ABSTRACT
Introduction
Studies on quadrivalent measles, mumps, rubella, and varicella (MMRV) vaccines have indicated a twofold increased relative risk of febrile convulsion (FC) after the first dose compared to MMR and V administered at the same medical visit (MMR+V).
Areas covered
This narrative review contextualizes FC occurrence after the first MMRV vaccine dose from a clinical perspective and outlines approaches to attenuate FC occurrence post-vaccination.
Expert opinion
While the relative FC risk increases after the first dose of MMRV compared to MMR+V vaccine in measles-naïve infants, the attributable risk is low versus the overall FC risk in the pediatric population triggered by other causes, like natural exposure to pathogens or routine vaccination. No increased risk of FC has been reported after MMRV co-administration with other routine vaccines compared to MMRV alone. Based on our findings and considering the MMRV vaccination benefits (fewer injections, higher coverage, better vaccination compliance), the overall benefit-risk profile of MMRV vaccine is considered to remain positive. Potential occurrence of FC in predisposed children (e.g. with personal/family history of FC) may be attenuated if they receive MMR+V instead of MMRV as the first dose. It is also important to monitor vaccinees for fever during the first 2 weeks post-vaccination.
Plain Language Summary
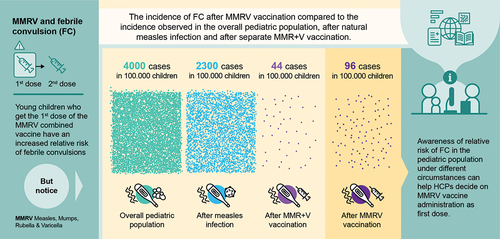
Children under 5 years of age can sometimes have convulsions when they get a fever during illness or after vaccination. These are called febrile convulsions, and, in most cases, they leave no lasting damage, and the child outgrows them. After a combined vaccine against four childhood illnesses (measles, mumps, rubella, and varicella) became available, concerns appeared that measles-naïve children who received a first dose of this vaccine had a higher risk of febrile convulsions than children vaccinated with two separate vaccines (one against measles, mumps, and rubella, and one against varicella) administered during the same medical visit. However, this risk is low: during the first or the second week after the first vaccine dose, 1 additional child out of approximately 2500 children who receive the combined vaccine will have a febrile convulsion compared to those receiving 2 separate vaccines. In comparison, febrile convulsions due to any cause will appear in 1 out of 25 children younger than 5 years, and in 1 out of 43 children with measles. The combined vaccine has certain advantages over separate vaccines: children receive fewer injections and are more likely to be fully vaccinated against all four diseases. Children who had febrile convulsions before, or with a close relative who had febrile convulsions could be at higher risk of febrile convulsions after the first dose of the combined vaccine. Provided the informed consent from their parents or legal guardians, these children must receive separate vaccines, while all other children may receive the combined vaccine.
1. Introduction
Febrile convulsion (FC), also called febrile seizure, is defined as an occasional seizure accompanied by fever >38°C that occurs in 2%–5% of children aged between 6 months and 5 years who do not have previous central nervous system (CNS) infection, a metabolic disturbance, or a history of afebrile seizures [Citation1–3]. It is the most common type of seizure in infants and young children. Although the exact cause of FC is unknown, it is likely to be multifactorial, involving both genetic and environmental factors. Besides these factors, underlying CNS disorders may also contribute [Citation4]. Previous studies have shown that male gender, a family history of FC, elevated temperature during illness, daycare attendance, neonatal nursery stay of more than 28 days, maternal smoking and stress, iron and zinc deficiencies, and low serum calcium, sodium, or blood sugar are among potential risk factors for FC onset [Citation1,Citation2,Citation5–7]. Moreover, viral infections with influenza, parainfluenza, adenovirus, measles, or herpesvirus may also trigger FC [Citation8–11]. A temporal increase in the relative risk of FC has also been detected following the administration of pediatric vaccines such as live-attenuated vaccines against measles, mumps, and rubella (MMR) combined or not with varicella (MMRV/MMR+V or MMR), the combined diphtheria – tetanus toxoids – pertussis-based vaccines (containing acellular or whole-cell pertussis antigens), the pneumococcal conjugate vaccines, and some formulations of meningococcal and inactivated influenza vaccines () [Citation12–20]. The attributable risk of FC after MMR administration was estimated to be between 1 per 1,700 and 1 per 1,150 administered doses in children aged up to 15 years [Citation21]. After the introduction of tetravalent MMRV vaccines, several studies have reported an approximately twofold increased relative risk of FC within 5–12 or 7–10 days after the administration of the first MMRV dose of the 2-dose vaccination schedule in infants previously unexposed to a natural measles infection or not vaccinated against measles, compared to the separate administration of MMR+V during the same medical visit [Citation22–25]. Also, although MMRV vaccination does not additionally increase the risk of FC in predisposed children (who are naturally at a higher risk of developing FC) [Citation23,Citation24], the occurrence of FC after vaccination cannot be predicted at the individual level. The observed increased relative risk of FC has hampered the use of MMRV vaccine for combined measles, mumps, rubella, and varicella vaccination in some countries where measles- and varicella-containing vaccines have been included in national immunization programs, and has slowed MMRV vaccine uptake, as illustrated by the situation in Germany [Citation26]. The aim of this manuscript is to contextualize the occurrence of FC after MMRV vaccination – when given as the first dose of a measles-containing vaccine in measles-naïve infants – from a clinical perspective and to outline approaches to attenuate FC occurrence after vaccination. The relevant literature was selected based on the expert opinion of the authors.
Table 1. Incidence of FC after immunization with vaccines against influenza, DT(a/w)P, IPV, Hib, HepB, PCV, rotavirus, and meningococcus.
2. Febrile convulsion/seizure
Fever is an expected response to infection and induces the release of high levels of cytokines that might trigger convulsions. The peak temperature is the most important risk factor for primary FC events [Citation27,Citation28]. FC is the most common convulsive event in toddlers, but the occurrence of FC is usually low in older children, as they typically outgrow this condition by the age of 5 years [Citation18]. Similarly, the risk for recurrence is 15%–70% within 2 years of an initial FC, particularly in children who experienced the primary event under the age of 18 months [Citation4]. Risk factors for recurrent FC are young age (<18 months) at the time of the first episode, family history of FC in first-degree relatives, temperature <40°C during the initial episode, and possibly also multiple seizures [Citation1,Citation29–31]. In addition to FCs, epileptic seizures can occur after vaccination, especially following MMR vaccines; these seizures have a monogenic cause, mostly as mutations of the sodium channel protein type 1 subunit alpha (SCN1A) or protocadherin 19 (PCDH19) genes (Dravet syndrome) [Citation32,Citation33].
