Abstract
Smoking is estimated to be responsible for at least 2/3 of chronic obstructive pulmonary disease (COPD) deaths. Mortality rates due to all causes and to COPD decline progressively after smoking cessation compared with continuing smoking. Nicotine replacement therapies (NRT) increase the likelihood of smoking abstinence by only 60%. Optimization of NRT is of importance in COPD patients because they may be more nicotine dependent and have more difficulties to quit than smokers without COPD. The objective was to critically review pharmacotherapeutic strategies to optimize the efficacy of NRT. Findings revealed that fixed high dose NRT does not convincingly result in higher abstinence rate compared with standard dose and increases the likelihood of adverse effects in smokers with low need for nicotine. Combination of NRT of different routes of administration versus single NRT provides a statistically significant benefit over a single NRT. A 2-week treatment by nicotine patch before quit day approximately doubles post-quit day abstinence. NRT augmentation with burpropion or nortriptyline, antidepressants with demonstrated efficacy for smoking cessation, does not seem to ameliorate further abstinence rates. Three months' and 6 months' NRT exposure was compared by only one but sufficiently powered study and found similar abstinence rates. Optimization strategies to increase the efficacy of NRT include combining NRT of different routes of administration and use of nicotine patch before target quit day. Uncertainties exist about the optimal length of NRT administration. Co-administration of NRT with bupropion or nortriptyline does not seem to lead to higher abstinence rate than NRT alone.
INTRODUCTION
During 2000–2005, COPD was the underlying cause of death for 718,077 persons overall aged 25 years or more in the United States and the overall age-standardized mortality rate from COPD was fairly stable varying around 120,000 deaths per year (Citation[1]). In 2005, approximately one in 20 deaths in the United States had COPD as the underlying cause. Smoking is estimated to be responsible for at least 75% of COPD deaths (Citation[2]).
Smoking cessation is the most effective way to reduce the risk of developing COPD and reduce the smoking-related excessive decline in FEV1 in both men and women. Smoking cessation decreases episodic respiratory symptoms in smokers without chronic respiratory symptoms; it decreases cough in patients with chronic bronchitis and it has beneficial effects on respiratory symptoms, airway hyperresponsiveness and inflammation (Citation[3]). Mortality rates due to all causes and to COPD decline progressively after smoking cessation compared with continuing smoking, but mortality risk in ex-smokers remains higher compared with never-smokers, even after many years of abstinence from smoking (Citation[4]). However, the effect of smoking cessation interventions by nicotine gum and by physician advice plus group therapy can be demonstrated even 11 years later (Citation[5], Citation[6]).
The efficacy of nicotine replacement therapies (NRT), bupropion, nortriptyline and more recently varenicline has clearly been demonstrated in smokers without co-morbidities. The first line pharmacological treatments to help smokers to quit or to maintain abstinence are NRT, bupropion and varenicline (Citation[7]). Nortriptyline, a tricyclic antidepressant with important anticholinergic properties, has demonstrated efficacy (Citation[8]) but is considered as a second-line treatment because of the frequency and type of adverse effects.
Relatively few studies testing pharmacotherapies for smoking cessation have been published in the specific population of COPD patients. Bupropion has been found to be effective in these patients to improve abstinence compared to placebo at medium term: at 6 months (Citation[9]) but not at 12 months (Citation[10]) and a phase III trial is going on with varenicline (Citation[11]). In smokers at risk for COPD or with COPD, in a study comparing bupropion and nortriptyline with placebo, bupropion significantly and nortriptyline non-significantly increased abstinence rate (Citation[12]). Results from the Lung Health Study (Citation[13]) showed that nicotine (polacrilex) gum ad libitum associated with individual counseling for 5 years increased smoking cessation rate and reduced decline in FEV1. This is the only sufficiently powered study to demonstrate long-term efficacy in smokers at high risk of for COPD or with COPD.
In smokers with COPD sublingual nicotine tablets (Citation[14]) nicotine gum, nicotine nasal spray and bupropion have a good safety profile (Citation[15]). Because of the very good safety profile of NRT, optimization strategies with NRT based on studies in smokers without co-morbidities can be applied also to patients with COPD.
The aim of this review is to present therapeutic strategies to optimize the effectiveness of NRT. Optimization strategies may be of importance in COPD patients because it has been suggested that this group of patients may be more nicotine dependent and may have more difficulties to quit than smokers without COPD (Citation[16]).
