Abstract
Context
The effect of the active ingredients in traditional Chinese medicines on the activity of cytochrome P450 enzymes (CYP450s) is a critical factor that should be considered in TCM prescriptions. Physcion, the major active ingredient of Rheum spp. (Polygonaceae), possesses wide pharmacological activities.
Objectives
The effect of physcion on CYP450 activity was investigated to provide a theoretical basis for use.
Materials and methods
The experiments were conducted in pooled human liver microsomes (HLMs). The activity of CYP450 isoforms was evaluated with corresponding substrates and probe reactions. Blank HLMs were set as negative controls, and typical inhibitors were employed as positive controls. The inhibition model was fitted with Lineweaver Burk plots. The concentration (0, 2.5, 5, 10, 25, 50 and 100 μM physcion) and time-dependent (0, 5, 10, 15 and 30 min) effects of physcion were also assessed.
Results
Physcion suppressed CYP2C9, 2D6 and 3A4 in a concentration-dependent manner with IC50 values of 7.44, 17.84 and 13.50 μM, respectively. The inhibition of CYP2C9 and 2D6 was competitive with the Ki values of 3.69 and 8.66 μM, respectively. The inhibition of CYP3A4 was non-competitive with a Ki value of 6.70 μM. Additionally, only the inhibition of CYP3A4 was time-dependent with the KI and Kinact parameters of 3.10 μM−1 and 0.049 min−1, respectively.
Conclusions
The inhibition of CYP450s by physcion should be considered in its clinical prescription, and the study design can be employed to evaluate the interaction of CYP450s with other herbs.
Introduction
Cytochrome P450 enzymes (CYP450s) are critical phase I enzymes in biotransformation and play vital roles in the metabolism of drugs, pollutions, carcinogens and endogenous substrates (Manikandan and Nagini Citation2018). CYP450s mainly exist in the human liver mediating the introduction of polar functional groups into drugs and promoting phase II reactions during drug metabolism (Kampe et al. Citation2014; Zimmerman et al. Citation2018). Due to their genetic diversity, wide substrate specificity, and catalytic versatility, CYP450s are responsible for about 3/4 of phase I-dependent drug metabolism. The CYP450 family includes a large number of members, and the CYP1, CYP2 and CYP3 families are most common in human liver microsomes (HLMs). The activity of CYP450s is one of the most susceptible factors during drug co-administration, which would induce drug-drug interaction and further affect drug efficacy (Lynch and Price Citation2007; Feng and He Citation2013). Drugs can be metabolized by a single CYP450 enzyme, or by several enzymes; inducers or inhibitors of CYP450s could also influence the activity of one or more CYP450 isoforms. Recently, the rapid development of traditional Chinese medicine, the use of Chinese patent medicine, and the widely accepted combination of traditional Chinese and western medicine, increases the occurrence of drug-drug interactions (Lu et al. Citation2015). Therefore, the effect of traditional Chinese medicine, Western medicine and their major active ingredients on the activity of CYP450s has attracted special attention.
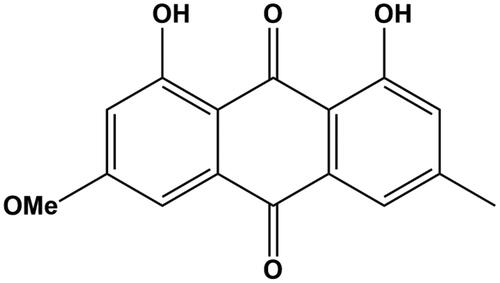
In the treatment of endocrine diseases, traditional Chinese medicine has been widely used. Rheum officinale Baill. (Polygonaceae) has various pharmacological activities, such as anticancer, regulating lipids metabolism and alleviating liver injury (Huang Q et al. Citation2007; Zhuang et al. Citation2020; Huang H et al. Citation2021; Gao and Nan Citation2022). Rhubarb possesses a number of active ingredients, such as anthraquinone, anthrone, organic acid, tannin and polysaccharides. Physcion () is an anthraquinone compound that widely exists in the Polygonaceae family. Previous studies demonstrated that physcion could alleviate brain and nerve injury induced by ischemia and hypoxia and possessed the activities of antibacteria, anti-inflammation and antioxidants (Adnan et al. Citation2021; Dong et al. Citation2021; Ji et al. Citation2021; Qi et al. Citation2022). In endocrine diseases, physcion could prevent ethanol-induced liver injury, reduce lipid accumulation and prevent obesity, implying its potential in the therapy of fatty liver (Lee et al. Citation2019; Yao et al. Citation2020). Decocting is the major form of administration for traditional Chinese medicine, therefore investigating the influence of herb extracts on CYP450 activity is of great significance. The effect of physcion on CYP450s activity is an important factor that should be considered during the prescription of physcion-containing or sourced herbs.