FC events have been classified as simple, complex, and prolonged FC or febrile status epilepticus [Citation4]. Most FCs (around 70%) are simple and typically benign, generalized, last <15 min, do not recur within 24 h, and do not cause any long-term health problems [Citation3]. Conversely, a complex FC lasts ≥15 min, is associated with focal neurologic findings, and usually recurs within 24 h [Citation3]. Febrile status epilepticus is defined as an FC that lasts >30 min and usually necessitates anticonvulsant treatment. This is the most severe type of complex FC, which refers to continuous or intermittent FC without consciousness for more than 30 min. Children with febrile status epilepticus are at an increased risk of recurrence of this event and of developing hippocampal abnormalities [Citation18].
Clinical signs and symptoms associated with FC are loss of consciousness, shaking of the arms and legs, generalized or focal twitching, difficulty in breathing, and foaming at the mouth. Following the seizure, the child might be confused and/or drowsy but will completely recover after approximately 30 min [Citation6,Citation18]. The majority of the simple FC episodes do not require medical treatment or hospitalization. Guidelines for the management of FC have been published by several organizations, notably, the National Institute of Neurological Disorders and Stroke (NINDS) [Citation29] and the American Academy of Pediatrics (AAP) [Citation34] in the United States, National Health Service (NHS) in the United Kingdom [Citation35], and the Japanese Society of Child Neurology (JSCN) [Citation36].
3. Combined measles, mumps, rubella, and varicella vaccines
Trivalent live-attenuated vaccines against MMR were licensed in the 1970s and have helped significantly reduce the incidence of these infectious diseases [Citation37]. Tetravalent vaccines that also include antigens against varicella have appeared at the beginning of the 2000s and are licensed in most developed countries (). The two live-attenuated tetravalent MMRV vaccines currently available worldwide are Priorix-Tetra (GSK) and ProQuad (Merck & Co., Inc.). Both MMRV vaccines were shown to be well tolerated and induce a high immunogenicity [Citation41,Citation42]. In the United States, MMRV vaccine was licensed in 2005 [Citation43] and recommended in the same year for a 2-dose varicella vaccination schedule, with the first dose administered at age 12–15 months and the second dose at 4–6 years [Citation44,Citation45]. The European Medicines Agency authorized one of the MMRV vaccines in 2006 [Citation46], though other vaccines against measles, mumps, rubella, and varicella have already been authorized by several European Union member states. In 2009, the German Standing Committee on Vaccinations (STIKO) included among its recommendations the potential use of MMRV for both doses of a varicella-containing vaccine within the national immunization schedule [Citation47].
Figure 1. Recommendations for vaccination against measles, mumps, rubella, and varicella [Citation21,Citation38–40,Citation43,Citation46,Citation51,Citation52,Citation82,Citation84] MMR – trivalent vaccine against measles, mumps, and rubella; V – monovalent varicella vaccine; MMRV – tetravalent vaccine against measles, mumps, rubella, and varicella. *one dose for individuals aged 18 years and older and born after 1970 with no vaccination or uncertain vaccination history or only one vaccination during childhood; **some Italian regions (Apulia, Friuli-Venezia-Giulia, Basilicata, Tuscany) have continued to use MMRV; #children at low risk of febrile convulsion may receive MMRV; &two catch-up doses up to 12 years of age.
![Figure 1. Recommendations for vaccination against measles, mumps, rubella, and varicella [Citation21,Citation38–40,Citation43,Citation46,Citation51,Citation52,Citation82,Citation84] MMR – trivalent vaccine against measles, mumps, and rubella; V – monovalent varicella vaccine; MMRV – tetravalent vaccine against measles, mumps, rubella, and varicella. *one dose for individuals aged 18 years and older and born after 1970 with no vaccination or uncertain vaccination history or only one vaccination during childhood; **some Italian regions (Apulia, Friuli-Venezia-Giulia, Basilicata, Tuscany) have continued to use MMRV; #children at low risk of febrile convulsion may receive MMRV; &two catch-up doses up to 12 years of age.](/cms/asset/49cdc4b8-98fc-48db-bace-133b0fa3d035/ierv_a_2252065_f0001_oc.jpg)
3.1. FC after MMRV administration
During the clinical development of MMRV vaccines, FC has been observed at low rates, though the frequency of fever was higher following MMRV vaccine administration than following MMR and MMR+V vaccinations [Citation41,Citation42]. Post-marketing studies have reported an approximately twofold increase in the relative risk of FC 7–10 or 5–12 days following the administration of MMRV first dose among measles-naïve children aged 12–60 months (of whom ≥70% were aged up to 24 months), as compared to children of the same age who had received MMR+V () [Citation16,Citation22–25]. This increased relative risk was not observed in children aged 4–6 years [Citation48,Citation49]. Based on the first post-marketing results, the United States Advisory Committee on Immunization Practices (ACIP) adopted new recommendations on the use of MMRV vaccines in 2009, expressing a preference for MMR+V as the first vaccine dose in children aged 12–47 months, unless the parent (or legal guardian) and health-care provider opted for MMRV. For the second dose, MMRV vaccine is preferred over MMR+V [Citation50]. In 2011, the STIKO and Italian Medicines Agency (AIFA) also changed their guidance, recommending MMR+V instead of MMRV as the first dose [Citation51,Citation52]. Nevertheless, some Italian regions have continued to use MMRV vaccine for the first varicella-containing dose () [Citation53,Citation54]. The Australian National Immunisation Program (NIP) recommended MMRV as the second dose of a measles-containing vaccine in children aged ≥18 months and as the first dose in children aged ≥4 years [Citation55].
Table 2. Summary of FC incidence after vaccination with measles – containing vaccines.
In 2015, a systematic review of eight post-marketing studies involving more than 3.2 million individuals showed that the increased relative risk of FC after the first dose of MMRV versus MMR+V vaccine is a class effect. While no significant differences in FC incidence between MMRV and MMR+V vaccine or MMR were observed in children aged 4–6 years, the risk of seizure or FC was increased by about twofold in 10–24-month-olds in the 7–10 or 5–12 days after the first dose of MMRV vaccine [Citation49]. A review of reviews published in 2022 indicated that the FC frequency after one-dose MMRV may be higher compared to MMR+V vaccination in young children. While this review also concluded that FC may occur after both monovalent and quadrivalent varicella vaccine administration, the numbers of varicella-containing vaccine doses (total and administered) were largely unknown from the source publications [Citation56]. Vaccination with MMR or MMRV vaccine in the second year of life has been associated with a similar relative risk of FC in children born preterm as in those who were born full-term [Citation57]. Nevertheless, there were some exceptions to the evidence of an increased risk of FC after MMRV vaccination. In the Puglia region, where the use of first-dose MMRV vaccination has been continued, despite the 2011 AIFA recommendation included in the Italian universal vaccination scheme, post-licensure surveillance data collected between 2009 and 2017 have confirmed the safety profile of the MMRV vaccine; none of the FC cases could be conclusively linked to the administration of the first dose of MMRV or MMR+V vaccine [Citation58]. Similar findings were reported for the 2017–2018 period [Citation53].
