Abstract
Mortality and other long-term outcomes of COPD from epidemiological studies of cohorts based on the general population are still rare. In contrast, data from follow-ups of patients from hospitals and general practices are more common and demonstrate often a 5-year mortality of about 50% and even higher. The aim was to study 20-year outcomes, mainly mortality, in a COPD cohort derived from a population study. The Obstructive Lung Disease in Northern Sweden (OLIN) Study's first postal survey was performed in 1985, and 5698 subjects (86%) responded. A stratified sample of symptomatic subjects and controls was invited to clinical examinations including lung function tests in 1986, 1506 (91%) of the invited participated and 266 subjects fulfilled the GOLD criteria of COPD. All alive and possible to trace had participated at least at two follow-up examinations. Of the 266 subjects with COPD 46% were still alive after 20 years. The proportion of survived among subjects with severe and very severe COPD at entry was 19%. Death was significantly related to age, male sex, disease severity and concomitant ischemic heart disease or cardiac failure at entry. Socioeconomic status (manual workers) was significant in the univariate analysis, but failed to reach statistical significance in the multivariate model. The annual decline in FEV1 among survivors was low to normal. Long-term follow-ups of subjects with COPD derived from population studies provide data reflecting the course of COPD in society better than follow-ups of hospital recruited patients, who represent the top of the iceberg. Surprisingly many with severe COPD were still alive after 20 years.
INTRODUCTION
Published studies of long term follow-ups of COPD are still rare. They mostly report data of outcome based on follow-ups of patients with COPD who have been hospitalized or subjects followed by specialists or general practitioners (1–3). Results from such studies show a more or less uniform outcome with a poor prognosis and a high 5-year mortality of 50% and higher among patients with mostly severe COPD followed at hospitals (Citation[1], Citation[2]) and somewhat lower when the patient cohorts are managed in general practice (Citation[3]).
The large under diagnosis of COPD with about only one out of three or four of all subjects fulfilling diagnostic criteria of COPD identified by the health care system (4–6) complicate assumptions based on patient registers of long term outcomes of COPD in society. As a consequence, death certificates with COPD as the underlying cause of death is underreported (Citation[7]) and may contribute to the large proportion of other causes than respiratory (Citation[8]) of death in COPD. There are still few publications about long term follow-ups including mortality of subjects with COPD identified in epidemiological studies of the general population (9–12).
The Obstructive Lung Disease in Northern Sweden (OLIN) Studies started in 1985 (Citation[13]). The first modern guidelines for COPD were developed from 1995 to 1997 (14–16), and we have reported the results of the prevalence of COPD from studies performed in 1996 (Citation[6]) and prevalence by disease severity (Citation[17]). Later on the cumulative incidence of COPD among symptomatic subjects (Citation[18]) and the incidence of COPD among middle-aged and elderly in the general population have been studied (Citation[19]), as well as the societal costs of COPD in a general population study setting (Citation[20]).
The subjects fulfilling the spirometric criteria of COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) and the American Thoracic Society/European Respiratory Society Standards for COPD (Citation[21], Citation[22]) in our first study sample from 1985 of the general population have retrospectively been identified using spirometric data collected in 1986. The aim of this study was to analyze the outcome of COPD from 1986 to 2006 in terms of survival and mortality by age, gender, smoking habits and lung function at entry. Further, change in lung function over time and progression of disease severity was studied as well as risk factors for mortality with multivariate analyses using Cox regression.
MATERIAL AND METHODS
Study sample
In 1985 a postal questionnaire survey was performed in an age-stratified sample of 6610 subjects, response rate 86%, in the northern most county of Sweden covering one-fourth of the area of Sweden (Citation[13]). Subjects reporting respiratory symptoms suggesting asthma or chronic bronchitis and a randomly selected sample of subjects with no suspicion of these diseases were invited to clinical validation studies in 1986 including mainly a structured interview and a lung function test. In subjects with obstruction defined as FEV1/VC < 0.7 or FEV1 < 80% of predicted values, a reversibility test was performed. Of a total of 1506 subjects (91%) of those who were invited actually participated (Citation[23]). Of the participants, 266 subjects fulfilled the spirometric criteria of COPD according to GOLD guidelines.