The influence of physcion on the activity of major CYP450 isoforms was investigated in this study in HLMs. The kinetics and models of CYP450 inhibitions were also disclosed, aiming to reveal more environmental risk factors and provide theoretical reference for the clinical application of physcion and its sourced herbs, especially for their co-administration.
Materials and methods
Study design
Pooled HLMs were obtained from BD Gentest Co. (Franklin Lakes, NJ) from 50 donors with the protein concentration of 5 mg/mL. The activity of CYP450 isoforms was evaluated with corresponding probe reactions and specific substrates summarized in (Mori et al. Citation2009; Dinger et al. Citation2014; Carrão et al. Citation2018). The HLMs were divided into negative control, positive control and physcion groups. The negative control group was the blank treatment incubated without inhibitors, while the positive control and physcion groups were treated with typical inhibitors of CYP450 isoforms and physcion, respectively. The concentration of inhibitors of different isoforms is listed in .
Table 1. Reaction conditions of CYP450 isoforms tested, including maker reactions, incubation conditions and Km used in the inhibition study.
Effect of physcion on the activity of CYP450 isoforms
The incubation system was composed of 100 mM potassium phosphate buffer (pH 7.4), NADPH generating system (1 mM NADP+, 10 mM glucose-6-phosphate, 1 U/mL of glucose-6-phosphate dehydrogenase and 4 mM MgCl2), typical inhibitors or physcion and HLM at appropriate protein concentration (). The incubation volume was 200 μL and was initiated by the NADPH-generating system after a pre-incubation of 3 min. The reaction was terminated by acetonitrile or trichloroacetic acid (for CYP2A6). After centrifugation, the supernatant was analysed with a Agilent 1260 HPLC system with DAD and FLD detectors.
Inhibition model fitting
The isoforms inhibited by physcion were further studied to fit the inhibition models. First, the half-inhibition concentrations of the inhibited isoforms were obtained by incubating with different physcion concentrations (0–100 μM). While the Ki values were obtained by incubation with different substrate concentrations by Lineweaver–Burk plots. The competitive inhibition was fitted with the equation: v = (VmaxS)/[Km(1+I/Ki)+S], while the non-competitive inhibition fitting was carried out with the equation: v = (VmaxS)/[Km+S(1+I/Ki)], where v indicates reaction rate, I indicates the concentration of physcion, Ki is the inhibition constant, S indicates the concentration of substrates and Km indicates the substrate concentration reaching the half of the maximum reaction rate (Vmax).
Time-dependent inhibition
HLMs were pre-incubated with physcion for 30 min and then 20 μL of aliquot was transferred to another incubation tube and initiated by the NADPH-generated system as described above. The concentrations of substrates were approximately to Km and the incubation was sustained for 5, 10, 15 and 30 min terminated by acetonitrile. The mixture was analysed by HPLC for residual activity after centrifugation.
Furthermore, the time-dependent inhibition was quantified by the values of KI and Kinact parameters. The incubation system was performed with different concentrations of physcion (0–50 μM) higher concentration of substrate approximately 4-fold of Km for 0–30 min. The two-step incubation scheme was conducted as described above.
Statistical analyses
Experiments were performed in triplicate, and data were analysed with GraphPad Prism version 9.0 (GraphPad Software, Inc, La Jolla, CA) and difference comparison was conducted with one-way ANOVA (p < 0.05). The values of IC50 and Ki were calculated by the Lineweaver–Burk plot.
