Abstract
We examined the effects of long-term smoking patterns on self-reported symptoms of chronic bronchitis within the Finnish adult twin cohort including 21, 609 individuals responding to questionnaires in 1975 and 1981, of which 11,015 respondents participated also in 1990. We also explored the association between smoking and bronchitis among discordant twin pairs. Among those without chronic bronchitis at baseline we examined incidence of chronic bronchitis in 1981 both by 1975 smoking status, but also based on subgroups formed according to change in smoking behaviors between 1975 and 1981. We conducted similar analyses in the longitudinal data including three consecutive measurements of smoking status. Logistic regressions demonstrated that among current smokers, the risk of chronic bronchitis increased about 1.5-fold by each amount category of daily cigarettes. When analyzing change of smoking status between 1975 and 1981, daily moderate and heavy smokers, smoking increasers and decreasers, as well as re-current smokers demonstrated elevated risks. In the analysis among discordant twin pairs the smoking co-twins had a 14-fold likelihood for chronic bronchitis compared to their never-smoking co-twins. Panel analyses showed that, not only moderate and heavy, but also former and light smokers, had significant risks for chronic bronchitis. Those with late smoking initiation, leisure time physical activity or over 10 years of smoking cessation were less likely to have chronic bronchitis. We conclude that in long term evaluation no safe level of smoking exists. Abstinence from tobacco seems to be the public health message justified by these results in prevention of chronic bronchitis.
INTRODUCTION
Chronic bronchitis is characterized by chronic cough and mucus production from airways for at least 3 months in each of 2 successive years without airway obstruction. Over 40% of smokers develop chronic bronchitis, while about half of those develop Chronic Obstructive Pulmonary Disease (COPD) (Citation[1], Citation[2]), which includes chronic bronchitis, emphysema and obstruction of small airways (Citation[3]). The diagnosis of COPD is based on spirometry: FEV1/FVC (forced vital capacity) < 70% is diagnostic according to the GOLD-standards (Global Initiative for Chronic Obstructive Lung Disease) (Citation[4]).
The prevalence of chronic bronchitis increases with pack-years of smoking (Citation[5]), and early smoking initiation is also a significant risk factor of COPD (Citation[6]). Farming, industrial work, asthma and airway hyper-responsiveness are risk factors for developing chronic bronchitis (Citation[1], Citation[7], Citation[8], Citation[9]). Interestingly, physically active smokers do not develop COPD as often as sedentary smokers (Citation[10]). However, the effect of exercise on chronic bronchitis has not been established.
In follow-up studies, individuals with chronic productive cough have demonstrated about 3-fold risks of developing COPD when compared to healthy controls (Citation[11], Citation[12]). Even without developing COPD, chronic bronchitis is associated with a decline in lung function, detected as decreased FEV1 values (Citation[1], Citation[2], Citation[13]). Chronic bronchitis has also been stated to be significantly related to increased mortality from all causes (Citation[1], Citation[12]).
One may recover from chronic bronchitis after smoking cessation (Citation[14]) but once COPD has developed, it usually progresses irreversibly, cessation only delaying the decline of lung function (Citation[14], Citation[15]). There is no consensus whether recovery from chronic bronchitis can be reached by smoking reduction. Smokers with chronic productive cough have been stated to live longer by decreasing their amount of cigarettes smoked per day (CPD) (Citation[1]). However, some other studies report that smoking reduction has no effect on mortality in smokers (Citation[16], Citation[17]). The connection between low-rate smoking patterns and pulmonary diseases remains also unclear although the proportions of light smokers are increasing in many countries (Citation[18], Citation[19]). Light smoking (< 5 CPD) is particularly common among adolescents, students and women (Citation[19], Citation[20]). Young age does not protect from either chronic bronchitis or COPD (Citation[11]). For example, a recent study detected a significantly increased prevalence of respiratory symptoms and decreased FEV1/FVC ratios in young adult smokers (Citation[21]).
Our aim was to examine the effects of different long-term smoking patterns on self-reported symptoms of chronic bronchitis. The prevalence, incidence and risks of these COPD-related outcomes were compared between smokers and non-smokers, as well as between different smoking categories within smokers. We also investigated those reporting low-rate smoking at any survey and examined long-term changes of these smoking patterns in relation to respiratory symptoms. Further aim was to explore causal nature of the association between smoking and bronchitis among twin pairs discordant for smoking and respiratory outcome.