Among the 266 subjects, 126 (47%) had been classified as having chronic bronchitis according to the WHO-criteria for chronic bronchitis, while 84 (32%) may have had a concomitant asthma, as in 1986 they either reported that they had asthma, had a positive reversibility test (increase > 15% in FEV1 from baseline), or were hyper-reactive to methacholine. Also these 84 subjects labeled as “asthma” are included in the analyses irrespectively of whether they had real asthma or not, as asthma and COPD may co-exist. Of the 266 subjects, all but 17 reported chronic or recurrent respiratory symptoms.
Follow-up surveys
Follow-up studies of the 1506 subjects were performed in 1996 and included spirometry (Citation[18], Citation[24]). The third survey of the cohort including spirometry was performed in 2003. The subjects who participated in the postal questionnaire survey in 1985 were followed-up by using the same questionnaire in 1992 and 1996 (Citation[25], Citation[26]). In 1996 the prevalence of COPD was studied using spirometry including reversibility testing and a structured interview in a random sample of 1500 questionnaire responders (Citation[6]), and they were followed-up in 2003 (Citation[19]). Time points of deaths were given by the population register of Norrbotten's local health authority.
Spirometry
At all examinations the same dry spirometer, the Dutch Mijnhardt Vicatest 5, was used. At examinations daily calibrations were performed. The ATS recommendations for spirometry were followed at all surveys with some exceptions; the subjects have been standing at the examinations with a few exceptions, and nose clip was not regularly used (Citation[27]). Forced vital capacity (FVC) maneuvers were performed at least 3 times at every examination, and the difference between the 2 best FVC and 2 two best FEV1 values had to be < 5%, or < 1 dl in case the values were < 2 liters. Up to 6 measurements were performed at each examination. Further, slow vital capacity (SVC) was also measured regularly. When calculating the ratio between FEV1 and vital capacity (VC), the highest value of FVC and SVC was used as the denominator. Swedish normal values (Citation[28]) that conform well to the population in the study area were used (Citation[23]).
Severity staging of COPD according to GOLD (Citation[21]), which conforms closely to the ATS/ERS Standards for COPD (Citation[22]) were used:
No COPD: FEV1/FVC ≥ 0.7,
Stage 1 COPD: FEV1/FVC < 0.7; FEV1 > 80% of predicted values,
Stage 2 COPD: FEV1/FVC < 0.7; FEV1 < 80% and ≥ 50% of predicted values;
Stage 3 COPD: FEV1/FVC < 0.7; FEV1 < 50% and ≥ 30% of predicted values; and
Stage 4 COPD: FEV1/FVC < 0.7; FEV1 < 30% of predicted values.
Questionnaire
The same basic questions were included in the interview questionnaires used at each survey (Citation[23]). The questionnaire was expanded in 1996 and 2003, and has been used also in comparative studies between Sweden, Finland and Estonia, the FinEsS-studies (Citation[29]). Questions about respiratory symptoms, heart disease and other co-morbidities, smoking habits, socioeconomic status and occupation were surveyed similarly at all surveys. Data about family history of obstructive airway disease, i.e., asthma and/or chronic bronchitis and/or emphysema was based on the initial self-administrated questionnaire (Citation[13]).
Ethical approval
The initial postal survey in 1985 and the three later performed surveys including clinical measures have each been approved by the Ethics Committee at the University and the University Hospital of Northern Sweden in Umeå.