Results
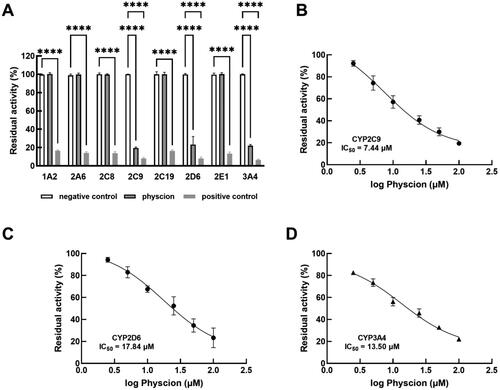
Physcion inhibited CYP2C9, 2D6 and 3A4 in a concentration-dependent manner
Compared with negative controls, the activity of all studied isoforms was significantly suppressed by their typical inhibitors (positive controls). Only the activity of CYP2C9, 2D6 and 3A4 was inhibited by physcion (). The inhibitory effect of physcion was weaker than the positive inhibitors of CYP2C9, 2D6 and 3A4. Additionally, the inhibitory effect of physcion was enhanced with the increasing concentrations, where the IC50 values of CYP2C9 (), 2D6 () and 3A4 () were 7.44, 17.84 and 13.50 μM obtained under 0, 2.5, 5, 10, 25, 50 and 100 μM physcion.
Figure 2. Effect of physcion on the major CYP450 isoforms (A) and its concentration-dependent manner in the inhibition of CYP2C9 (B), 2D6 (C) and 3A4 (D). ****p < 0.0001 relative to the negative control. Negative control: blank treatment; physcion: treated with 100 μM physcion; positive control: treated with typical inhibitors (inhibitor types and concentrations are listed in ).
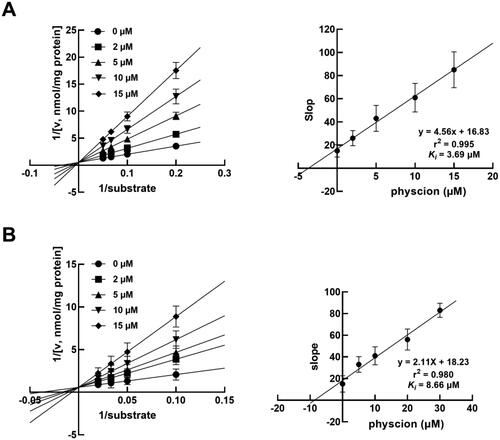
Physcion served as a competitive inhibitor of CYP2C9 and 2D6
In the presence of 0, 2, 5, 10 and 15 μM physcion, the Lineweaver–Burk results fitted the inhibition of CYP2C9 () and 2D6 () with a competitive inhibition model with a constant value of Vmax. Meanwhile, the Ki values of CYP2C9 and 2D6 were obtained as 3.69 and 8.66 μM, respectively.
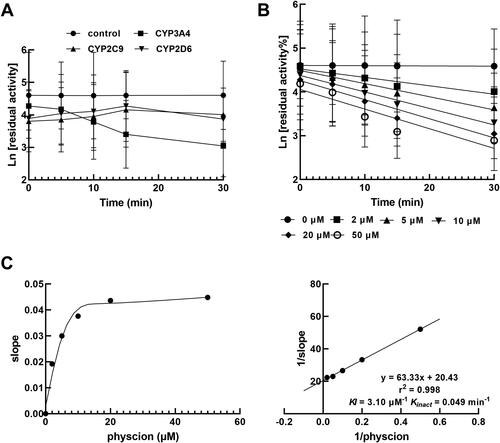
The inhibition of CYP3A4 by physcion was non-competitively and time-dependent
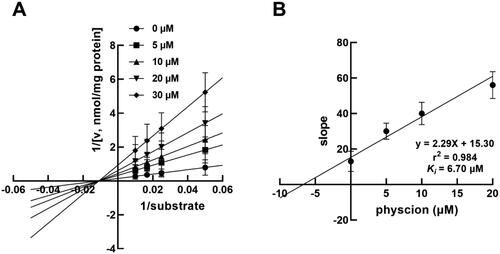
The inhibition of CYP3A4 by 0, 5, 10, 20 and 30 μM physcion was fitted with the non-competitive inhibition model with a constant value of Km (). Moreover, the Ki value of CYP3A4 by physcion was obtained as 6.07 μM ().