4. FC risk contextualization
4.1. Vaccine safety profile
To contextualize the risk of FC after MMR or MMRV vaccination, the overall adverse event (AE) profile of these vaccines should be considered. Several post-licensure (passive) surveillance studies and reviews have not indicated new significant safety concerns following MMR or MMRV vaccine administration [Citation21,Citation37,Citation41,Citation42,Citation53,Citation58–61].
In a study comparing the two vaccination strategies (MMR+V versus MMRV as the first dose), local AEs were reported more commonly in the MMR+V group (9.6% versus 2.9% of participants), while no difference was observed regarding the occurrence of general AEs or FC (50.0% versus 52.0% and 14.0% versus 17.0% of participants) [Citation59]. Fever was the most common general AE in both groups. Fever episodes were more commonly associated with MMRV vaccine, but the number of FC episodes was similar between the MMRV and MMR+V groups [Citation59]. In general, fever is a well-documented AE after administration of measles-containing vaccines, with genetic and biological factors potentially influencing susceptibility to fever following vaccination [Citation62]. A ubiquitous assumption among vaccination experts is that a more frequent occurrence of fever is associated with a higher titer of the measles virus component in MMRV versus MMR vaccines. Previous studies have indicated that fewer individuals experience high fever after the second dose of a measles-containing vaccine than after the first dose [Citation63–70]. Moreover, the timing of fever coincides with the timing of FC [Citation23]. These observations suggest that the observed more frequent febrile reactions may be related to a greater reactogenicity and a higher immune response toward the measles component of the vaccine rather than the other vaccine components.
No increase in the relative risk of FC has been observed after MMRV co-administration with other routine vaccines compared to MMRV alone [Citation71–75]. When co-administered, MMRV and monovalent or tetravalent meningococcal vaccines (MenC or MenACWY, respectively) have been shown to be immunogenic and well tolerated in toddlers aged ≥12 months; in addition, non-inferiority in terms of immune responses has been demonstrated for all vaccine antigens as compared to either MMRV or MenC or MenACWY administered alone [Citation76]. MMRV vaccine can also be concomitantly used with the combined diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus-Haemophilus influenzae type b conjugate vaccine [Citation71,Citation72,Citation75], hepatitis A vaccine [Citation74], and pneumococcal conjugate vaccines [Citation73,Citation74].
4.2. FC manifestation in the clinical practice
The risk of FC occurrence is not associated solely with tetravalent MMRV vaccines. Physiological predisposition, genetic background, and familial history of FC have been suggested as risk factors for FC onset [Citation18]. The occurrence of FC has also been shown to be higher in children who received the MMR vaccine than in unvaccinated individuals [Citation77–79]. According to one systematic review and meta-analysis of clinical trial data, the incidence of FC (all and vaccine-related) in the second week after the first vaccine dose was 2.61‰ and 0.52‰ for MMRV versus 0.00‰ and 0.00‰ for MMR+V vaccination, in children aged ≤2 years [Citation49]. In contrast, it is estimated that 40.00‰ of children will have had an episode of FC before the age of 5 years [Citation50,Citation80]; for instance, in children who developed measles, an FC incidence of 1.00‰–23.00‰ has been reported in the United States and England [Citation11]. Other childhood vaccines have also been associated with increased relative risks of FC, such as the combined diphtheria – tetanus toxoids – acellular pertussis – inactivated poliovirus-Haemophilus influenzae type b and conjugated pneumococcal vaccine () [Citation12–20]. Nevertheless, the overall safety profile of these vaccines remains acceptable. In the 2010–2011 season, vaccination with one dose of trivalent influenza vaccine (TIV) led to an estimated FC rate of 3.3 per 1,000 doses in Western Australia (200 times higher than previous population-based estimates) [Citation13], or an incidence rate ratio of FC of 4.0 (versus unexposed control interval) in the United States [Citation20], at 0–3 days following vaccination in children aged 6–59 months. When TIV was concomitantly administered with pneumococcal conjugate vaccines in the United States, a relative risk of 3.5–5.9 and an attributable risk of 16–17.5 cases per 100,000 vaccinated persons were reported compared to TIV administration alone [Citation15,Citation20]. Finally, infections with certain herpesvirus or coronavirus strains, pharyngitis, otitis media, and Shigella gastroenteritis are significant risk factors for FC occurrence [Citation18].
FC episodes after MMRV administration thus account for a small proportion of FC occurring in children, as also discussed by Gabutti et al. [Citation81]. Data from the regional monitoring system of post-vaccination AEs from the Veneto region of Italy have shown that the burden of FC after MMRV vaccination is low as compared to other causes of FC [Citation82]. Similarly, a review of the medical history of 90,294 MMR and 8,344 MMRV Israeli vaccinees revealed that the MMRV-specific attributable risk of FC was not significant at any point of the observation period and was very low compared to other risk factors such as age, low birth weight, or preterm birth [Citation16].
4.3. Populations susceptible to FC
In general, children at high risk of seizure are more prone to post-vaccination FC. As indicated in a retrospective population-based cohort study involving more than 277,000 Canadian children aged 12–23 months, the MMRV vaccine first dose was associated with a twofold increased risk of FC (relative risk ratio 1.99 [95% CI: 1.30–3.05]) relative to MMR+V at 7–10 days post-vaccination when the entire study population was considered () [Citation24]. The excess absolute risk for MMRV versus MMR+V was 3.52 seizures per 10,000 doses [Citation24]. However, in high-risk children, in whom the baseline FC incidence is higher than in the general population, MMRV vaccination did not result in a significantly increased relative risk of FC as compared to MMR+V (relative risk ratio 1.30 [0.60–2.79]). Children considered at high risk in this study were those with personal history of FC or who had seizure disorder, infection, CNS injury, encephalopathy, or progressive, evolving, or unstable neurological condition [Citation24]. Similar results have been observed in children from the United States with a personal history of seizures [Citation23]. Moreover, the modeling post-hoc analysis of a matched cohort study of a similar sample size (more than 226,000 children aged <2 years) from Germany estimated that in children without personal or family history of FC, the risk of FC after the first MMRV dose is likely similar to MMR+V given as separate injections [Citation83]. The authors reported similar findings when MMRV was compared to MMR vaccine or the combined exposure MMR/MMR+V [Citation83]. The conclusion of this study was that, to minimize the risk of FC, children with a personal or family history of FC should receive the MMR+V vaccine for the first dose, and be closely monitored for the occurrence of FC and/or fever during the known risk period post-vaccination. In contrast, a first dose of the MMRV vaccine could be administered to children at low risk. This strategy is aligned with the label of the available MMRV vaccines [Citation46,Citation84]. Currently, ACIP recommends this approach [Citation50]. The prophylactic administration of antipyretics is not indicated as it might lower the immune response, and there is no scientific evidence indicating any significant reduction in the rate of FC [Citation85].