Analyses
Death over time has been expressed using Kaplan–Meier curves in the total material as well as stratified by age group. Survival in subjects fulfilling criteria for chronic bronchitis in 1986 have been compared with survival among subjects with COPD also having an asthmatic component in their disease. Determinants of mortality were calculated by Cox regression analysis, and risk factors of death have been expressed with Hazard ratios (HR) using 95% confidence intervals (CI) for statistical significance. Age, sex, lung function (FEV1), smoking habits, heart disease divided in ischaemic heart disease and other heart diseases, family history of obstructive airway disease (i.e., asthma, COPD, chronic bronchitis or emphysema) among parents and siblings, and socioeconomic status at entry have been used as independent variables. Age and FEV1 have been used both as continuous variables and categorical variables, i.e., age group and COPD stage according to GOLD. In univariate analyses the chi2-test was used. All analyses were made using the Statistical Package for Social Science (SPSS) software version 14.5.
RESULTS
Baseline characteristics
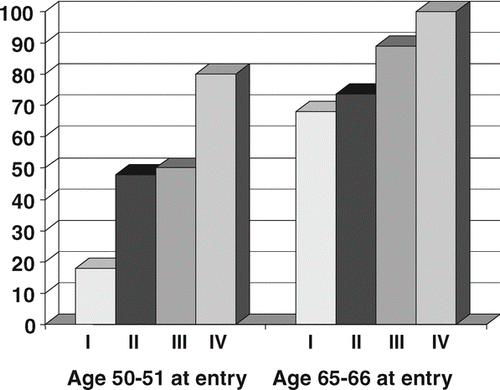
Of the 266 subjects fulfilling the spirometric GOLD criteria for COPD, 171 (64%) were men and 95 were women. By age at entry in 1986, 55% belonged to age group aged 65–66 years (in 1986), 32% to middle age group of 50–51 year olds, and 13% or 35 subjects belonged to the youngest age group aged 35–36 years (). Current smokers constituted 46%, ex-smokers 30%, and 24% were non-smokers at base line. Of men with COPD, 15% were non-smokers, while 39% of women fulfilling the spirometric criteria of COPD reported they never had been smokers (p < 0.001). Ten percent had had cardiac infarction or heart failure, while 25% reported having or having had a heart disease at entry. Heart disease was strongly correlated with increasing age, and 55 out of 66 subjects with heart disease came from the oldest age group ().
Table 1 Baseline characteristics of the COPD cohort in 1986 by age and sex
Half of the subjects with COPD had GOLD severity stage II, and 14% belonged to GOLD stage III or IV. The distribution by severity was similar in men and women (). The distribution of smoking habits by severity of COPD showed that ex-smokers comprised a majority of subjects with severe and very severe COPD (GOLD III and IV). Among all subjects, mean FEV1 was 70.7% of predicted at entry, and there were no major differences between men and women or between the two oldest age groups, while FEV1 was slightly higher in the youngest age group ().
Table 2 Deaths in relation to anthropometric data, severity of COPD and mean FEV1 in percent of predicted values
Mortality by age, sex and severity of COPD
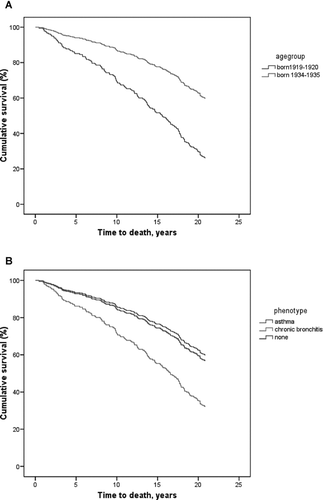
Of the 266 subjects, 144 (54%) had died, while 122 were still alive in January 2006. The survival curve showed an accelerating decline by year of follow-up. This was true particularly in the oldest age group (). Before January 1997, 63 subjects had died. From 1997 to January 2004 a further 56 subjects died, and thereafter from January 2004 to January 2006 an additional 25 subjects had died ().
Figure 1 Kaplan-Maiyer curves expressing survival in subjects with COPD over 20 years by age-group (a), and in subjects with either chronic bronchitis, asthma-like phenotype, or without either chronic bronchitis and asthma-like phenotype (b). Sex, age-group (not in ), COPD severity stage, smoking habits, socioeconomic status and atopy were used as covariates.