Figure 4. The inhibition of CYP3A4 by physcion was best fitted the non-competitive inhibition model by Lineweaver Burk plot in the presence of 0, 5, 10, 20 and 30 μM physcion (A) and the Ki value was 6.70 μM (B).
CYP3A4 activity was decreased with the increasing incubation time, but the inhibition of CYP2C9 and 2D6 was not influenced, indicating the time-dependent characteristic of CYP3A4 inhibition by physcion (). Additionally, the time-dependent manner was observed in the presence of different concentrations of physcion (), and the KI and Kinact parameters were obtained as 3.10 μM−1 and 0.049 min−1 ().
Discussion
Due to the complex composition of active ingredients, the therapeutic efficiency of TCM is always a result of the combined action of various components (Tang et al. Citation2008; Zhou P. Citation2010; Li et al. Citation2020). The activity of CYP450s might be suppressed or induced by co-administrated herbs and influence drug pharmacokinetics, therefore affecting the curative effects of TCM prescriptions (Almazroo et al. Citation2017). As a critical herb of liver injury treatment and anticancer therapy, the effect of rhubarb active ingredients on CYP450s activity is an important factor for its co-prescription with other herbs. Herein, the effect of physcion, a major extract of rhubarb, on CYP450s was investigated in HLMs. The activity of CYP2C9, 2D6 and 3A4 was dramatically suppressed by physcion, but the inhibitory effects of physcion were weaker than their positive inhibitors. The observed inhibitory effects of positive inhibitors are consistent with previous studies (Sun et al. Citation2021; Zhou J et al. Citation2022).
Both CYP2C9 and 2D6 are major members of the CYP2 subfamily. CYP2C9 is a critical mediator in the metabolism of non-steroidal anti-inflammatory drugs, oral anticoagulants, oral hypoglycemics and other therapeutic agents (Ma et al. Citation2008; Yildirim et al. Citation2008; Theken et al. Citation2020; Velizarova et al. Citation2022). CYP2D6 is responsible for the biotransformation of about a quarter of prescription drugs (Maréchal et al. Citation2008; Taylor et al. Citation2020). The activities of CYP2C9 and 2D6 were revealed to induce adverse drug-drug interaction during the co-administration of CYP2C9- or 2D6-metabolized drugs with CYP2C9 or 2D6 inhibitors or inducers (Bahar et al. Citation2017; Wang K et al. Citation2020). For example, the inhibitory effect of thymoquinone and its sourced herb, Nigella sativa L. (Ranunculaceae) on CYP2C9 resulted in their adverse interactions with phenytoin, and this kind of interaction might also occur during their combination with other CYP2C9 substrates (Wang Z et al. Citation2022). The genetic polymorphism of CYP2D6 significantly affected the metabolism of dacomitinib, and CYP2D6 was demonstrated to mediate the interaction between dacomitinib and trazodone (Han et al. Citation2022). Through fitting by Lineweaver Burk plots, the inhibition of CYP2C9 and 2D6 by physcion was revealed to be performed in a competitive manner, competing for binding sites with substrates and therefore suppressed enzyme activity.