4.4. (Expected) benefits of MMRV vaccination
The tetravalent MMRV vaccine can have some advantages compared to MMR+V. This formulation reduces the number of injections and the percentage of AEs and facilitates compliance with the 2-dose vaccination strategy. In Germany, 1 year after STIKO changed indications and recommended MMR+V over MMRV as the first dose, varicella vaccination coverage declined in some regions [Citation26]. Lower vaccination coverage may lead to more hospitalizations. A modeling study performed by Bauchau et al. predicted that transitioning from MMR+V to MMRV vaccination would induce 225 vaccine-related FC hospitalization days but would prevent 1,976 varicella-related hospitalization days per year [Citation86]. The authors concluded that despite the increased risk of FC after the first MMRV dose, MMRV vaccination can substantially reduce the length of hospital stay by increasing vaccination coverage against varicella [Citation86]. Thus, the risk of FC must be balanced against the benefits and coverage achieved with the MMRV formulation. The Italian National Plan for Vaccine Prevention introduced in 2017 has expanded the number of mandatory vaccinations from four to ten, including the MMRV vaccine [Citation87]. One year later, considerable increases in MMRV vaccination coverage were identified [Citation88]. Similarly, a study from Canada found that varicella vaccine coverage increased 4 years after the introduction of the MMRV vaccine into the national immunization program [Citation89]. While the coverage of measles-containing vaccines remained comparable to that before MMRV introduction, most parents/legal guardians opted for the tetravalent vaccine instead of MMR+V [Citation89].
5. Conclusion
The overall safety profile of the measles-containing vaccine formulations – MMRV, MMR, and MMR+V – was deemed acceptable. Co-administration of MMRV vaccine with other routine pediatric vaccines did not increase the risk of FC post-vaccination compared to MMRV vaccination alone. When evaluated in a broader context, the risk of FC following the first dose of MMRV vaccine was low compared to the overall risk of FC seen among the pediatric population aged <5 years. MMR and other pediatric vaccines, measles disease, age, low birth weight, preterm birth, and personal or family history of FC are also associated with an increased FC risk. Based on these findings and considering the benefits of MMRV vaccination (fewer injections, higher vaccination coverage, increased vaccination compliance), MMRV vaccination administered as first dose remains a viable option for all children who are not at risk of FC.
6. Expert opinion
In children aged <5 years, FCs are most commonly triggered by fever, which accompanies numerous childhood diseases or routine pediatric vaccinations. Although an FC is transient and often without long-term consequences, its high prevalence, especially in the second year of life, raises serious concerns in parents worried about their children’s wellbeing. The introduction of MMRV vaccines has created expectations of several logistic and practical benefits, the most important of which is a high vaccination coverage against measles, mumps, and rubella, and an increased coverage against varicella, due to convenient combination of antigens in one vaccine [Citation90]. However, retrospective evidence of an increased relative risk of FC after the first dose has hindered the universal implementation of MMRV vaccines and led to changes in official vaccine recommendations. The mechanisms underlying the elevated risk of FC after MMRV vaccination are currently speculative (e.g. higher titer of the measles component, interaction of measles and varicella components in the same preparation) and warrant further investigation. First-dose MMRV vaccination has been correlated with FC risk based on largely retrospective studies. Therefore, well-designed prospective surveillance studies are needed to corroborate these observations and thoroughly characterize individuals at risk of FC.
Literature data available to date suggest that the risk of FC can be perceived differently when it is put in the overall perspective of FC seen in the pediatric population. According to some estimates, approximately 4.0% of children aged <5 years [Citation50,Citation80], and up to 2.3% of children who contract measles [Citation11] experience at least one FC episode. In this context, the observed FC incidence following MMRV vaccine administration (0.3‰–3.0‰) likely contributes marginally to the overall rate of FC in children aged ≤24 months [Citation22,Citation42,Citation49,Citation60]. Additionally, although rates of FC are generally increased by all measles-containing vaccines as well as several routinely administered pediatric vaccines [Citation13,Citation14,Citation20,Citation77–79], neither the relative risk of FC nor the overall safety profile of MMRV vaccine was negatively affected by the co-administration of MMRV with other vaccines.
Children with personal or family history of seizure, underlying medical conditions, or neurological disorders are generally more susceptible to FC. Though an increased relative risk of FC after MMRV vaccination was observed in the overall population, this risk is comparable between MMRV, MMR+V, and MMR in the high-risk population. Therefore, possible risk factors should be considered when a vaccination strategy against measles, mumps, rubella, and varicella is established for a certain pediatric population. MMRV vaccines could be considered as a complete 2-dose schedule for children who are not at risk or have no history of personal or familial FC. Conversely, in children who are at an elevated risk of FC or have a history of FC, the risk of post-vaccination FC might be lowered if MMR+V is administered as a first dose. For the second dose, MMRV is still preferred in this population to extend the benefit of combined vaccines [Citation83].
In the context of FC risk, results should be interpreted by considering the differences between relative risk and attributable risk. Some studies report the attributable risk of FC after MMRV vaccination, while others use relative risk. Attributable risk measures the difference between the absolute risk (incidence of the event) in the vaccinated group and the absolute risk in the comparator group [Citation91]. Conversely, relative risk is the ratio of the two absolute risks [Citation91]. Though vaccination with certain routinely available vaccines has been associated with an increased relative risk of FC shortly after administration, the absolute risk of FC post-vaccination with these vaccines is low [Citation15,Citation92]. Thus, post-vaccination FC should not be a concern for most children receiving vaccines, including measles-containing formulations.
Physicians and parents are often concerned about FC and perceive a convulsion episode as a moderate or serious event. This leads to parental vaccine hesitancy, which results in a negative impact on vaccination coverage and public health [Citation6,Citation93,Citation94]. Previous studies have described several socio-economic risk factors associated with low parental acceptance rates of vaccination [Citation95,Citation96], but also lack of appropriate information among health-care professionals (HCPs) [Citation97]. These observations strengthen the importance of providing timely, relevant, and accurate information to HCPs. Improving awareness and information levels of HCPs will lead to more confident use of measles-containing vaccines and enable HCPs to guide parents on the management of their child’s vaccination schedule, as well as potential occurrences of fever after vaccination.