The large majority of the deceased subjects belonged to the oldest age group (75%), while only one subject in the youngest age group had died (). Of all men, 57% had died versus 48% of all women (ns). The mortality was significantly higher among manual workers. Disease severity at entry based on GOLD stages was highly related to death (). Two subjects out of twelve in GOLD stage IV were still alive after 20 years. Of those in GOLD stage III 79% had died, of those in stage II 57%, while 41% had died of those with GOLD stage I at entry in 1986 (). About half were still alive in 2006 of those in the age group of 50-51 year olds in 1986 with GOLD stage II and III.
Mortality by FEV1
Initial FEV1 in 1986 was significantly related to death. Of those who died during the 20 years of follow-up, the initial FEV1 in 1986 was 66% of predicted versus 76% among those who still were alive in 2006 (p < 0.001). The subjects who survived kept their lung function relatively stable and had a close to stable mean FEV1 in percent of predicted values measured at all three surveys. Among those who died, mean FEV1 continuously decreased more or less rapidly (). Among all subjects who survived until 1996, the mean annual decline in FEV1 from 1986 to 1996 was limited, or 24 ml/y. The mean decline from 1996 to 2003 among those who also survived that period was 36 ml/y.
Symptoms and conditions in relation to death
Chronic respiratory symptoms were significantly more common at baseline among those who died during the follow-up period. On the other hand, rhinitis had a protective effect and was more common among the survivors. The same was found with concomitant asthma (). BMI was not related to death.
Table 3 Deaths in relation to heart disease, family history of obstructive airways disease (OAD), atopy, and chronic respiratory symptoms
Risk factors for death
Increasing age and initial lung function were both significantly related to death. Increased age year by year yielded a hazard ratio (HR) of 1.085 (95% CI 1.059–1.112). A decrease in initial FEV1 of one percent unit of predicted value increased the risk of death with HR 1.023 (95% CI 1.015–1.032).
When using age and lung function as categorical variables, the impact of age group and severity stage according to GOLD is apparent. As shown by figures, the effect of age on mortality increased with increasing age (, Table 5b). The age group 50–51 years yielded a HR of 14.99, and the HR for the age group 65–66 years at entry was 37.17, both highly and significantly related to death. When using the GOLD severity stage, instead of using FEV1, as independent categorical variables with GOLD I as reference, GOLD stage II and III increased the risk of death with HR 1.52 and HR 2.53, respectively, while GOLD stage IV increased the risk with HR 7.29 ().
Table 4 Risk factors for death by Cox regression; Age (age group) and FEV1 (GOLD severity stage) as categorical variables
Cardiac infarction and/or heart failure were highly related to death, HR 3.16 (95% CI 1.94–5.16), while heart diseases other than cardiac infarction or heart failure were not significantly related to death (). Further, as expected, male sex was a risk factor for death during the 20 years' follow-up time.
Smoking habits at entry were not significantly related to death. However, the majority of those with severe and very severe COPD at entry had already quit smoking before 1986. Socioeconomic status was not statistically significant in the multivariate model as a cause of death.
When comparing subjects with an asthma-like phenotype (n = 84), the mortality among them was significantly lower compared with the 123 subjects who fulfilled the criteria of chronic bronchitis at entry. This difference remained also after correction of the influence of smoking habits and severity grade of COPD and the other covariates used in the analyses. Non chronic bronchitis –non asthma-like phenotype had a survival curve similar to the asthma-like phenotype ().
DISCUSSION
In comparison with data of hospitalized subjects with COPD (Citation[1],Citation[2]) and subjects with COPD identified by the health care system (Citation[30]), our study found a considerably better survival among the subjects with COPD identified by an epidemiologic population study. Although our study confirms the poor prognosis of COPD, 46% of the subjects with COPD were still alive after the 20-year follow-up period. A large majority of subjects having severe or very severe COPD at entry according to the GOLD criteria had died, however, a surprisingly large proportion of them, 19%, were still alive after the 20-year follow-up period. The survivors had a normal or even low annual decline in lung function. As expected, mortality was highly associated with age and disease severity at entry. Also ischemic heart disease and heart failure at entry were highly related to death during the follow-up period.