METHODS
The author identified the most recent (2007–2008) publications of NRT with the highest level of evidence for evaluation of treatment effectiveness according to the Centre for Evidence Based Medicine Levels of evidence (Citation[17]). Level 1 evidence includes systematic reviews (meta-analyses) with testing for homogeneity (level 1a) and individual randomized controlled studies (level 1b). This was powered by an internet search (ClinicalTrials.gov) for relevant ongoing randomized controlled trials for smoking cessation. The usual outcome measure in these publications is biochemically verified smoking abstinence in the active treatment and control groups.
RESULTS
NRT increase abstinence rate by around 60% compared to a placebo or to a control no treatment situation () (Citation[18]). The standard NRT strategy in most countries is a three month course with decreasing nicotine's daily dose monthly. However, smokers may need higher than usual or individualized daily doses, individualized treatment duration, and combination treatments to increase efficacy.
Table 1 Efficacy of nicotine replacement therapies expressed as risk ratio (percent abstinent on active/control: placebo or no treatment) assessed at 6 months or longer (from ref. 18)
Fixed high dose of nicotine replacement therapy
The most recent Cochrane review of NRT shows (Citation[18]) that fixed high dose nicotine (nicotine patch ≥ 22 mg/day and up to 56 mg/day) provides some weak advantage over standard dose (nicotine patch < 22 mg/day) with a risk ratio (RR) of 1.01 (95% confidence interval: 1.01 to 1.3). This moderate advantage comes exclusively from the CEASE trial (Citation[19]) which has a substantial weight in this meta-analysis. The CEASE trial tested as high dose 25 mg/day versus 15 mg/day nicotine patches. It shows a RR for abstinence of 1.23 (95% CI: 1.03 to 1.48). However, today 25 mg/day of nicotine cannot be considered as a high dose. Exclusion of this study from the meta-analysis shows that high fixed dose of nicotine patch does not result in higher abstinence rates than standard fixed dose nicotine patch even in heavy smokers. Fixed high dose of NRT in patients led to more nicotine related adverse effects especially in smokers with low need for nicotine.
In smokers with high nicotine dependence 4 mg nicotine gum may lead to a better outcome compared with the 2 mg nicotine gum (RR = 1.85, 95% CI: 1.36 to 2.5) but no superiority has been demonstrated in smokers with low nicotine dependence (Citation[18]).
Combination of nicotine replacement therapies of different routes of administration
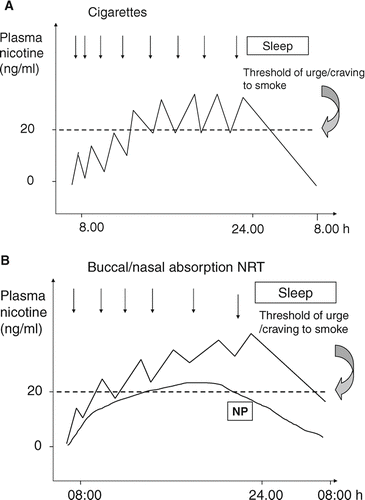
Although most individual studies did not show clear superiority of combining NRT of different routes of administration (nicotine patch plus buccal/nasal absorption products) versus single NRT, the meta-analysis of all available studies shows a statistically significant benefit of combined NRT over a single NRT (RR = 1.35, 95% CI: 1.11 to 1.63) (Citation[18]). The combination of a slow delivery and long acting pharmaceutical product (nicotine patch) with a rapid delivery and short-acting nicotine product (nicotine gum, lozenge, inhaler, spray) may suit well to a certain number of smokers. Nicotine patch assures a continuous, progressively increasing and declining plasma nicotine concentration which is sometimes just above the threshold of feeling urge to smoke/craving for cigarettes. Co-administration of rapid, buccal/nasal absorption NRT results in peak plasma nicotine concentrations similar to that smokers experience with cigarettes and may reduce more smoking urges than nicotine patch alone ().
Figure 1 Schematic presentation of plasma nicotine concentrations in venous blood of a smoker over a 24-hour period (A) and in an abstinent smoker using nicotine patch (NP) and buccal/nasal absorption nicotine replacement products (B). Combination of NP with rapid, buccal/nasal absorption NRT leads to higher area under the plasma nicotine concentration curve and peak nicotine concentrations. These result in more time spent above the craving/urge to smoke plasma nicotine concentration threshold and a better mimicking of self-titrated nicotine peaks.