In conclusion, although present, the relative risk of FC after the first dose of MMRV vaccine is acceptable when assessed in a broader context of general risk of FC seen in the pediatric population. The increased risk of FC after MMRV vaccination can be mitigated by limiting the administration of the first MMRV vaccine dose to children who are not at elevated risk of FC and closely monitoring the vaccinees during the FC risk period. Policymakers should balance these findings with the potential advantages of using combination vaccines to ensure maximum protection of children from measles, mumps, rubella, and varicella.
Article highlights
The relative risk of febrile convulsion after the first MMRV dose is twofold higher compared to MMR+V in measles-naïve infants.
However, the incidence of febrile convulsion after the first MMRV dose contributes marginally to the overall rate of febrile convulsion in toddlers.
The overall safety profile of MMRV vaccine as first dose remains acceptable when assessed in a broader context.
Declaration of interests
G Casabona, O Berton, and T Singh are employees of GSK, hold shares in this company, and declare financial/non-financial relationships and activities. P Bonanni received payment or honoraria as a participant at advisory boards and speaker sponsored by GSK, MSD, Pfizer, Seqirus, AstraZeneca, Janssen, and Sanofi Pasteur and as a member of a Data Safety Monitoring Committee for a Shigella investigational vaccine with GSK Vaccines Institute for Global Health (GVGH). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Authors’ contributions
All authors were involved in the conception and design of this review article. All authors were involved in data analyses and interpretation, and in writing the article. All authors contributed to the review of the paper for important intellectual content and have approved it for submission and publication.
Trademark
Priorix-Tetra is a trademark owned by or licensed to the GSK group of companies. ProQuad is a trademark of Merck & Co., Inc..
Acknowledgments
Authors thank Akkodis platform for editorial assistance and manuscript coordination, on behalf of GSK. Botond Nagy provided medical writing support and Gil Costa provided graphic support.
This work has already been presented at the 40th Annual meeting of the European Society for Paediatric Infectious Diseases (9–13 May 2022) as an E-poster EP079/#1126 entitled “Combined Measles-Mumps-Rubella-Varicella vaccine and febrile convulsions: the risk considered in the broad context” and authored by Giacomo Casabona (presenting author), Olivia Berton, Markus Knuf, and Paolo Bonanni.
Additional information
Funding
References
- Sadleir LG, Scheffer IE. Febrile seizures. BMJ. 2007;334(7588):307–311. doi: 10.1136/bmj.39087.691817.AE
- Smith DK, Sadler KP, Benedum M. Febrile seizures: risks, evaluation, and prognosis. Am Fam Physician. 2019;99(7):445–450.
- Steering Committee on Quality Improvement Management; Subcommittee on Febrile Seizures. Febrile seizures: clinical practice guideline for the long-term management of the child with simple febrile seizures. Pediatrics. 2008;121(6):1281–1286. doi: 10.1542/peds.2008-0939
- Mewasingh LD, Chin RFM, Scott RC. Current understanding of febrile seizures and their long-term outcomes. Dev Med Child Neurol. 2020;62(11):1245–1249. doi: 10.1111/dmcn.14642
- Bethune P, Gordon K, Dooley J, et al. Which child will have a febrile seizure? Am J Dis Child. 1993;147(1):35–39. doi: 10.1001/archpedi.1993.02160250037013
- Laino D, Mencaroni E, Esposito S. Management of pediatric febrile seizures. Int J Environ Res Public Health. 2018;15(10):2232. doi: 10.3390/ijerph15102232
- Naveed Ur R, Billoo AG. Association between iron deficiency anemia and febrile seizures. J Coll Physicians Surg Pak. 2005;15(6):338–340.
- Chiu SS, Tse CY, Lau YL, et al. Influenza a infection is an important cause of febrile seizures. Pediatrics. 2001;108(4):E63. doi: 10.1542/peds.108.4.e63
- Chung B, Wong V. Relationship between five common viruses and febrile seizure in children. Arch Dis Child. 2007;92(7):589–593. doi: 10.1136/adc.2006.110221
- Hall CB, Long CE, Schnabel KC, et al. Human herpesvirus-6 infection in children. A prospective study of complications and reactivation. N Engl J Med. 1994;331(7):432–438. doi: 10.1056/NEJM199408183310703
- Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis. 2004;189 Suppl 1(Supplement_1):S4–16. doi: 10.1086/377712
- Andrews N, Stowe J, Wise L, et al. Post-licensure comparison of the safety profile of diphtheria/tetanus/whole cell pertussis/haemophilus influenza type b vaccine and a 5-in-1 diphtheria/tetanus/acellular pertussis/haemophilus influenza type b/polio vaccine in the United Kingdom. Vaccine. 2010;28(44):7215–7220. doi: 10.1016/j.vaccine.2010.08.062
- Armstrong PK, Dowse GK, Effler PV, et al. Epidemiological study of severe febrile reactions in young children in Western Australia caused by a 2010 trivalent inactivated influenza vaccine. BMJ Open. 2011;1(1):e000016. doi: 10.1136/bmjopen-2010-000016
- Duffy J, Hambidge SJ, Jackson LA, et al. Febrile seizure risk after vaccination in children one to five months of age. Pediatr Neurol. 2017;76:72–78. doi: 10.1016/j.pediatrneurol.2017.08.005
- Duffy J, Weintraub E, Hambidge SJ, et al. Febrile seizure risk after vaccination in children 6 to 23 months. Pediatrics. 2016;138(1):e20160320. doi: 10.1542/peds.2016-0320
- Gavrielov-Yusim N, Hoshen M, Singer SR, et al. The weight of MMRV-related febrile convulsions among other clinical factors contributing to febrile convulsions in children. Vaccine. 2014;32(39):4954–4959. doi: 10.1016/j.vaccine.2014.07.024
- Hall GC, Douglas I, Heath PT, et al. Post-licensure observational safety study after meningococcal B vaccine 4CMenB (Bexsero) vaccination within the routine UK immunisation program. Vaccine. 2021;39(24):3296–3303. doi: 10.1016/j.vaccine.2021.02.065