The CYP3A subfamily is also a critical CYP450 enzyme in the liver and extrahepatic tissues, such as the intestinal and kidney, which plays vital roles in the oxidant of xenobiotics and contributes to the biotransformation of over 50% of clinical therapeutic drugs (Zhou SF Citation2008; Hohmann et al. Citation2016; Zhang N et al. Citation2018). CYP3A4 is the most important member of the CYP3A subfamily and could mediate various drug–drug interactions in recent studies (Kato Citation2008; Tian and Hu Citation2014; Kondža et al. Citation2021). For instance, the inhibition of CYP3A4 by tilmicosin was demonstrated to mediate its interaction with enrofloxacin (Zhang L et al. Citation2022). A clinical study in healthy individuals revealed that the inhibitory effect of itraconazole on CYP3A4 resulted in the increased systemic exposure of tropifexor (Chen et al. Citation2022). The inhibition of CYP3A4 by physcion was also observed in this study, which was non-competitive. Moreover, the inhibition of CYP3A4 by physcion showed a time-dependent manner. Therefore, the administration time and the half-life of physcion should be considered in its prescription. According to previous reports on the pharmacokinetics of physcion, the plasma concentration of physcion was 0.018 μg/mL after 1 h of rhubarb extract administration, and the maximum concentration was only 0.032 μg/mL, reached at 2 h of administration (Ding et al. Citation2003; Xun et al. Citation2019). Another study reported that physcion in rats reached the maximum plasma concentration of 461.85 ± 266.77 ng/mL at 0.167 ± 0.002 h after administration of Semen Cassiae extract (Yang et al. Citation2015). Although the pharmacokinetic parameters of physcion are much lower than the observed IC50 and Ki in this study, its inhibitory effect on CYP2C9, CD6 and 3A4 should also be considered in its co-prescription with other drugs or herbs, especially the substrates metabolized by these CYP450s.
There were still several points that need to be confirmed in future studies. First, the activity of CYP450s is regulated by a series of genes, of which the expression influenced by physcion might be the potential molecular mechanism underlying the effect of physcion. The assessment of CYP450s activity is diverse, and some direct methods have been developed based on high throughput sequencing, fluorescence methods and molecular biological methods (Buters et al. Citation1993; Kozakai et al. Citation2014; Mattinen et al. Citation2014). Currently, molecular docking has attracted special attention, which could reveal the molecular mechanism underlying the inhibitory effect of physcion. Therefore, future studies could further explore the molecular mechanism and provide more information on the pharmacological characteristics of physcion and its source herbs and explore more direct evaluations of CYP450s.
CYP450s mainly exist in the human liver. The effect of physcion on the activity of CYP450s might also indicate changes in liver function. Hence, the effect of physcion on liver function should also attract special attention in future studies to further complete the attention in the prescription of physcion and its sourced or containing herbs.
Taken together, physcion was shown to be a competitive inhibitor of CYP2C9 and 2D6 and a non-competitive inhibitor of CYP3A4. The inhibitory effect of physcion was concentration-dependent and its inhibitory effect on CYP3A4 was time-dependent. The combined prescription of physcion or its sourced herbs should be careful, especially for their co-administration with CYP2C9-, 2D6- and 3A4-metabolized herbs or drugs. Further studies are needed to confirm the potential herb-herb or herb–drug interactions. Moreover, this study also provides a reference experimental design in the research direction of CYP450 isoforms.
Disclosure statement
The authors have no relevant financial or non-financial interests to disclose.
Data availability statement
The datasets used and/or analysed during this study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Adnan M, Rasul A, Hussain G, Shah MA, Sarfraz I, Nageen B, Riaz A, Khalid R, Asrar M, Selamoglu Z, et al. 2021. Physcion and physcion 8-O-β-d-glucopyranoside: natural anthraquinones with potential anticancer activities. Curr Drug Targets. 22(5):488–504. doi: 10.2174/1389450121999201013154542.
- Almazroo OA, Miah MK, Venkataramanan R. 2017. Drug metabolism in the liver. Clin Liver Dis. 21(1):1–20. doi: 10.1016/j.cld.2016.08.001.
- Bahar MA, Setiawan D, Hak E, Wilffert B. 2017. Pharmacogenetics of drug-drug interaction and drug-drug-gene interaction: a systematic review on CYP2C9, CYP2C19 and CYP2D6. Pharmacogenomics. 18(7):701–739. doi: 10.2217/pgs-2017-0194.
- Buters JT, Schiller CD, Chou RC. 1993. A highly sensitive tool for the assay of cytochrome P450 enzyme activity in rat, dog and man. Direct fluorescence monitoring of the deethylation of 7-ethoxy-4-trifluoromethylcoumarin. Biochem Pharmacol. 46(9):1577–1584. doi: 10.1016/0006-2952(93)90326-r.
- Carrão DB, de Oliveira AR, Magalhães IR. 2018. Challenges of probe cocktail approach for human drug-drug interaction assays. Bioanalysis. 10(24):1969–1972. doi: 10.4155/bio-2018-0247.