Precessation nicotine replacement therapy
A 2-week pretreatment by nicotine patch before target quit day is a useful strategy to improve post-quit day abstinence. The two existing meta-analyses provide the same estimation of efficacy. Shiffman and Ferguson (Citation[20]) report an odds ratio (OR) of 2.17 (95% CI: 1.46 to 3.22) and Stead et al. (Citation[18]) a RR of 1.79 (95% CI: 1.17 to 2.72) for the 6 months abstinence rate between pre-quit day treatment with nicotine or placebo patch. Precessation nicotine patch does not seem to increase adverse effects (Citation[21]) and its efficacy seems to be independent of the type of cigarettes smoked in the presence of nicotine (Citation[22]). The precessation NRT administration is not far from the clinical practice in which frequently occurs that smokers are unable to be abstinent on and following their quit date, so they use, concomitantly, a nicotine patch and cigarettes for certain periods of time.
Extended duration of nicotine replacement therapy
Compared to placebo both 8, or more than 8, weeks' nicotine patch exposure yields higher abstinence rates (Citation[18]). The only sufficiently powered and planned comparison of treatment duration was the CEASE trial. It showed that 28 weeks nicotine patch exposure did not better than 12 weeks exposure (RR: 1.05, 95% CI: 0.88 to1.26) (Citation[19]). No specific study tested the more and more usual clinical situation: nicotine patch or buccal absorption NRT alone or in combination followed by ad libitum use of buccal absorption NRT. This strategy is frequently used if the standard 3 months treatment course is insufficient to maintain abstinence. This approach may be helpful with smokers who report persistent withdrawal symptoms during the course of medications, who have relapsed in the past after stopping medication, or who desire long-term therapy (Citation[7]).
Add-on treatments
The combination of NRT with an antidepressant with demonstrated efficacy for smoking cessation is an attractive option to increase abstinence rates. However, only one study (Citation[23]) showed that bupropion plus nicotine patch resulted in higher abstinence rate than nicotine patch alone. Other studies did not confirm this and a meta-analysis of 4 studies yielded an odds ratio of 1.37 with 95% CI: 0.65 to 2.91 (Citation[8]), suggesting that adding bupropion to NRT is of low or no interest. The same Cochrane review (Citation[8]) reported also that nortriptyline augmentation of NRT was not more effective than NRT alone (). Add-on nortriptyline was assessed in a recent sufficiently powered study. It showed again that nortriptyline augmentation of nicotine patch did not increase abstinence rate. More adverse effects were observed with the association nicotine patch plus nortriptyline than with nicotine patch plus placebo (Citation[24]).
Table 2 Nicotine patch augmentation by bupropion or nortriptyline (from ref. 8)
With the accessibility of varenicline for smoking cessation the question arises whether NRT augmentation of varenicline is more effective than varenicline alone. As of today, no study has been published to answer this question so only speculation prevails. Varenicline is a selective α 4β 2 nicotinic acetylcholine receptor partial agonist with higher affinity to this receptor than nicotine. Consequently, it inhibits the action of exogenous nicotine (from tobacco or NRT). Moreover, high dose nicotine can theoretically displace varenicline from the α 4β 2 nicotinic acetylcholine receptor binding sites leading to decreased varenicline efficacy. According to the manufacturer's information the co-administration of varenicline 2 mg/day plus nicotine patch 21 mg/day for 12 days led to greater frequency of nausea, headache, vomiting, dizziness, dyspepsia, and fatigue in the group receiving combination therapy, compared to the group receiving nicotine alone (Citation[25]).
DISCUSSION
A systematic review of smoking cessation interventions in COPD found evidence that a combination of psychosocial interventions and pharmacological interventions is superior to no treatment or to psychosocial interventions alone (Citation[26]). Therefore, it is of interest to optimize pharmacological interventions in smoking COPD patients. NRT have an excellent benefit/risk profile and are largely cost effective. They increase the likelihood of abstinence by only around 60% (Citation[18]) leaving substantial space for treatment strategies to improve their efficacy.
As the recent U.S. Clinical Practice Guideline pointed out (Citation[7]), there is evidence that combining NRT of different routes of administration may lead to higher abstinence rates than a single NRT. Precessation nicotine patch results in higher abstinence rates compared to the traditional method: starting nicotine patch on target quit day. However, there are no data about the efficacy of precessation use of buccal/nasal absorption NRT. The use of nicotine patches during the precessation period along with smoking seems to be well tolerated.