- Leung AK, Hon KL, Leung TN. Febrile seizures: an overview. Drugs Context. 2018;7:212536. doi: 10.7573/dic.212536
- Sun Y, Christensen J, Hviid A, et al. Risk of febrile seizures and epilepsy after vaccination with diphtheria, tetanus, acellular pertussis, inactivated poliovirus, and haemophilus influenzae type B. JAMA. 2012;307(8):823–831. doi: 10.1001/jama.2012.165
- Tse A, Tseng HF, Greene SK, et al. Signal identification and evaluation for risk of febrile seizures in children following trivalent inactivated influenza vaccine in the vaccine safety datalink project, 2010–2011. Vaccine. 2012;30(11):2024–2031. doi: 10.1016/j.vaccine.2012.01.027
- Di Pietrantonj C, Rivetti A, Marchione P, et al. Vaccines for measles, mumps, rubella, and varicella in children. Cochrane Database Syst Rev. 2020;4(4):CD004407. doi: 10.1002/14651858.CD004407.pub4
- Jacobsen SJ, Ackerson BK, Sy LS, et al. Observational safety study of febrile convulsion following first dose MMRV vaccination in a managed care setting. Vaccine. 2009;27(34):4656–4661. doi: 10.1016/j.vaccine.2009.05.056
- Klein NP, Fireman B, Yih WK, et al. Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizures. Pediatrics. 2010;126(1):e1–8. doi: 10.1542/peds.2010-0665
- MacDonald SE, Dover DC, Simmonds KA, et al. Risk of febrile seizures after first dose of measles-mumps-rubella-varicella vaccine: a population-based cohort study. CMAJ. 2014;186(11):824–829. doi: 10.1503/cmaj.140078
- Schink T, Holstiege J, Kowalzik F, et al. Risk of febrile convulsions after MMRV vaccination in comparison to MMR or MMR+V vaccination. Vaccine. 2014;32(6):645–650. doi: 10.1016/j.vaccine.2013.12.011
- Streng A, Liese JG. Decline of varicella vaccination in German surveillance regions after recommendation of separate first-dose vaccination for varicella and measles–mumps–rubella. Vaccine. 2014; 32(8):897–900. doi: 10.1016/j.vaccine.2013.12.065
- Gontko-Romanowska K, Żaba Z, Panieński P, et al. The assessment of risk factors for febrile seizures in children. Neurol Neurochir Pol. 2017;51(6):454–458. doi: 10.1016/j.pjnns.2017.07.011
- Sharawat IK, Singh J, Dawman L, et al. Evaluation of risk factors associated with first episode febrile seizure. J Clin Diagn Res. 2016;10(5):SC10–13. doi: 10.7860/JCDR/2016/18635.7853
- National Institute of Neurological Disorders and Stroke. Febrile seizures fact sheet. [cited 2021 Sep 2]. Available from: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Febrile-Seizures-Fact-Sheet.
- Offringa M, Bossuyt PM, Lubsen J, et al. Risk factors for seizure recurrence in children with febrile seizures: a pooled analysis of individual patient data from five studies. J Pediatr. 1994;124(4):574–584. doi: 10.1016/S0022-3476(05)83136-1
- Waruiru C, Appleton R. Febrile seizures: an update. Arch Dis Child. 2004;89(8):751–756. doi: 10.1136/adc.2003.028449
- Verbeek NE, Jansen FE, Vermeer-de Bondt PE, et al. Etiologies for seizures around the time of vaccination. Pediatrics. 2014;134(4):658–666. doi: 10.1542/peds.2014-0690
- Verbeek NE, van der Maas NA, Sonsma AC, et al. Effect of vaccinations on seizure risk and disease course in Dravet syndrome. Neurology. 2015;85(7):596–603. doi: 10.1212/WNL.0000000000001855
- Committee on Infectious Diseases. Febrile seizures: guideline for the neurodiagnostic evaluation of the child with a simple febrile seizure. Pediatrics. 2011;127(2):389–394. doi: 10.1542/peds.2010-3318
- National Health Service. Febrile seizures. [cited 2021 Sep 22]. Available from: https://www.nhs.uk/conditions/febrile-seizures/.
- Natsume J, Hamano SI, Iyoda K, et al. New guidelines for management of febrile seizures in japan. Brain Dev. 2017;39(1):2–9. doi: 10.1016/j.braindev.2016.06.003
- Demicheli V, Rivetti A, Debalini MG, et al. Vaccines for measles, mumps and rubella in children. Cochrane Database Syst Rev. 2012;2012(2):CD004407. doi: 10.1002/14651858.CD004407.pub3
- Australian Government-Department of Health and Aged Care. National immunisation program schedule. [cited 2022 Sep 20]. Available from: https://www.health.gov.au/health-topics/immunisation/when-to-get-vaccinated/national-immunisation-program-schedule.
- Government of Canada. Measles vaccine: Canadian immunization guide. [cited 2022 Sep 20]. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-12-measles-vaccine.html.
- Robert-Koch-Institut. Masern. RKI-Ratgeber. [cited 2022 Jan 14]. Available from: https://www.rki.de/DE/Content/Infekt/EpidBull/Merkblaetter/Ratgeber_Masern.html.
- Czajka H, Schuster V, Zepp F, et al. A combined measles, mumps, rubella and varicella vaccine (Priorix-Tetra™): immunogenicity and safety profile. Vaccine. 2009;27(47):6504–6511. doi: 10.1016/j.vaccine.2009.07.076
- Kuter BJ, Hoffman Brown ML, Hartzel J, et al. Safety and immunogenicity of a combination measles, mumps, rubella and varicella vaccine (ProQuad). Hum Vaccin. 2006;2(5):205–214. doi: 10.4161/hv.2.5.3246
- Advisory Committee on Immunization Practices. ACIP provisional recommendations for prevention of varicella. [cited 2022 Jul 25]. Available from: https://oeps.wv.gov/varicella/documents/lhd/varicella_acip_recs_prov.pdf.
- CDC. Licensure of a combined live attenuated measles, mumps, rubella, and varicella vaccine. MMWR Weekly. 2005;54(47):1212–1214.
- Marin M, Güris D, Chaves SS, et al. Prevention of varicella: recommendations of the Advisory Committee on immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR–4):1–40.
- European Medicines Agency. ProQuad. [cited 2021 Sep 22]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/proquad.
- Robert-Koch-Institut. Impfung gegen Varizellen im Kindesalter: Empfehlung einer zweiten Varizellenimpfung Empfehlung und Begründung. [cited 2022 Jul 25]. Available from: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2009/Ausgaben/32_09.pdf?__blob=publicationFile.
