ngassapa OD, Pieters L, Vlietinck AJ. 2007. Ethnopharmacological survey of the Bunda district, Tanzania: plants used to treat infectious diseases. J Ethnopharmacol. 113(3):457–470. doi: 10.1016/j.jep.2007.07.006.
- Maroyi A. 2013. Traditional use of medicinal plants in south-central Zimbabwe: review and perspectives. J Ethnobiol Ethnomed. 9:31.
- Martinello F, Soares SM, Franco JJ, Santos AC, Sugohara A, Garcia SB, Curti C, Uyemura SA. 2006. Hypolipemic and antioxidant activities from Tamarindus indica L. pulp fruit extract in hypercholesterolemic hamsters. Food Chem Toxicol. 44(6):810–818. doi: 10.1016/j.fct.2005.10.011.
- Mbinile SD, Munishi LK, Ngondya IB, Ndakidemi PA. 2020. Spatial distribution and anthropogenic threats facing medicinal plant Zanthoxylum chalybeum in Simanjiro area, Northern Tanzania. Sci Afr. 10:e00562. doi: 10.1016/j.sciaf.2020.e00562.
- Memvanga PB, Tona GL, Mesia GK, Lusakibanza MM, Cimanga RK. 2015. Antimalarial activity of medicinal plants from the Democratic Republic of Congo: a review. J Ethnopharmacol. 169:76–98. doi: 10.1016/j.jep.2015.03.075.
- Mesfin F, Demissew S, Teklehaymanot T. 2009. An ethnobotanical study of medicinal plants in Wonago Woreda, SNNPR, Ethiopia. J Ethnobiol Ethnomed. 5:28.
- Midiwo JO, Omoto FM, Yenesew A, Akala HM, Wangui J, Liyala P, Wasunna C, Waters NC. 2006. The first 9-hydroxyhomoisoflavanone, and antiplasmodial chalcones, from the aerial exudates of Polygonum senegalense. ARKIVOC. 2007(9):21–27. doi: 10.3998/ark.5550190.0008.904.
- Mogha NG, Kalokora OJ, Amir HM, Kacholi DS. 2022. Ethnomedicinal plants used for treatment of snakebites in Tanzania – a systematic review. Pharm Biol. 60(1):1925–1934. doi: 10.1080/13880209.2022.2123942.
- Moshi MJ, Otieno DF, Mbabazi PK, Weisheit A. 2009. The ethnomedicine of the Haya people of Bugabo ward, Kagera Region, north western Tanzania. J Ethnobiol Ethnomed. 5:24.
- Moshi MJ, Otieno DF, Mbabazi PK, Weisheit A. 2010. Ethnomedicine of the Kagera Region, north western Tanzania. Part 2: the medicinal plants used in Katoro Ward, Bukoba District. J Ethnobiol Ethnomed. 6:19.
- Moshi MJ, Otieno DF, Weisheit A. 2012. Ethnomedicine of the Kagera Region, north western Tanzania. Part 3: plants used in traditional medicine in Kikuku village, Muleba District. J Ethnobiol Ethnomed. 8:14.
- Muganga R, Angenot L, Tits M, Frédérich M. 2010. Antiplasmodial and cytotoxic activities of Rwandan medicinal plants used in the treatment of malaria. J Ethnopharmacol. 128(1):52–57. doi: 10.1016/j.jep.2009.12.023.
- Muregi FW, Chhabra SC, Njagi ENM, Lang'at-Thoruwa CC, Njue WM, Orago ASS, Omar SA, Ndiege IO. 2003. In vitro antiplasmodial activity of some plants used in Kisii, Kenya against malaria and their chloroquine potentiation effects. J Ethnopharmacol. 84(2–3):235–239. doi: 10.1016/s0378-8741(02)00327-6.
- Murugan K, Panneerselvam C, Subramaniam J, Madhiyazhagan P, Hwang J-S, Wang L, Dinesh D, Suresh U, Roni M, Higuchi A, et al. 2016. Eco-friendly drugs from the marine environment: spongeweed-synthesized silver nanoparticles are highly effective on Plasmodium falciparum and its vector Anopheles stephensi, with little non-target effects on predatory copepods. Environ Sci Pollut Res Int. 23(16):16671–16685. doi: 10.1007/s11356-016-6832-9.
- Muthaura CN, Keriko JM, Mutai C, Yenesew A, Gathirwa JW, Irungu BN, Nyangacha R, Mungai GM, Derese S. 2015. Antiplasmodial potential of traditional antimalarial phytotherapy remedies used by the Kwale community of the Kenyan Coast. J Ethnopharmacol. 170:148–157. doi: 10.1016/j.jep.2015.05.024.