MATERIALS AND METHODS
Subjects
The data for this study were collected as part of the Finnish Twin Cohort established in 1974. The cohort was compiled from the Central Population Registry and it includes all same-sex twin pairs born before 1958 with both members alive in 1967 (Citation[22]). Three questionnaire studies were carried out in 1975, 1981 and 1990 with response rates of 89%, 84% and 77%, respectively. In 1981 the questionnaire was sent to all twins still alive in the cohort, whereas in 1990 only to those born 1930–1957, with both co-twins resident in Finland, if they had responded in at least one of the previous surveys. For the present study, we included those subjects who had participated in the survey in both 1975 and 1981 (n = 21,609). In further analyses including also the 1990 survey, the sample consisted of 11,015 respondents.
Definition of the outcome
We investigated incidence of self-reported chronic bronchitis symptoms. Those COPD-related respiratory symptoms were monitored using the British Medical Research Council (MRC) series of questions (Citation[23]). A person was considered suffering from chronic bronchitis if he/she answered positively to the MRC question “Do you regularly for extended periods of time have a cough?” and reported suffering from the symptoms (“How many months in a row do you cough per year?,” “For how many months in a row do you bring up phlegm from your chest per year?”) at least for over three successive months a year. The wording of the questions was identical in each survey.
A variable designing the incidence of chronic bronchitis was created. Among those without chronic bronchitis at baseline in 1975 we examined the risks of chronic bronchitis in 1981 according to 1975 smoking status and amount smoked, and according to the subgroups describing change in smoking behaviors between 1975 and 1981. Individuals with chronic bronchitis in 1975 were not examined in this incidence analysis, because sufficient data on their prior smoking history was not available and their current smoking behavior may have changed after developing symptoms. A further analysis was conducted among twin pairs discordant for smoking in 1975–1981 and for incident chronic bronchitis in 1990, i.e., where one twin had incident chronic bronchitis and the other was symptom free. Finally, we analyzed the relationship of chronic bronchitis to smoking in the longitudinal data including three consecutive measurements.
Definition of the predictors
Among subjects who participated in the survey in 1975 and 1981, we first identified never smokers, former smokers, occasional smokers and current daily smokers, i.e. those who had smoked at least 5–10 packs of cigarettes over their lifetime, and who were smoking daily or almost daily. The classification was identical in each survey and the questions asked were: “Have you ever smoked more than 5–10 packs of cigarettes in your lifetime?” Those responding positively were asked, “Do you smoke or have you smoked cigarettes regularly, say daily, or almost daily during your lifetime?” If one replied ‘yes,’ he/she was further asked if still smoking regularly. If one replied ‘no’ he/she was regarded as a former smoker, and if ‘yes’ he/she was classified as a current smoker, whose average daily cigarette consumption was then defined. The exact question was: “How many cigarettes do you smoke daily on average?” The amount categories were as follows: < 5, 5–9, 10–14, 15–19, 20–24, 25–39, and > 40. According to those amount categories, we collapsed the 1975 current smokers into three groups, i.e., daily light (< 5), daily moderate (5–19), and daily heavy (≥20 CPD) smokers. Those subjects who answered negatively the question: “Have you ever smoked more than 5–10 packs of cigarettes in your lifetime?” were regarded as never smokers. Further, those answering positively to this question, but negatively to the question “Do you smoke or have you smoked cigarettes regularly, say daily, or almost daily during your lifetime?,” were classified as occasional smokers. Similar smoker classifications have been used in our earlier studies based on the same cohort (Citation[24], Citation[25]).
In addition to forming groups according to the smoking status reported in a certain year, we further formed more detailed subgroups by taking into account the change of smoking status and amount smoked between 1975 and 1981. These groups included constant never smokers; occasional/non-daily smokers; constant former smokers (i.e. former smokers in 1975 and 1981), initiators of daily smoking (i.e., never smokers in 1975 but daily smokers in 1981), quitters (i.e., current smokers in 1975 but former smokers in 1981), recurrent smokers (i.e., former smokers in 1975 but current smokers in 1981), constant daily light, moderate and heavy smokers (same consumption in 1975 and 1981), as well as ‘increasers’ and ‘decreasers’ (according to the change of daily consumption between 1975 and 1981), plus others (including illogical reports and misclassifications). The proportions of these specific groups are presented in . Similar classification was also made according to the change of smoking status between 1981 and 1990.