- Klein NP, Lewis E, Baxter R, et al. Measles-containing vaccines and febrile seizures in children age 4 to 6 years. Pediatrics. 2012;129(5):809–814. doi: 10.1542/peds.2011-3198
- Ma SJ, Xiong YQ, Jiang LN, et al. Risk of febrile seizure after measles–mumps–rubella–varicella vaccine: a systematic review and meta-analysis. Vaccine. 2015;33(31):3636–3649. doi: 10.1016/j.vaccine.2015.06.009
- Marin M, Broder KR, Temte JL, et al. Use of combination measles, mumps, rubella, and varicella vaccine: recommendations of the Advisory Committee on immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR–3):1–12.
- Robert-Koch-Institut. Zur kombinationsimpfung gegen masern, mumps röteln und varizellen (MMRV). Epidemiologisches Bull. 2011;38:352–353.
- AIFA - Working Group Pediatrico. Raccomandazioni del working group pediatrico dell’AIFA in relazione all’ utilizzo dei vaccini MPRV. [cited 2021 Sep 22]. Available from: https://www.aifa.gov.it/sites/default/files/raccomandazione_vaccino_mprv_14_novembre_2011.pdf.
- Stefanizzi P, Stella P, Ancona D, et al. Adverse events following measles-mumps-rubella-varicella vaccination and the case of seizures: a post marketing active surveillance in Puglia Italian region, 2017-2018. Vaccines (Basel). 2019;7(4):140. doi: 10.3390/vaccines7040140
- Vitali Rosati G. Chickenpox vaccination. Ital J Pediatr. 2014;40(S1):A3. doi: 10.1186/1824-7288-40-S1-A3
- Deng L, Wood N, Danchin M. Seizures following vaccination in children: risks, outcomes and management of subsequent revaccination. Aust J Gen Pract. 2020;49(10):644–649. doi: 10.31128/AJGP-02-20-5236
- Ahern S, Walsh KA, Paone S, et al. Safety of varicella vaccination strategies: an overview of reviews. Rev Med Virol. 2023;33(2):e2416. doi: 10.1002/rmv.2416
- McClure DL, Jacobsen SJ, Klein NP, et al. Similar relative risks of seizures following measles containing vaccination in children born preterm compared to full-term without previous seizures or seizure-related disorders. Vaccine. 2019;37(1):76–79. doi: 10.1016/j.vaccine.2018.11.038
- Stefanizzi P, De Nitto S, Patano F, et al. Post-marketing surveillance of adverse events following measles, mumps, rubella and varicella (MMRV) vaccine: retrospective study in Apulia region (ITALY), 2009-2017. Hum Vaccin Immunother. 2020;16(8):1875–1883. doi: 10.1080/21645515.2019.1704124
- Cocchio S, Zanoni G, Opri R, et al. A postmarket safety comparison of 2 vaccination strategies for measles, mumps, rubella and varicella in Italy. Hum Vaccin Immunother. 2016;12(3):651–654. doi: 10.1080/21645515.2015.1101198
- Klopfer SO, Stek JE, Petrecz M, et al. Analysis of safety data in children after receiving two doses of ProQuad® (MMRV). Vaccine. 2014;32(52):7154–7160. doi: 10.1016/j.vaccine.2014.08.067
- Woo EJ, Winiecki SK, Arya D, et al. Adverse events after MMR or MMRV vaccine in infants under nine months old. Pediatr Infect Dis J. 2016;35(8):e253–257. doi: 10.1097/INF.0000000000001201
- Klein NP, Zerbo O, Goddard K, et al. Genetic associations with a fever after measles-containing vaccines. Hum Vaccin Immunother. 2021;17(6):1763–1769. doi: 10.1080/21645515.2020.1849520
- Gillet Y, Steri GC, Behre U, et al. Immunogenicity and safety of measles-mumps-rubella-varicella (MMRV) vaccine followed by one dose of varicella vaccine in children aged 15 months–2 years or 2–6 years primed with measles-mumps-rubella (MMR) vaccine. Vaccine. 2009;27(3):446–453. doi: 10.1016/j.vaccine.2008.10.064
- Goh P, Lim FS, Han HH, et al. Safety and immunogenicity of early vaccination with two doses of tetravalent measles-mumps-rubella-varicella (MMRV) vaccine in healthy children from 9 months of age. Infection. 2007;35(5):326–333. doi: 10.1007/s15010-007-6337-z
- Knuf M, Zepp F, Meyer CU, et al. Safety, immunogenicity and immediate pain of intramuscular versus subcutaneous administration of a measles–mumps–rubella–varicella vaccine to children aged 11–21 months. Eur J Pediatr. 2010;169(8):925–933. doi: 10.1007/s00431-010-1142-6
- Prymula R, Bergsaker MR, Esposito S, et al. Protection against varicella with two doses of combined measles-mumps-rubella-varicella vaccine versus one dose of monovalent varicella vaccine: a multicentre, observer-blind, randomised, controlled trial. Lancet. 2014;383(9925):1313–1324. doi: 10.1016/S0140-6736(12)61461-5
- Rüger G, Gabutti G, Rümke H, et al. Safety of a 2-dose regimen of a combined measles, mumps, rubella and varicella live vaccine manufactured with recombinant human albumin. Pediatr Infect Dis J. 2012;31(11):1166–1172. doi: 10.1097/INF.0b013e318267fd8b
- Rümke HC, Loch HP, Hoppenbrouwers K, et al. Immunogenicity and safety of a measles–mumps–rubella–varicella vaccine following a 4-week or a 12-month interval between two doses. Vaccine. 2011;29(22):3842–3849. doi: 10.1016/j.vaccine.2011.02.067
- Schuster V, Otto W, Maurer L, et al. Immunogenicity and safety assessments after one and two doses of a refrigerator-stable tetravalent measles-mumps-rubella-varicella vaccine in healthy children during the second year of life. Pediatr Infect Dis J. 2008;27(8):724–730. doi: 10.1097/INF.0b013e318170bb22
- Shinefield H, Black S, Digilio L, et al. Evaluation of a quadrivalent measles, mumps, rubella and varicella vaccine in healthy children. Pediatr Infect Dis J. 2005;24(8):665–669. doi: 10.1097/01.inf.0000172902.25009.a1
- Deichmann KA, Ferrera G, Tran C, et al. Immunogenicity and safety of a combined measles, mumps, rubella and varicella live vaccine (ProQuad®) administered concomitantly with a booster dose of a hexavalent vaccine in 12–23-month-old infants. Vaccine. 2015;33(20):2379–2386. doi: 10.1016/j.vaccine.2015.02.070
- Kiely M, Billard MN, Toth E, et al. Investigation of an increase in large local reactions following vaccine schedule change to include DTaP-HB-IPV-Hib (infanrix-hexa®) and MMRV (ProQuad®) at 18 months of age. Vaccine. 2018;36(45):6688–6694. doi: 10.1016/j.vaccine.2018.09.049