- Nanyingi M, Kipsengeret KB, Wagate C, Langat B, Asaava L, Midiwo J. 2009. In vitro and in vivo antiplasmodial activity of Kenyan medicinal plants. In: Midiwo JO, Clough J, editors. Aspects of African biodiversity: Proceedings of the Pan-Africa Chemistry Network. Cambridge (UK): RCS Publishing; p. 20–28.
- Ngarivhume T, Van't Klooster CIEA, de Jong JTVM, Van der Westhuizen JH. 2015. Medicinal plants used by traditional healers for the treatment of malaria in the Chipinge district in Zimbabwe. J Ethnopharmacol. 159:224–237. doi: 10.1016/j.jep.2014.11.011.
- Nguta JM, Mbaria JM. 2013. Brine shrimp toxicity and antimalarial activity of some plants traditionally used in treatment of malaria in Msambweni district of Kenya. J Ethnopharmacol. 148(3):988–992. doi: 10.1016/j.jep.2013.05.053.
- Nigussie G, Wale M. 2022. Medicinal plants used in traditional treatment of malaria in Ethiopia: a review of ethnomedicine, anti-malarial and toxicity studies. Malar J. 21(1):262. doi: 10.1186/s12936-022-04264-w.
- Nondo RSO, Moshi MJ, Erasto P, Masimba PJ, Machumi F, Kidukuli AW, Heydenreich M, Zofou D. 2017. Anti-plasmodial activity of Norcaesalpin D and extracts of four medicinal plants used traditionally for treatment of malaria. BMC Complement Alternat Med. 17:167.
- Nondo RSO, Zofou D, Mainen JM, Erasto P, Samuel S, Ngemenya NM, Titanji PKV, Kidukuli WA, Masimba JP. 2015. Ethnobotanical survey and in vitro antiplasmodial activity of medicinal plants used to treat malaria in Kagera and Lindi regions, Tanzania. J Med Plants Res. 9(6):179–192. doi: 10.5897/JMPR2014.5685.
- Ntie-Kang F, Onguéné PA, Lifongo LL, Ndom JC, Sippl W, Mbaze LM. 2014. The potential of anti-malarial compounds derived from African medicinal plants, part II: a pharmacological evaluation of non-alkaloids and non-terpenoids. Malar J. 13(1):81. doi: 10.1186/1475-2875-13-81.
- Obbo CJD, Kariuki ST, Gathirwa JW, Olaho-Mukani W, Cheplogoi PK, Mwangi EM. 2019. In vitro antiplasmodial, antitrypanosomal and antileishmanial activities of selected medicinal plants from Ugandan flora: refocusing into multi-component potentials. J Ethnopharmacol. 229:127–136. doi: 10.1016/j.jep.2018.09.029.
- Odugbemi TO, Akinsulire OR, Aibinu IE, Fabeku PO. 2006. Medicinal plants useful for malaria therapy in Okeigbo, Ondo State, Southwest Nigeria. Afr J Tradit Complement Altern Med. 4:191–198.
- Okaiyeto K, Oguntibeju OO. 2021. African herbal medicines: adverse effects and cytotoxic potentials with different therapeutic applications. Int J Environ Res Public Health. 18(11):5988. doi: 10.3390/ijerph18115988.
- Okello D, Kang Y. 2019. Exploring antimalarial herbal plants across communities in Uganda based on electronic Data. Evid Based Complement Alternat Med. 2019:1–27. doi: 10.1155/2019/3057180.
- Oketch-Rabah HA, Dossaji SF, Mberu EK. 1999. Antimalarial activity of some Kenyan medicinal plants. Pharm Biol. 37(5):329–334. doi: 10.1076/phbi.37.5.329.6053.
- Olanlokun JO, David OM, Afolayan AJ. 2017. In vitro antiplasmodial activity and prophylactic potentials of extract and fractions of Trema orientalis (Linn.) stem bark. BMC Complement Alternat Med. 17:407.
- Omara T, Kagoya S, Openy A, Omute T, Ssebulime S, Kiplagat KM, Bongomin O. 2020. Antivenin plants used for treatment of snakebites in Uganda: ethnobotanical reports and pharmacological evidences. Trop Med Health. 48(1):6. doi: 10.1186/s41182-019-0187-0.
- Omara T. 2020. Antimalarial plants used across Kenyan communities. Evid Based Complement Alternat Med. 2020:1–31. doi: 10.1155/2020/4538602.
- Omulokoli E, Khan B, Chhabra SC. 1997. Antiplasmodial activity of four Kenyan medicinal plants. J Ethnopharmacol. 56(2):133–137. doi: 10.1016/s0378-8741(97)01521-3.