There is very few information about the optimal length of NRT use and even less about the characteristics of smokers who may need longer than 3 or 6 months' NRT exposure. The need for nicotine may be stable over a long period of time. Data from the Lung Health Study show that saliva cotinine concentrations after 1 or 5 years were similar in abstinent ex-smokers using nicotine gum, in continuing smokers and in smokers using both cigarettes and nicotine gum (Citation[27]). This is in accordance with the fact that NRT substitutes for tobacco's nicotine but does not cure nicotine dependence. For this reason, it is important to assess the risk of relapse after cessation of NRT. A study based on a mathematical model found that the risk of relapse is higher after stopping NRT than after stopping placebo (Citation[28]). Further studies analysing raw data are needed to assess the effect of stopping NRT on post-NRT abstinence rates.
Clinical practitioners empirically adapt nicotine's daily dose on the number and intensity of urge to smoke or nicotine withdrawal symptoms. An ongoing study addresses the question whether 100% substitution according to precessation saliva cotinine improves or not abstinence rate in smokers with smoking related disorders, in particular COPD and previous, unsuccessful attempts to quit (Citation[29]).
The co-administration of nicotine and the monoamine reuptake inhibitors bupropion and nortriptyline seemed to be very attractive to increase abstinence rate compared to nicotine alone. One of the main pharmacological action of nicotine being stimulation of monoamine release, inhibition of monoamine reuptake was hypothesized to increase further monoamine neurotransmission and consequently abstinence rate. Experimental data from randomized clinical trials did not confirm this hypothesis and augmentation of nicotine patch treatment by bupropion or nortriptyline did not result in higher abstinence rate than nicotine patch alone (Citation[8], Citation[24]).
Limitations. Because the aim of this paper was to review and synthesize optimization strategies of NRT applicable in clinical practice and provide information about potential future optimization strategies on a descriptive way, a specific meta-analysis with adequate methodology could not be conducted. This may have led to selection bias or omissions.
CONCLUSIONS
Optimization strategies to increase the efficacy of NRT include combining NRT of different routes of administration and the use of nicotine patch before target quit day. Uncertainties exist about the optimal length of NRT administration. Co-administration of NRT with bupropion or nortriptyline does not seem to lead to higher abstinence rate than NRT alone. As of today, there is no experimental evidence to co-administer NRT and varenicline.
Declaration of interest:
The author has served as a consultant for Pfizer Inc. and Sanofi-Aventis. The author alone is responsible for the content and writing of the paper.
Permanent affiliation: Université P. and M. Curie, Faculté de médecine-Hôpital Pitié-Salpêtrière-INSERM Unité 894, Paris, France
REFERENCES
- CDC. Deaths from Chronic Obstructive Pulmonary Disease. United States, 2000–2005. MMWR 2008; 57: 1229–1232
- CDC. Annual smoking-attributable mortality, years of potential life lost, and productivity losses. United States, 1997–2001. MMWR 2005; 54: 625–8
- Willemse B WM, Postma D S, Timens W, ten Hacken N HT. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur Resp J 2005; 23: 464–76
- Godtfredsen N S, Lam T H, Hansel T T, Leon M E, Gray N, Dresler C, Burns D M, Prescott E, Vestbo J. COPD-related morbidity and mortality after smoking cessation: status of the evidence. Eur Resp J 2008; 32: 844–53
- Anthonisen N R, Connett J E, Murray R P. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med 2002; 166: 675–9
- Murray R P, Connett J E, Rand C S, Pan W, Anthonisen N R. Persistence of the effect of the Lung Health Study (LHS) smoking intervention over eleven years. Prev Med. 2002; 35: 314–9
- U.S. Department of Health and Human Services, Public Health Service. Clinical Practice Guideline. May, 2008, http://www.surgeongeneral.gov/tobacco/treating_tobacco_use08.pdf Treating Tobacco Use and Dependence: 2008 Update