- Leonardi M, Bromberg K, Baxter R, et al. Immunogenicity and safety of MMRV and PCV-7 administered concomitantly in healthy children. Pediatrics. 2011;128(6):e1387–e1394. doi: 10.1542/peds.2010-2132
- Yetman RJ, Shepard JS, Duke A, et al. Concomitant administration of hepatitis A vaccine with measles/mumps/rubella/varicella and pneumococcal vaccines in healthy 12- to 23-month-old children. Hum Vaccin Immunother. 2013;9(8):1691–1697. doi: 10.4161/hv.24873
- Zepp F, Behre U, Kindler K, et al. Immunogenicity and safety of a tetravalent measles-mumps-rubella-varicella vaccine co-administered with a booster dose of a combined diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus-haemophilus influenzae type b conjugate vaccine in healthy children aged 12-23 months. Eur J Pediatr. 2007;166(8):857–864. doi: 10.1007/s00431-007-0506-z
- Bonanni P, Boccalini S, Bechini A, et al. Co-administration of vaccines: a focus on tetravalent measles-mumps-rubella-varicella (MMRV) and meningococcal C conjugate vaccines. Hum Vaccin Immunother. 2020;16(6):1313–1321. doi: 10.1080/21645515.2019.1688032
- Barlow WE, Davis RL, Glasser JW, et al. The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. N Engl J Med. 2001;345(9):656–661. doi: 10.1056/NEJMoa003077
- Gold M, Dugdale S, Woodman RJ, et al. Use of the Australian childhood immunisation register for vaccine safety data linkage. Vaccine. 2010;28(26):4308–4311. doi: 10.1016/j.vaccine.2010.04.021
- Vestergaard M, Hviid A, Madsen KM, et al. MMR vaccination and febrile seizures: evaluation of susceptible subgroups and long-term prognosis. JAMA. 2004;292(3):351–357. doi: 10.1001/jama.292.3.351
- National Institute of Neurological Disorders and Stroke. Febrile seizures information page. [cited 2022 Jan 14]. Available from: https://www.ninds.nih.gov/Disorders/All-Disorders/Febrile-Seizures-Information-Page.
- Gabutti G, Kuhdari P, Ferioli S, et al. Hospital admissions for seizure in Italy: a decennial retrospective analysis with a special focus on the burden in the pediatric age. Neurol Sci. 2015;36(9):1667–1673. doi: 10.1007/s10072-015-2230-1
- Opri R, Moretti U, Zanoni G XVI Relazione sull’attivita` del ‘‘Canale Verde’’. Dati relativi al 2012. [cited 2021 Sep 22]. Available from: https://www.ospedaleuniverona.it/extfiles/internet/93101/attachment/16a-relazione-dati-2012-17-07-13-finale.pdf.
- Gvozdenovic E, Vetter V, Willame C, et al. Impact of history of febrile convulsions on the risk difference of febrile convulsions with the tetravalent measles-mumps-rubella-varicella vaccine: post-hoc exploratory analysis of results from a matched-cohort study. Vaccine. 2018;36(39):5803–5806. doi: 10.1016/j.vaccine.2018.08.018
- GlaxoSmithKline. Priorix-Tetra. [cited 2022 Aug 8]. Available from: https://gskpro.com/content/dam/global/hcpportal/en_NA/PI/Priorix-Tetra-GDS13.pdf.
- Monfries N, Goldman RD. Prophylactic antipyretics for prevention of febrile seizures following vaccination. Can Fam Physician. 2017;63(2):128–130.
- Bauchau V, Van Holle L, Cohen C. Modelling hospitalisation ratios for febrile convulsions and severe varicella under combined measles, mumps, rubella, and varicella (MMRV—Priorix-Tetra™) compared to separate MMR + V vaccination. Drug Saf. 2015;38(11):1095–1102. doi: 10.1007/s40264-015-0326-4
- Di Pietro A, Visalli G, Antonuccio GM, et al. Today’s vaccination policies in Italy: the National plan for vaccine prevention 2017-2019 and the law 119/2017 on the mandatory vaccinations. Ann Ig. 2019;31(2 Supple 1):54–64. doi: 10.7416/ai.2019.2277
- Costantino C, Casuccio A, Sannasardo CE, et al. Public health strategies adopted to manage the increase of accesses to vaccination services, as a result of the application of the law 119/2017. Acta Biomed. 2020;91(3–S):35–40. doi: 10.23750/abm.v91i3-S.9413
- MacDonald SE, Tough S, Guo X, et al. Impact of combination MMRV vaccine on first-dose coverage for measles and varicella: a population-based study. J Public Health. 2022;30(5):1063–1068. doi: 10.1007/s10389-020-01379-9
- Vesikari T, Sadzot-Delvaux C, Rentier B, et al. Increasing coverage and efficiency of measles, mumps, and rubella vaccine and introducing universal varicella vaccination in Europe: a role for the combined vaccine. Pediatr Infect Dis J. 2007;26(7):632–638. doi: 10.1097/INF.0b013e3180616c8f
- Hennekens CH, Buring JE. Epidemiology in medicine. 1 ed. Vol. 20. Philadelphia (USA): Lippincott Williams & Wilkins; 1987. (Mayrent SL. editor)
- Francis JR, Richmond P, Robins C, et al. An observational study of febrile seizures: the importance of viral infection and immunization. BMC Pediatr. 2016;16(1):202. doi: 10.1186/s12887-016-0740-5
- Saada A, Lieu TA, Morain SR, et al. Parents’ choices and rationales for alternative vaccination schedules: a qualitative study. Clin Pediatr. 2015;54(3):236–243. doi: 10.1177/0009922814548838
- Williams SE. What are the factors that contribute to parental vaccine-hesitancy and what can we do about it? Hum Vaccin Immunother. 2014;10(9):2584–2596. doi: 10.4161/hv.28596
- Brown KF, Kroll JS, Hudson MJ, et al. Factors underlying parental decisions about combination childhood vaccinations including MMR: a systematic review. Vaccine. 2010;28(26):4235–4248. doi: 10.1016/j.vaccine.2010.04.052
- Tabacchi G, Costantino C, Napoli G, et al. Determinants of European parents’ decision on the vaccination of their children against measles, mumps and rubella: a systematic review and meta-analysis. Hum Vaccin Immunother. 2016;12(7):1909–1923. doi: 10.1080/21645515.2016.1151990
- O’Leary ST, Suh CA, Marin M. Febrile seizures and measles–mumps–rubella–varicella (MMRV) vaccine: what do primary care physicians think? Vaccine. 2012;30(48):6731–6733. doi: 10.1016/j.vaccine.2012.08.075