- Orwa JA, Ngeny L, Mwikwabe NM, Ondicho J, Jondiko IJO. 2013. Antimalarial and safety evaluation of extracts from Toddalia asiatica (L) Lam. (Rutaceae). J Ethnopharmacol. 145(2):587–590. doi: 10.1016/j.jep.2012.11.034.
- Owuor BO, Ochanda JO, Kokwaro JO, Cheruiyot AC, Yeda RA, Okudo CA, Akala HM. 2012. In vitro antiplasmodial activity of selected Luo and Kuria medicinal plants. J Ethnopharmacol. 144(3):779–781. doi: 10.1016/j.jep.2012.09.045.
- Oyeyemi IT, Akinseye KM, Adebayo SS, Oyetunji MT, Oyeyemi OT. 2019. Ethnobotanical survey of the plants used for the management of malaria in Ondo State, Nigeria. S Afr J Bot. 124:391–401. doi: 10.1016/j.sajb.2019.06.003.
- Parasuraman S. 2011. Toxicological screening. J Pharmacol Pharmacother. 2(2):74–79. doi: 10.4103/0976-500X.81895.
- Pattanayak S, Das DC, Sinha NK, Parida S. 2017. Use of medicinal plants for the treatment of urinary tract infections: a study from Paschim Medinipur District, West Bengal, India. Int J Pharm Bio Sci. 8(3):250–259. doi: 10.22376/ijpbs.2017.8.3.p250-259.
- Perez H, Rosa M, Apitz R. 1994. In vivo activity of ajoene against rodent malaria. Antimicrob Agents Chemother. 38(2):337–339. doi: 10.1128/AAC.38.2.337.
- Phillipson JD, Wright CW. 1991. Can ethnopharmacology contribute to the development of antimalarial agents? J Ethnopharmacol. 32(1–3):155–165. doi: 10.1016/0378-8741(91)90113-r.
- Pierre S, Fernand-N TF, Alexandre-Michel NN, Jean M. 2011. Medicinal plants used in traditional treatment of malaria in Cameroon. J Ecol Nat Environ. 3:104–117.
- Pillay P, Maharaj VJ, Smith PJ. 2008. Investigating South African plants as a source of new antimalarial drugs. J Ethnopharmacol. 119(3):438–454. doi: 10.1016/j.jep.2008.07.003.
- Rahmatullah M, Hossan S, Khatun A, Seraj S, Jahan R. 2012. Medicinal plants used by various tribes of Bangladesh for treatment of malaria. Malar Res Treat. 2012:e371798. doi: 10.1155/2012/371798.
- Ramathal DC, Ngassapa OD. 2001. Medicinal plants used by Rwandese traditional healers in refugee camps in Tanzania. Pharm Biol. 39(2):132–137. doi: 10.1076/phbi.39.2.132.6251.
- Rasmussen C, Alonso P, Ringwald P. 2022. Current and emerging strategies to combat antimalarial resistance. Expert Rev Anti Infect Ther. 20(3):353–372. doi: 10.1080/14787210.2021.1962291.
- Ruffo C. 1991. A survey of medicinal plants in Tabora region, Tanzania. In: Mshigeni KE, Nkunya MHH, Fupi V, Mahunnah RLA, Mshiu EN, editors. Traditional medicinal plants. 1st ed. Dar es Salaam: Dar es Salaam University Press – Ministry of Health, Tanzania; p. 391–406.
- Rukunga GM, Muregi FW, Tolo FM, Omar SA, Mwitari P, Muthaura CN, Omlin F, Lwande W, Hassanali A, Githure J, et al. 2007. The antiplasmodial activity of spermine alkaloids isolated from Albizia gummifera. Fitoterapia. 78(7–8):455–459. doi: 10.1016/j.fitote.2007.02.012.
- Rumisha SF, Shayo EH, Mboera LEG. 2019. Spatio-temporal prevalence of malaria and anaemia in relation to agro-ecosystems in Mvomero district, Tanzania. Malar J. 18(1):228. doi: 10.1186/s12936-019-2859-y.
- Salinitro M, Vicentini R, Bonomi C, Tassoni A. 2017. Traditional knowledge on wild and cultivated plants in the Kilombero Valley (Morogoro Region, Tanzania). J Ethnobiol Ethnomed. 13(1):17.
- Shangali CF, Zilihona IJE, Mwang'ingo PLP, Nummelin M. 2008. Use of medicinal plants in the Eastern Arc Mountains with special reference to the Hehe Ethnic Group in the Udzungwa Mountains, Tanzania. J East Afr Nat Hist. 97(2):225–254. doi: 10.2982/0012-8317-97.2.225.