Table 1 Incident chronic bronchitis in 1981 by smoking status in 1975–1981 with number of subjects in each category, proportion of subjects affected, odds ratios (OR) and confidence intervals (95% CI) among Finnish twin cohort individuals without chronic bronchitis in 1975 Footnote1
Confounders
All analyses were adjusted for age and sex. As additional covariates and potential confounders we considered ‘pack-years,’ age of regular smoking initiation, duration of cessation (among former smokers), physical activity level, occupation, as well as asthma and allergic rhinitis. Age of regular smoking initiation was categorized as ‘no initiation,’ indicating never and occasional smokers; and ‘early/late onset,’ early regarded as smoking initiation before or at the age of 16. For former smokers, duration of cessation was categorized as ‘long’ (> 10 years), ‘intermediate’ (2–10 years) and ‘short’ (< 2 years). We categorized physical activity as sedentary, intermediate or active, based on frequency, duration and intensity of leisure physical activity (Citation[26]). Because physical activity was measured differently in 1990, this covariate was used only in the 1975 and 1981 data. Occupation was coded as follows: 1) upper-level employee or large entrepreneur, 2) Lower-level employee or small entrepreneur, 3) trained worker, 4) untrained worker, 5) farmer, fisher or gardener, and 6) unknown. Presence of asthma and allergic rhinitis were asked in similar way in each survey with the following question: “Has your doctor ever told you that you have or have had asthma/allergic rhinitis?”
Statistical analyses
All data analyses were performed with Stata (version 9.1) (Citation[27]). First, we conducted logistic regression models, where the statistical significances of the risks of incident chronic bronchitis between different smoking categories were tested adjusted for age, sex and for selected significant confounders among subjects without chronic bronchitis in 1975. The Odds Ratios (OR) with 95% Confidence Intervals (CI) for chronic bronchitis in 1981 (event) versus not having such condition (non event) were computed. Since observations on twin individuals within twin pairs may be correlated, robust estimators of variance and the cluster option from Stata were used, when estimating standard errors (Citation[28]).
Second, in order to further explore causal nature of the association between smoking and bronchitis, we analyzed twin pairs discordant for both smoking and chronic bronchitis using a matched pair case-control design. Twin pairs were regarded as discordant for incident bronchitis, if one twin had incident outcome (case) whereas the co-twin did not (control). Among such pairs, we examined the distribution of smoking behavior. Smoking discordant pairs, where one twin was persistent or recurrent smoker and the co-twin never smoker, were considered informative, as these represented the extremes of risk. The ORs are given by the ratio of the number of case-control pairs in which the case smokes and the control does not smoke to the number of case-control pairs in which the case does not smoke and the control does smoke. The significance of the association within such pairs is tested using McNemar's test and the effect of confounders can be assessed using conditional logistic regression (Citation[29]). The other smoking categories were excluded in this analysis.
Third, given the longitudinal and repeated nature of measurements and in order to fully benefit from the information of this 15-year follow-up survey, the observations were further analyzed as longitudinal panel data. The data were reshaped and panel analyses were carried out. Using random-effects model logistic regressions, the ORs for reporting chronic bronchitis were computed for different smoker subgroups considering never-smokers as a reference group. For currents smokers, the risks were also examined with amount of smoking as a multilevel variable and a trend test was performed. Finally, fixed effects models were used to compare the between- and within-subjects variations (Citation[30]). Pack-years, age of regular smoking initiation, duration of cessation, physical activity, asthma, rhinitis and occupation were considered as potential confounders. These models use information on smoking, symptoms and confounders from all 3 surveys at the same time.
RESULTS
Basic characteristics
The mean ages of the study participants were 34.2 years in 1975, 40.2 years in 1981, and 49.2 years in 1990 with standard deviation of 13.4 each year. For light smokers, the average (mean) smoking duration was 9 years in 1975, 15 years in 1981 and 19 years in 1990, while for moderate smokers 11, 17 and 22 years and for heavy smokers 16, 19 and 25 years, respectively.
Incidence of chronic bronchitis in different smoking groups
First, we analyzed the risk of incidence of chronic, productive cough in 1981 according to smoking status and quantity in 1975. Those having symptoms at baseline, i.e. in 1975, were excluded from the analysis. When analyzing all subjects, the incidence of symptoms in 1981 was significantly elevated among moderate (5-19 CPD) (OR = 3.03, 95% CI 2.26-4.06) and heavy (20+ CPD) smokers (OR = 6.75, 95% CI 4.88–9.35) if compared to never smokers. If the analysis was restricted among 1975 current smokers only, there was a linear trend in risk of having symptoms in 1981, increasing about 1.5-fold (OR 1.54, 95% CI 1.38, 1.73) by each amount category of cigarettes on the original scale asked in the questionnaire (not shown in tables/figures).