How common is death from COPD? The very large under diagnosis of COPD, which probably is the case in most countries (4–6), contributes to uncertainty, and register database on death certificates may thus give underestimated results. According to death certificates, the number of causes of death due to COPD in Sweden amounted to about 2500 in 2005 corresponding to 2–3% of all deaths, and it is now similar in men and women after a continuous increase in women, while deaths among men due to COPD have reached a plateau (Citation[31]). In Denmark, a 25-year follow-up within the Copenhagen City Heart Study found COPD responsible for 3.7% of all deaths according to death certificates (Citation[11]).
Lower figures have been published from France. During the same time period COPD was the underlying cause of only 1.4% of all deaths, while COPD was mentioned in 3.0% on the death certificates (Citation[32]). In England and Wales during the 1990s “obstructive lung disease,” including both COPD and asthma, was mentioned as a contributing cause of death in 8% of all death certificates (Citation[33]). Also in the US, obstructive lung disease was listed on death certificates in 8%, and among them 43% was recorded as the underlying cause of death (Citation[7]). Interestingly, similar to the United Kingdom (Citation[5]) and Sweden (Citation[31]), the number of women dying from COPD in the year 2000 surpassed the corresponding number of men (Citation[34]).
The relatively low mortality of COPD in Sweden can be explained by a lower proportion of smokers than in most other countries, including westernized countries. In 1985 about 30% of both men and women were current smokers in our study area (Citation[13]), while in 2006 17% of the adult population (women 20%; men 14%) smoked at least once a week. Over the past 5 to 10 years, the mortality caused by COPD has reached a plateau among men, while there is a continuous increase in women (Citation[31]).
There are some large population studies allowing comparison with our results. Two of them are from the USA. Analyses of death within the large US National Health and Nutrition Survey (NHANES) have resulted in strikingly similar results (Citation[10]). After a median follow-up time of 18 years (maximum 22 years) the mortality in subjects with severe and very severe COPD (GOLD) was 71% versus 81% in our study. When comparing COPD defined as GOLD stage ≥ II, a prevalence of COPD of 8% was found both in northern Sweden (Citation[6]) and in a later phase of the NHANES (Citation[4]). Further, both studies found that up to 50% of smokers sooner or later develop COPD (Citation[6], Citation[35]). The proportion of deaths in our study also corresponds fairly well with another U.S. study, the Cardiovascular Health Study, which included subjects ≥ 65 years at baseline with 1292 subjects (26%) fulfilling spirometric criteria of COPD defined as GOLD stage ≥ II (Citation[12]). Among those subjects, death occurred in 34% over a 7-year period versus 75% during 20 years in our study among subjects aged 65–66 years at entry.
Some large COPD studies conducted as clinical trials and their follow-ups provide important mortality data of COPD. Moderate to severe COPD (FEV1 < 60% of predicted values) was studied over three years in the TORCH trial (Citation[36]). Mortality was related to disease severity based on lung function and was overall 14%. The ISOLDE study performed in the UK had included subjects with severe COPD (FEV1 < 50% of predicted values) and 375 subjects or a half of the participating subjects could be analysed in a 13 year follow-up (Citation[37]). The mortality of 56% is in line with ours, taking follow-up time and disease severity at baseline into account. Long term conclusions are not yet ready to be taken from the TORCH study. Follow-up surveys of mild-to-moderate COPD clinical trials have also been performed. The U.S. Lung Health Study found the mortality over 14.5 years to be 12%, a result reflecting the relatively mild disease with FEV1 55–90% of predicted values and age ≤ 60 years at entry (Citation[38]).