- Shukla G, Bhatt JA, Chakravarty S. 2018. Folk therapeutic uses of ethnomedicinal plants to cure gynecological disorders: a meta-analysis of West Bengal State in India. Ethnobot Res Appl. 24:1–19.
- Silva MGB, Aragão TP, Vasconcelos CFB, Ferreira PA, Andrade BA, Costa IMA, Costa-Silva JH, Wanderley AG, Lafayette SSL. 2011. Acute and subacute toxicity of Cassia occidentalis L. stem and leaf in Wistar rats. J Ethnopharmacol. 136(2):341–346. doi: 10.1016/j.jep.2011.04.070.
- Singh SV, Manhas A, Kumar Y, Mishra S, Shanker K, Khan F, Srivastava K, Pal A. 2017. Antimalarial activity and safety assessment of Flueggea virosa leaves and its major constituent with special emphasis on their mode of action. Biomed Pharmacother. 89:761–771. doi: 10.1016/j.biopha.2017.02.056.
- Smolenski SJ, Silinis H, Farnsworth NR. 1975. Alkaloid screening. VIII. Lloydia. 38(6):497–528.
- Somsak V, Borkaew P, Klubsri C, Dondee K, Bootprom P, Saiphet B. 2016. Antimalarial properties of aqueous crude extracts of Gynostemma pentaphyllum and Moringa oleifera leaves in combination with artesunate in Plasmodium berghei-infected Mice. J Trop Med. 2016:8031392–8031396. doi: 10.1155/2016/8031392.
- Stangeland T, Alele PE, Katuura E, Lye KA. 2011. Plants used to treat malaria in Nyakayojo sub-county, western Uganda. J Ethnopharmacol. 137(1):154–166. doi: 10.1016/j.jep.2011.05.002.
- Tabuti JRS, Obakiro SB, Nabatanzi A, Anywar G, Nambejja C, Mutyaba MR, Omara T, Waako P. 2023. Medicinal plants used for treatment of malaria by indigenous communities of Tororo District, Eastern Uganda. Trop Med Health. 51(1):34. doi: 10.1186/s41182-023-00526-8.
- Taek M, Prajogo B, Agil M. 2018. Plants used in traditional medicine for treatment of malaria by Tetun ethnic people in West Timor Indonesia. Asian Pac J Trop Med. 11(11):630–637. doi: 10.4103/1995-7645.246339.
- Teklay A, Abera B, Giday M. 2013. An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J Ethnobiol Ethnomed. 9:65.
- Thu AM, Phyo AP, Landier J, Parker DM, Nosten FH. 2017. Combating multidrug‐resistant Plasmodium falciparum malaria. FEBS J. 284(16):2569–2578. doi: 10.1111/febs.14127.
- Tiko GH, Adamou R, Amoussa AMO, Medjigbodo AA, Sanni A, Djogbénou LS, Lagnika L. 2020. Antiplasmodial, antioxidant, hemolytic activities and acute toxicity of Costus afer Ker Gawl (Costaceae) used in malaria healing in Benin. Res J Med Plants. 14(1):24–34.
- Tona L, Mesia K, Ngimbi NP, Chrimwami B, Cimanga K, de Bruyne T, Apers S, Hermans N, Totte J, Pieters L, et al. 2001. In-vivo antimalarial activity of Cassia occidentalis, Morinda morindoides and Phyllanthus niruri. Ann Trop Med Parasitol. 95(1):47–57. doi: 10.1080/00034983.2001.11813614.
- Turner PV, Brabb T, Pekow C, Vasbinder MA. 2011. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci. 50(5):600–613.
- Weenen H, Nkunya MH, Bray DH, Mwasumbi LB, Kinabo LS, Kilimali VA. 1990. Antimalarial activity of Tanzanian medicinal plants. Planta Med. 56(4):368–370. doi: 10.1055/s-2006-960984.
- White NJ. 2004. Antimalarial drug resistance. J Clin Invest. 113(8):1084–1092. doi: 10.1172/JCI21682.
- Wicht KJ, Mok S, Fidock DA. 2020. Molecular mechanisms of drug resistance in Plasmodium falciparum Malaria. Annu Rev Microbiol. 74(1):431–454. doi: 10.1146/annurev-micro-020518-115546.
- [WHO] World Health Organization. 2014. WHO traditional medicine strategy: 2014–2023. Geneva, Switzerland: World Health Organization Press; p. 76.
- [WHO] World Health Organization. 2021. World malaria report 2021. Geneva, Switzerland: World Health Organization Press; p. 263.