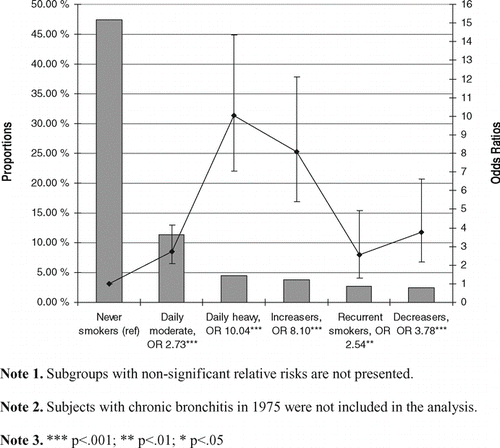
Based on information of smoking status in 1975 and 1981, more detailed smoking subgroups were formed. Among those subgroups, the risk of incident chronic bronchitis was elevated significantly not only among moderate and heavy daily smokers, but also among recurrent smokers, increasers and decreasers (). The proportional sizes and the ORs of those subgroups with statistically significant risks for incident chronic bronchitis are shown in . Similarly, when taken into account the change of smoking patterns between 1981 and 1990 and examining the incidence of symptoms in 1990, the same subgroups demonstrated elevated risks when compared to never smokers (data not shown).
Figure 1 Odds Ratios (OR) and proportional sizes (bars) of those smoking subgroups with statistically significant relative risks for incident chronic bronchitis in 1981 (n = 14,602; total n = 20,208). Data points (joined by a line) represent OR with 95% confidence intervals in each smoking subgroup.
Discordant twin pairs
Among those twins who participated in all surveys (1975, 1981 and 1990), we indentified 112 pairs discordant for incident chronic bronchitis in 1990 (no one had chronic bronchitis in 1975 or 1981, whereas in 1990 one twin had bronchitis but the co-twin did not have). Among those pairs 15 pairs were discordant also for 1975–1981 smoking status. Conditional logistic regression models showed that the persistently or re-currently smoking co-twins had a 14-fold relative risk (OR = 14, 95% CI 1.8, 107) for incident chronic bronchitis compared to their never-smoking co-twins; i.e. in 14 pairs out of 15 the smoker had developed symptoms and the non-smoker had not. Among 12 dizygotic pairs, the OR was 11 (95% CI 1.4, 85). Among the three monozygotic pairs, only the persistently or re-currently smoking co-twins had chronic bronchitis, and none of the never smokers had chronic bronchitis.
Longitudinal panel analyses
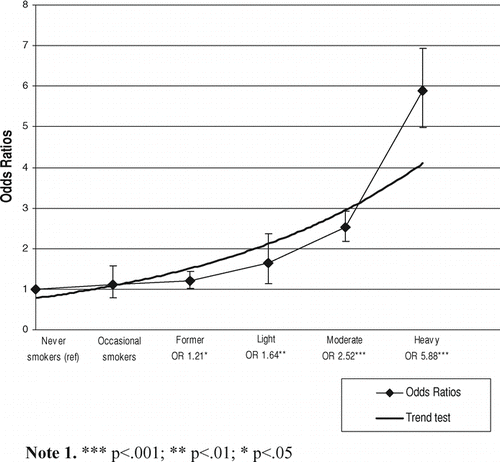
Panel analyses based on the longitudinal repeated measures data suggested that former smokers, light, moderate and heavy smokers all had a significantly elevated risk of reporting symptoms of chronic bronchitis. The smallest risk was observed in former smokers, while among current smokers, the risks were increased with increasing quantity of cigarettes smoked ().
Figure 2 Odds Ratios (OR) of chronic bronchitis in different smoking groups based on random-effects model analysis carried out for longitudinal data. Data points represent OR with 95% confidence intervals in each smoking category. Continuous line represents the result of the trend test.