- Hughes J R, Stead L F, Lancaster T. Antidepressants for smoking cessation (Review), http://www.thecochranelibrary.com The Cochrane Library, 2007, Issue 4
- Tashkin D P, Kanner R, Bailey W, Buist S, Anderson P, Nides M A, Gonzales D, Dozier G, Patel M K, Jamerson B D. Smoking cessation in patients with chronic obstructive pulmonary disease: a double-blind, placebo-controlled, randomised trial. Lancet 2001; 357: 1571–5
- Wagena E J, van der 1Meer R M, Ostelo R J, Jacobs J E, van Schayck C P. The efficacy of smoking cessation strategies in people with chronic obstructive pulmonary disease: results from a systematic review. Respir Med. 2004; 98: 805–15
- ClinicalTrials.gov. Smoking Cessation in Subjects With Mild-to-Moderate Chronic Obstructive Pulmonary Disease (COPD). ClinicalTrials.gov Identifier: NCT00285012. Accessed on November 24, 2008
- Wagena E J, Knipschild P G, Huibers M JH, Wouters E FM, van Schayck C P. Efficacy of Bupropion and Nortriptyline for smoking cessation among people at risk for or with chronic obstructive pulmonary disease. Arch Intern Med. 2005; 165: 2286–92
- Anthonisen N R, Connett J E, Kiley J P, Altose M D, Bailey W C, Buist A S, Conway W A, Jr, Enright P L, Kanner R E, O'Hara P, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 1994; 272: 1497–505
- Tønnesen P, Kim Mikkelsen K, Bremann L. Nurse-conducted smoking cessation in patients with COPD using nicotine sublingual tablets and behavioral support. Chest 2006; 130: 334–42
- Wagena E J, Zeegers M PA, van Schayck C P, Wouters E FM. Benefits and risks of pharmacological smoking cessation therapies in chronic obstructive pulmonary disease. Drug Safety 2003; 26: 381–403
- Jimenez-Ruiz C A, Masa F, Miravitlles M, Gabriel R, Viejo J L, Villasante C, Sobradillo V. Smoking characteristics: differences in attitudes and dependence between healthy smokers and smokers with COPD. Chest 2001; 119: 1365–70
- Centre for Evidence Based Medicine, Oxford Centre for Evidence-based Medicine. May, 2001, www.cebm.net Levels of Evidence
- Stead L F, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation (Review). The Cochrane Library 2008, http://www.thecochranelibrary.com Issue 4
- Tønnesen P, Paoletti P, Gustavsson G, Russell M A, Saracci R, Gulsvik A, Rijcken B, Sawe U. Higher dosage nicotine patches increase one-year smoking cessation rates: results from the European CEASE trial. Collaborative European Anti-Smoking Evaluation. European Respiratory Society. Eur Respir J 1999; 13: 238–46
- Shiffman S, Ferguson S G. Nicotine patch therapy prior to quitting smoking: a meta-analysis. Addiction 2008; 103: 557–63
- Schuurmans M M, van Diacon A HBX, Bolliger C. Effect of pre-treatment with nicotine patch on withdrawal symptoms and abstinence rates in smokers subsequently quitting with the nicotine patch: a randomized controlled trial. Addiction 2004; 99: 634–40
- Rose J E, Behm F M, Westman E C, Kukovich P. Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine Tob Res 2006; 8: 89–101
- Jorenby D E, Leischow S J, Nides M A, Rennard S I, Johnston J A, Hughes A R, Smith S S, Muramoto M L, Daughton D M, Doan K, Fiore M C, Baker T B. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. New Engl J Med 1999; 340: 685–691
- Aveyard P, Johnson C, Fillingham S, Parsons A, Murphy M. Nortriptyline plus nicotine replacement versus placebo plus nicotine replacement for smoking cessation: pragmatic randomised controlled trial. BMJ 2008; 336(7655)1223–1227
- http://www.pfizer.com/files/products/uspichantix.pdf Accessed on December 1, 2008
- van d er, Meer R M, Wagena E J, Ostelo R WJG, Jacobs J E, van Schayck C P. Smoking cessation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2001, Issue 1. Art. No.: CD002999. DOI: 10.1002/14651858. CD002999
- Murray R P, Nides M A, Istvan J A, Daniels K. Levels of cotinine associated with long-term ad-libitum nicotine polacrilex use in a clinical trial. Addict Behav 1998; 23: 529–535
- Medioni J, Berlin I, Mallet A. Increased risk of relapse after stopping nicotine replacement therapies: a mathematical modelling approach. Addiction. 2005; 100: 247–254
- ClinicalTrials.gov. Adjustment of DOses of NIcotine in Smoking Cessation (ADONIS). ClinicalTrials.gov Identifier: NCT00235313. Accessed on December 1, 2008