- Chen J, Stringer R, Shah B, Gu J, Zhang Y, Hackling M, Prince W, Woessner R. 2022. Drug-drug interaction studies to evaluate the effect of inhibition of UGT1A1 and CYP3A4 and induction of CYP3A4 on the pharmacokinetics of tropifexor in healthy subjects. Clin Pharmacol Drug Dev. 11(11):1253–1263. doi: 10.1002/cpdd.1140.
- Ding M, Ma S, Liu D. 2003. Simultaneous determination of hydroxyanthraquinones in rhubarb and experimental animal bodies by high-performance liquid chromatography. Anal Sci. 19(8):1163–1165. doi: 10.2116/analsci.19.1163.
- Dinger J, Meyer MR, Maurer HH. 2014. Development of an in vitro cytochrome P450 cocktail inhibition assay for assessing the inhibition risk of drugs of abuse. Toxicol Lett. 230(1):28–35. doi: 10.1016/j.toxlet.2014.08.004.
- Dong X, Wang L, Song G, Cai X, Wang W, Chen J, Wang G. 2021. Physcion protects rats against cerebral ischemia-reperfusion injury via inhibition of TLR4/NF-κB signaling pathway. Drug Des Devel Ther. 15:277–287. doi: 10.2147/DDDT.S267856.
- Feng S, He X. 2013. Mechanism-based inhibition of CYP450: an indicator of drug-induced hepatotoxicity. Curr Drug Metab. 14(9):921–945. doi: 10.2174/138920021131400114.
- Gao Y, Nan Z. 2022. Mechanistic insights into the use of rhubarb in diabetic kidney disease treatment using network pharmacology. Medicine. 101(1):e28465. doi: 10.1097/MD.0000000000028465.
- Han M, Zhang X, Ye Z, Wang J, Kong Q, Hu X, Qian J, Cai J, Hu G. 2022. Effects of CYP2D6 genetic polymorphism and drug interaction on the metabolism of dacomitinib. Chem Res Toxicol. 35(2):265–274. doi: 10.1021/acs.chemrestox.1c00327.
- Hohmann N, Haefeli WE, Mikus G. 2016. CYP3A activity: towards dose adaptation to the individual. Expert Opin Drug Metab Toxicol. 12(5):479–497. doi: 10.1517/17425255.2016.1163337.
- Huang H, Liu Z, Qi X, Gao N, Chang J, Yang M, Na S, Liu Y, Song R, Li L, et al. 2021. Rhubarb granule promotes diethylnitrosamine-induced liver tumorigenesis by activating the oxidative branch of pentose phosphate pathway via G6PD in rats. J Ethnopharmacol. 281:114479. doi: 10.1016/j.jep.2021.114479.
- Huang Q, Lu G, Shen HM, Chung MC, Ong CN. 2007. Anti-cancer properties of anthraquinones from rhubarb. Med Res Rev. 27(5):609–630. doi: 10.1002/med.20094.
- Ji XW, Lyu HJ, Zhou GH, Wu B, Zhu YY, Wu TH, Zhang F, Jin SN, Cho KW, Wen JF. 2021. Physcion, a tetra-substituted 9,10-anthraquinone, prevents homocysteine-induced endothelial dysfunction by activating Ca2+- and Akt-eNOS-NO signaling pathways. Phytomedicine. 81:153410. doi: 10.1016/j.phymed.2020.153410.
- Kampe T, König A, Schroeder H, Hengstler JG, Niemeyer CM. 2014. Modular microfluidic system for emulation of human phase I/phase II metabolism. Anal Chem. 86(6):3068–3074. doi: 10.1021/ac404128k.
- Kato M. 2008. Intestinal first-pass metabolism of CYP3A4 substrates. Drug Metab Pharmacokinet. 23(2):87–94. doi: 10.2133/dmpk.23.87.
- Kondža M, Bojić M, Tomić I, Maleš Ž, Rezić V, Ćavar I. 2021. Characterization of the CYP3A4 enzyme inhibition potential of selected flavonoids. Molecules. 26(10):3018. doi: 10.3390/molecules26103018.