The effects of potential confounders, such as physical activity, asthma, rhinitis, occupation, age of regular smoking initiation and duration of cessation among former smokers, were also examined. The effect of pack-years was initially considered as well, but because of the high correlation between smoking categories and pack-years, it was not included into the final analyses. Random effect logistic regression models suggested that likelihood of suffering from chronic productive cough was increased among smokers with early age as well as late age of initiation (OR 2.94; 95% CI 2.45, 3.53 and OR 2.14; 95% CI 1.90, 2.41) versus never/occasional smokers. However, when analyzing the potential confounding effect of smoking initiation age was restricted among current smokers, no significant confounding effect was discovered. Among former smokers the risk of symptoms was increased among those having been abstinent for less than two years versus those being abstinent for over ten years (OR 1.77; 95% CI 1.21, 2.60). The risk was decreased among smokers exhibiting intermediate (OR 0.71; 95% CI 0.62, 0.82) and high physical activity (OR 0.40; 95% CI 0.31, 0.51) versus sedentary smokers. However, when testing physical activity as a potential confounder in the model examining association of smoking and incident chronic bronchitis, those risk estimates of different smoking categories did not change dramatically.
Although both asthma (OR 8.26; 95% CI 6.62, 10.3) and rhinitis (OR 2.62, 95% CI 2.26, 3.02) as such had significant direct effects on the outcome, those effects of smoking on the outcome were only very slightly attenuated when adjusted for asthma and rhinitis. When observing the risks of chronic bronchitis among various occupations, for example farmers (OR 2.67; 95% CI 1.94, 3.69) demonstrated an elevated risk. Adjustment for occupation slightly strengthened the effects of different smoking patterns.
Finally, the fixed effects models to compare the between- and within-subjects variations showed that among current smokers, the risk of symptoms increased 1.25-fold (95% CI 1.10, 1.43) by each amount category. Similar analysis made by the random-effects model suggested the risk of symptoms increasing 1.46-fold (95% CI 1.38, 1.54) by each category. When former, occasional and never smokers were included into the above-mentioned fixed-effects model, only heavy smokers demonstrated a significant risk for chronic bronchitis (OR 2.56, 95% CI 1.49, 4.41) (not shown in tables) compared with a corresponding OR of 5.88 in the random effects models ().
DISCUSSION
The risk of chronic bronchitis increased among current smokers about 1.5-fold by each amount category of daily cigarettes. In addition to daily smokers, also smoking increasers, decreasers and re-current smokers demonstrated elevated risks. In panel analyses, significant risks for chronic bronchitis were found for former, light, moderate and heavy smokers. Those with late smoking initiation, leisure time physical activity or over 10 years of cessation were less likely to have chronic bronchitis, whereas asthma, rhinitis and farming work increased this risk. However, adjustment for these factors did not significantly modify the effects of smoking.
According to the analyses comparing smoking subgroups describing change in smoking behaviors, the risk of chronic bronchitis was elevated not only among regular smokers, but also for example in those smokers who had quit but relapsed. Also those who reduced from moderate to light smoking manifested an increased risk of respiratory symptoms, which was even higher than among relapsed former smokers. It is possible that those with the most symptoms are motivated to reduce their smoking, while those previously asymptomatic easily start smoking again.
Despite the very large cohort there were only 15 complete twin pairs extremely discordant for both 1975–1981 smoking and incident bronchitis in 1990. We observed that persistently or re-currently smoking twins had a 14-fold relative risk for incident chronic bronchitis compared to their never-smoking co-twins. However, although such effect turned out to be statistically significant, the 95% confidence intervals were relatively large. This was due to a small number of observations included in the model, because we considered only those twin pairs who were extremely discordant for both risk factor and outcome. However, we think that the effect size is the most important one, particularly because observed even when familial factors were controlled for.
The recent genome-wide study of common genetic variants associated with COPD (Citation[31]) found one gene to have an association with COPD based on a genome-wide test of statistical significance. This association was with the acetylcholine nicotinic receptor gene complex on chromosome 15, which has been shown to associate with smoking and nicotine dependence (32–34). It is likely that much of the genetic predisposition to COPD may be mediated through the genetics of smoking and nicotine dependence; this would be consistent with our discordant twin pair analyses.
Panel analyses provided evidence that in addition to a very strong dose-response relationship for amount smoked, also very low-rate smoking patterns, even constant light smoking, can result in chronic respiratory symptoms. Concerning the effects of other factors, random-effects models demonstrated that the risk of chronic bronchitis was smaller among late smoking initiators, physically active smokers, as well as among former smokers having quit smoking over 10 years earlier. Physical activity, age of regular smoking initiation and duration of cessation were tested as potential confounders also in the standard model. These analyses confirmed the risk of chronic bronchitis being increased among former, light, moderate and heavy smokers independently of the effects of potential confounders as they did not clearly attenuate the association between smoking and respiratory symptoms.
Between-subjects variance was examined with fixed-effects models where the within-subject effect was controlled for. Such an analysis is equivalent to a single cross-sectional snapshot. When analyzing the risk of chronic bronchitis with such fixed-effects model, only heavy smokers demonstrated increased risk of symptoms, whereas the random-effects models, which uses all the data from all the surveys, was more sensitive in showing relationships of lesser smoking exposure with symptoms. These results might reflect the instability of smoking patterns over such a long follow-up time. Our earlier analyses within the same twin cohort have suggested that particularly the pattern of light smoking (< 5 CPD) is relatively unstable in long term follow-up (Citation[35]). However, when examining with fixed-effects model current smokers only, the risk increased in a linear fashion by each category of CPD indicating the greater strength of the relationship of symptoms with amount smoked.
This study had several strengths. The large longitudinal data provided by the Finnish Twin Cohort let us examine the changes that happened in smoking patterns over a 15-year period. By measuring the smoking status and respiratory symptoms equally at 3 time points, we were able to examine the risks of chronic bronchitis in more sophisticated subgroups. Development of chronic bronchitis has not been studied earlier in such a large sample in relation to the subjects' smoking status and changes therein being taken into account.
The criteria used for chronic bronchitis in this study may miss some new patients with less than 2 years of symptoms (Citation[36]). These criteria were however chosen because we wanted to be certain about the chronic status of productive cough. It should also be noticed that although chronic bronchitis builds up the clinical picture of COPD, not all those with the diagnosis report chronic productive cough. Cough in COPD patients can be absent in early stages (Citation[37]), occur intermittently, be unproductive and neglected when asked (Citation[38]). Results on discrimination of healthy patients from those with COPD by self-reported chronic bronchitis are controversial (39–41). Thus, spirometry is central to the diagnosis of COPD, while respiratory symptoms only predict an increased risk of the disease. The extent of predicting power these symptoms have for COPD remains a challenge for further validating analyses.
The connection between chronic bronchitis and potential confounders considered; physical activity, age of regular smoking initiation, duration of cessation, asthma, rhinitis and occupation, was in line with previous studies. However, we did not adjust for BMI, although high BMI has also been shown to be prognostic for COPD (Citation[39]). The effect of pack-years was not tested because of its high correlation with our smoking categories. Further, our analyses considered cigarette smokers and we did not examine separately those who may have been using also other tobacco products simultaneously; however, the proportion of regular pipe and cigar users was very small.
The most aged participants were excluded from the 1990 survey in order to avoid possible bias caused by their increased morbidity. Measured with GOLD-standards, the COPD prevalence in those having smoked more than 40 pack-years is 5-fold higher than in general population (Citation[40]). Accordingly, the number of chronic bronchitis diagnoses might have been higher without age restriction of the sample in 1990. It can also be argued that the drop-out subjects were more likely smokers than non-smokers. This has been suggested in an earlier study within the same cohort (Citation[25]). However, when we observed the drop-out subjects within the current data set similar proportions of subjects reported being smokers in the previous survey than was the share of smokers in the cohort in general.
We conclude that our results strengthen the view that in long term evaluation there is no safe level of cigarette smoking. Thus, abstinence from tobacco seems to be the public health message justified by these results in prevention of chronic bronchitis.
Declaration of interest
The Finnish Twin Cohort study is funded by the Academy of Finland Centre of Excellence in Complex Disease Genetics. The present analyses were supported by the research grant from the Yrjö Jahnsson Foundation. The authors reported having no conflicts of interests. The authors are responsible for the content and writing of the paper.
This work was performed at the University of Helsinki, Department of Public Health, Helsinki, Finland and at the National Institute for Health and Welfare (formerly National Public Health Institute).