- Kozakai K, Yamada Y, Oshikata M, Kawase T, Suzuki E, Haramaki Y, Taniguchi H. 2014. Cocktail-substrate approach-based high-throughput assay for evaluation of direct and time-dependent inhibition of multiple cytochrome P450 isoforms. Drug Metab Pharmacokinet. 29(2):198–207. doi: 10.2133/dmpk.dmpk-13-rg-093.
- Lee SJ, Cho SJ, Kwon EY, Choi MS. 2019. Physcion reduces lipid accumulation and prevents the obesity in mice. Nutr Metab. 16(1):31. doi: 10.1186/s12986-019-0362-7.
- Li L, Wang L, Fan W, Jiang Y, Zhang C, Li J, Peng W, Wu C. 2020. The application of fermentation technology in traditional Chinese medicine: a review. Am J Chin Med. 48(4):899–921. doi: 10.1142/S0192415X20500433.
- Lu TL, Su LL, Ji D, Gu W, Mao CQ. 2015. Interaction between CYP450 enzymes and metabolism of traditional Chinese medicine as well as enzyme activity assay. Zhongguo Zhong Yao Za Zhi. 40(18):3524–3529.
- Lynch T, Price A. 2007. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician. 76(3):391–396.
- Ma J, Yang XY, Qiao L, Liang LQ, Chen MH. 2008. CYP2C9 polymorphism in non-steroidal anti-inflammatory drugs-induced gastropathy. J Dig Dis. 9(2):79–83. doi: 10.1111/j.1751-2980.2008.00326.x.
- Manikandan P, Nagini S. 2018. Cytochrome P450 structure, function and clinical significance: a review. Curr Drug Targets. 19(1):38–54.
- Maréchal JD, Kemp CA, Roberts GC, Paine MJ, Wolf CR, Sutcliffe MJ. 2008. Insights into drug metabolism by cytochromes P450 from modelling studies of CYP2D6-drug interactions. Br J Pharmacol. 153(1):S82–S89.
- Mattinen L, Kublbeck J, Rechardt O, Honkakoski P, Rautio J. 2014. Direct and rapid transcript analysis assay for CYP mRNA expression and inducibility in human primary hepatocytes. Drug Metab Lett. 8(2):77–87. doi: 10.2174/1872312808666140606101422.
- Mori K, Hashimoto H, Takatsu H, Tsuda-Tsukimoto M, Kume T. 2009. Cocktail-substrate assay system for mechanism-based inhibition of CYP2C9, CYP2D6, and CYP3A using human liver microsomes at an early stage of drug development. Xenobiotica. 39(6):415–422. doi: 10.1080/00498250902822204.
- Qi F, Zhang W, Xue Y, Geng C, Jin Z, Li J, Guo Q, Huang X, Lu X. 2022. Microbial production of the plant-derived fungicide physcion. Metab Eng. 74:130–138. doi: 10.1016/j.ymben.2022.10.007.
- Sun Y, He M, Sun Y, Wei J. 2021. 4-O-Galloylalbiflorin inhibits the activity of CYP3A, 2C9, and 2D in human liver microsomes. Xenobiotica. 51(8):871–876. doi: 10.1080/00498254.2021.1936688.
- Tang JL, Liu BY, Ma KW. 2008. Traditional Chinese medicine. Lancet. 372(9654):1938–1940. doi: 10.1016/S0140-6736(08)61354-9.
- Taylor C, Crosby I, Yip V, Maguire P, Pirmohamed M, Turner RM. 2020. A review of the important role of CYP2D6 in pharmacogenomics. Genes. 11(11):1295. doi: 10.3390/genes11111295.
- Theken KN, Lee CR, Gong L, Caudle KE, Formea CM, Gaedigk A, Klein TE, Agúndez JAG, Grosser T. 2020. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2C9 and nonsteroidal anti-inflammatory drugs. Clin Pharmacol Ther. 108(2):191–200. doi: 10.1002/cpt.1830.
- Tian D, Hu Z. 2014. CYP3A4-mediated pharmacokinetic interactions in cancer therapy. Curr Drug Metab. 15(8):808–817. doi: 10.2174/1389200216666150223152627.
- Velizarova M, Abedinov P, Svinarov D, Nikolov V, Hristova J. 2022. Frequency distribution of CYP2C9 and VKORC1 mutations among Bulgarian patients and their importance for anticoagulant therapy. Clin Lab. 68(12):2527–2532. doi: 10.7754/Clin.Lab.2022.220213.
- Wang K, Gao Q, Zhang T, Rao J, Ding L, Qiu F. 2020. Inhibition of CYP2C9 by natural products: insight into the potential risk of herb-drug interactions. Drug Metab Rev. 52(2):235–257. doi: 10.1080/03602532.2020.1758714.
- Wang Z, Wang X, Wang Z, Lv X, Yin H, Li W, Li W, Jiang L, Liu Y. 2022. Potential herb-drug interaction risk of thymoquinone and phenytoin. Chem Biol Interact. 353:109801. doi: 10.1016/j.cbi.2022.109801.
- Xun L, Liu Y, Chu S, Yang S, Peng Y, Ren S, Wen B, Chen N. 2019. Physcion and physcion 8-O-β-glucopyranoside: a review of their pharmacology, toxicities and pharmacokinetics. Chem Biol Interact. 310:108722. doi: 10.1016/j.cbi.2019.06.035.
- Yang C, Wang S, Guo X, Sun J, Liu L, Wu L. 2015. Simultaneous determination of seven anthraquinones in rat plasma by Ultra High Performance Liquid Chromatography-tandem Mass Spectrometry and pharmacokinetic study after oral administration of Semen Cassiae extract. J Ethnopharmacol. 169:305–313. doi: 10.1016/j.jep.2015.04.008.
- Yao Y, Zuo A, Deng Q, Liu S, Zhan T, Wang M, Xu H, Ma J, Zhao Y. 2020. Physcion protects against ethanol-induced liver injury by reprogramming of circadian clock. Front Pharmacol. 11:573074. doi: 10.3389/fphar.2020.573074.
- Yildirim H, Tamer L, Sucu N, Atik U. 2008. The role of CYP2C9 gene polymorphisms on anticoagulant therapy after heart valve replacement. Med Princ Pract. 17(6):464–467. doi: 10.1159/000151568.
- Zhang L, Wang X, Wang L, Badawy S, Liu Z, Xie C, Wang X, Tao Y. 2022. A new drug-drug interaction-tilmicosin reduces the metabolism of enrofloxacin through CYP3A4. Res Vet Sci. 148:33–41. doi: 10.1016/j.rvsc.2022.05.004.
- Zhang N, Shon J, Kim MJ, Yu C, Zhang L, Huang SM, Lee L, Tran D, Li L. 2018. Role of CYP3A in oral contraceptives clearance. Clin Transl Sci. 11(3):251–260. doi: 10.1111/cts.12499.
- Zhou J, Qian X, Zhou Y, Xiong S, Ji S, Wang Y, Zhao P. 2022. Human liver microsomes study on the inhibitory effect of plantainoside D on the activity of cytochrome P450 activity. BMC Complement Med Ther. 22(1):197. doi: 10.1186/s12906-022-03671-5.
- Zhou P. 2010. Traditional Chinese medicine. Comb Chem High Throughput Screen. 13(10):836–836. doi: 10.2174/138620710793360329.
- Zhou SF. 2008. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab. 9(4):310–322. doi: 10.2174/138920008784220664.
- Zhuang T, Gu X, Zhou N, Ding L, Yang L, Zhou M. 2020. Hepatoprotection and hepatotoxicity of Chinese herb rhubarb (Dahuang): how to properly control the "general (Jiang Jun)" in Chinese medical herb. Biomed Pharmacother. 127:110224. doi: 10.1016/j.biopha.2020.110224.
- Zimmerman AD, Breckenridge CB, Yi KD, Sawhney Coder P, Wanders D, Judd RL, Foradori CD. 2018. Changes in hepatic phase I and phase II biotransformation enzyme expression and glutathione levels following atrazine exposure in female rats. Xenobiotica. 48(9):867–881. doi: 10.1080/00498254.2017.1374486.