REFERENCES
- Pelkonen M, Notkola I L, Nissinen A, Tukiainen H, Koskela H. Thirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a follow-up in middle-aged rural men. Chest Oct, 2006; 130(4)1129–1137
- Pelkonen M. Smoking: relationship to chronic bronchitis, chronic obstructive pulmonary disease and mortality. Curr Opin Pulm Med Mar, 2008; 14(2)105–109
- Chronic obstructive pulmonary disease (COPD), Available on the Internet at www.kaypahoito.fi(online). Current Care Summary. Working group set up by the Finnish Medical Society Duodecim and the Finnish Respiratory Society. Helsinki: The Finnish Medical Society Duodecim, 1999 (updated on 1 March 2004)
- Pauwels R. Global initiative for chronic obstructive lung diseases (GOLD): time to act. Eur Respir J Dec, 2001; 18(6)901–902
- Viegi G, Paoletti P, Prediletto R, Carrozzi L, Fazzi P, Di Pede F, Pistelli G, Giuntini C, Lebowitz M D. Prevalence of respiratory symptoms in an unpolluted area of northern Italy. Eur Respir J Apr, 1988; 1(4)311–318
- Kotaniemi J. Asthma, Chronic Obstructive Pulmonary Disease and Respiratory Symptoms among Adults: Prevalence and Risk Factors: The FinEsS Study in Northern Finland. University of Helsinki, Helsinki 2006, http://ethesis.helsinki.fi/julkaisut/laa/kliin/vk/kotaniemi2/
- Mannino D M. Chronic obstructive pulmonary disease: definition and epidemiology. Respir Care Dec, 2003; 48(12)1185–1191, discussion 1191–1193
- Terho E O, Koskenvuo M, Kaprio J. Atopy: a predisposing factor for chronic bronchitis in Finland. J Epidemiol Community Health Jun, 1995; 49(3)296–298
- Laasonen K, Uitti J. Bronchitis and emphysema as occupational diseases. Duodecim 2001; 117(2)156–161
- Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto J M. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med Mar 1, 2007; 175(5)458–463
- de Marco R, Accordini S, Cerveri I, Corsico A, Anto J M, Kunzli N, Janson C, Sunyer J, Jarvis D, Chinn S, Vermeire P, Svanes C, Ackermann-Liebrich U, Gislason T, Heinrich J, Leynaert B, Neukirch F, Schouten J P, Wjst M, Burney P. Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am J Respir Crit Care Med Jan 1, 2007; 175(1)32–39
- Lange P, Parner J, Prescott E, Vestbo J. Chronic bronchitis in an elderly population. Age Ageing Nov, 2003; 32(6)636–642
- Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med May, 1996; 153(5)1530–1535
- Willemse B W, Postma D S, Timens W, ten Hacken N H. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur Respir J Mar, 2004; 23(3)464–476
- Cooper C B, Dransfield M. Primary care of the patient with chronic obstructive pulmonary disease-part 4: understanding the clinical manifestations of a progressive disease. Am J Med Jul, 2008; 121(7 Suppl)S33–45
- Tverdal A, Bjartveit K. Health consequences of reduced daily cigarette consumption. Tob Control Dec, 2006; 15(6)472–480
- Godtfredsen N S, Vestbo J, Osler M, Prescott E. Risk of hospital admission for COPD following smoking cessation and reduction: a Danish population study. Thorax Nov, 2002; 57(11)967–972
- National Cancer Institute. What's in a name? Examination of light and intermittent smokers. U.S. Department of Health and Human Services. 2007, Retrieved from http://cancercontrol.cancer.gov/TCRB/LITSExecSummary.pdf(NIH Publication No 07-6253)
- Okuyemi K S, Harris K J, Scheibmeir M, Choi W S, Powell J, Ahluwalia J S. Light smokers: issues and recommendations. Nicotine Tob Res 2002; 4(Suppl 2)S103–112
- Owen N, Kent P, Wakefield M, Roberts L. Low-rate smokers. Prev Med Jan, 1995; 24(1)80–84
- Vianna E O, Gutierrez M R, Barbieri M A, Caldeira R D, Bettiol H, Da Silva A A. Respiratory effects of tobacco smoking among young adults. Am J Med Sci Jul, 2008; 336(1)44–49
- Kaprio J, Koskenvuo M. Genetic and environmental factors in complex diseases: the older Finnish Twin Cohort. Twin Res Oct, 2002; 5(5)358–365
- Lebowitz M D, Burrows B. Comparison of questionnaires: the BMRC and NHLI respiratory questionnaires and a new self-completion questionnaire. Am Rev Respir Dis May, 1976; 113(5)627–635
- Kaprio J, Koskenvuo M. A prospective study of psychological and socioeconomic characteristics, health behavior and morbidity in cigarette smokers prior to quitting compared to persistent smokers and non-smokers. J Clin Epidemiol 1988; 41(2)139–150
- Korhonen T, Broms U, Varjonen J, Romanov K, Koskenvuo M, Kinnunen T, Kaprio J. Smoking behaviour as a predictor of depression among Finnish men and women: a prospective cohort study of adult twins. Psychol Med May, 2007; 37(5)705–715
- Kujala U M, Kaprio J, Koskenvuo M. Modifiable risk factors as predictors of all-cause mortality: the roles of genetics and childhood environment. Am J Epidemiol Dec 1, 2002; 156(11)985–993
- StataCorp. Stata Statistical Software. Stata Corporation, College Station, TX 2005
- Williams R L. A note on robust variance estimation for cluster-correlated data. Biometrics Jun, 2000; 56(2)645–646
- Thomas D C. Statistical Methods in Genetic Epidemiology. Oxford University Press, New York 2004
- Twisk J WR. Applied Longitudinal Data Analysis for Epidemiology: A Practical Guide. Cambridge University Press, Cambridge, UK; New York 2003
- Pillai S G, Ge D, Zhu G, Kong X, Shianna K V, Need A C, Feng S, Hersh C P, Bakke P, Gulsvik A, Ruppert A, Lodrup Carlsen K C, Roses A, Anderson W, Rennard S I, Lomas D A, Silverman E K, Goldstein D B, ICGN Investigators. A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet Mar, 2009; 5(3)e1000421
- Bierut L J, Madden P A, Breslau N, Johnson E O, Hatsukami D, Pomerleau O F, Swan G E, Rutter J, Bertelsen S, Fox L, Fugman D, Goate A M, Hinrichs A L, Konvicka K, Martin N G, Montgomery G W, Saccone N L, Saccone S F, Wang J C, Chase G A, Rice J P, Ballinger D G. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet Jan 1, 2007; 16(1)24–35
- Thorgeirsson T E, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson K P, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, Stacey S N, Bergthorsson J T, Thorlacius S, Gudmundsson J, Jonsson T, Jakobsdottir M, Saemundsdottir J, Olafsdottir O, Gudmundsson L J, Bjornsdottir G, Kristjansson K, Skuladottir H, Isaksson H J, Gudbjartsson T, Jones G T, Mueller T, Gottsater A, Flex A, Aben K K, de Vegt F, Mulders P F, Isla D, Vidal M J, Asin L, Saez B, Murillo L, Blondal T, Kolbeinsson H, Stefansson J G, Hansdottir I, Runarsdottir V, Pola R, Lindblad B, van Rij A M, Dieplinger B, Haltmayer M, Mayordomo J I, Kiemeney L A, Matthiasson S E, Oskarsson H, Tyrfingsson T, Gudbjartsson D F, Gulcher J R, Jonsson S, Thorsteinsdottir U, Kong A, Stefansson K. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature Apr 3, 2008; 452(7187)638–642
- Weiss R B, Baker T B, Cannon D S, von Niederhausern A, Dunn D M, Matsunami N, Singh N A, Baird L, Coon H, McMahon W M, Piper M E, Fiore M C, Scholand M B, Connett J E, Kanner R E, Gahring L C, Rogers S W, Hoidal J R, Leppert M F. A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet Jul, 2008; 4(7)e1000125
- Hukkinen M, Kaprio J, Broms U, Koskenvuo M, Korhonen T. Characteristics and consistency of light smoking: long-term follow-up among Finnish adults. Nicotine Tob Res 7 May, 2009, [Epub ahead of print]
- Bobadilla A, Guerra S, Sherrill D, Barbee R. How accurate is the self-reported diagnosis of chronic bronchitis?. Chest Oct, 2002; 122(4)1234–1239
- Vestbo J, Lange P. Can GOLD Stage 0 provide information of prognostic value in chronic obstructive pulmonary disease?[see comment]. Am J Respir Crit Care Med Aug 1, 2002; 166(3)329–332
- Pauwels R A, Rabe K F. Burden and clinical features of chronic obstructive pulmonary disease (COPD).[see comment]. Lancet Aug 14–20, 2004; 364(9434)613–620
- van Schayck C P, Halbert R J, Nordyke R J, Isonaka S, Maroni J, Nonikov D. Comparison of existing symptom-based questionnaires for identifying COPD in the general practice setting. Respirology Jun, 2005; 10(3)323–333
- Kotaniemi J, Sovijarvi A, Lundback B. Chronic obstructive pulmonary disease in Finland: prevalence and risk factors. COPD Sep, 2005; 2(3)331–339
- Marsh S E, Travers J, Weatherall M, Williams M V, Aldington S, Shirtcliffe P M, Hansell A L, Nowitz M R, McNaughton A A, Soriano J B, Beasley R W. Proportional classifications of COPD phenotypes. Thorax Sep, 2008; 63(9)761–767