When studying mortality in COPD, the risks factors for death vary considerably depending on the age composition of the subjects under study and the severity of COPD. Further, when studies are limited to subjects with COPD, the risk factor pattern appear different from studies based on representative samples of the general population. In population studies smoking appears to be a major risk factor yielding a great impact both on development of COPD and mortality in subjects with COPD (Citation[11], Citation[39]). Correspondingly, outdoor air pollution and airborne occupational exposures contribute to both development and death in COPD (39–42), however, yielding risk estimates generally lower than risks attributed to smoking. Other important risk factors for death include cardiovascular disease (Citation[38], Citation[43]) and co-morbid conditions with both bronchial and systemic inflammation. For instance, increases in concomitant chronic bronchitis and the risk of death in COPD (Citation[44]). Further, among several other respiratory conditions, exacerbations of COPD (Citation[30]) and rapid decline in lung function (Citation[12]) are both markers of increased risk for death. Family history of obstructive airway disease is associated with development of COPD (Citation[6]); however, its impact on a severe outcome has not so far been evaluated.
When the study is limited to subjects having COPD, the impact of smoking cannot be sufficiently evaluated. For instance in our study and the follow-up of the ISOLDE study (Citation[37]), both studies of similar size and of similar follow-up time of about 20 years including only subjects with COPD, smoking did not appear as a significant risk factor for death. In our study most subjects with severe and very severe COPD were ex-smokers already at baseline. Similar risk factors for death appeared in both the ISOLDE follow-up and in our study, and included male sex, increasing age, and a low lung function at entry, however, co-morbid conditions were not analysed in the ISOLDE study. In our study also ischemic heart disease and/or heart failure was significantly related to death. BMI was not related to death in our study, possibly due to the low number of subjects with severe disease and socioeconomic status failing to reach significance in the multivariate model. As expected, male sex was a significant risk factor for death.
Also, causes of death among subjects with COPD differ considerably depending on disease severity and the age composition of the studied samples. Generally, cardiovascular disease, cancer and respiratory disease are the three most common causes of death in COPD. In severe COPD, respiratory causes dominate, in TORCH they comprised 52%, and in the ISOLDE follow-up 38% (Citation[36], Citation[37]), while in mild COPD among middle-aged subjects in the U.S. Lung Health Study, cancer was the most common cause of death, 32%, and in contrast a respiratory cause accounted only for 8% (Citation[38]). Among elderly with mild-to-moderate COPD, cardiovascular diseases appear often as cause of death (Citation[43]). We have not yet explored the causes of death in detail. We have found a large discrepancy between statistics based on death certificates and the data we find from the ongoing population studies. Piloting so far suggests cardiovascular disease to be the most common cause of death in our study, but the impact of respiratory cause of death in COPD seems to be largely underestimated in death certificates.
There are several strengths in our study. The participation has been high over the 20-year observation period. In the initial postal survey, 86% participated (Citation[13]), and in the follow-up surveys including spirometry, the participation among the subjects possible to trace has been close to 90% (Citation[6], Citation[17], Citation[18], Citation[19], Citation[20], Citation[23], Citation[24]). The results of the initial lung function measurements have been confirmed, as the subjects have been followed over time with spirometry. Thus, occasionally measured low values at entry cannot explain the good prognosis among the one fifth of subjects with severe COPD at entry. Further, the same research team have with few exceptions performed the lung function tests over the 20 years. The internal validity must thus be judged as high. When calculating risks for death, our study has limitations. As the study included only subjects with COPD, there are no real possibilities to study risk factors that are causal for COPD, such as smoking. The limitation allows risk estimates only between different severity grades of COPD, and in calculations of risks we have used GOLD stage I as reference.
We can conclude that COPD identified by population studies have a considerably better long term prognosis than found by follow-up studies of general practice and hospital based cohorts. Our results are in line with the so far few other longitudinal population based studies including outcomes of COPD. The main reason to the better outcome is the greater proportion of subjects with mild and moderate disease. Early detection is important, as it opens for preventive measures aimed at slowing down disease progression. The long follow-up time points out that even subjects with severe COPD may have a good prognosis. Pooling of data from several cohorts is in progress aiming to identify positive prognostic factors in severe and very severe COPD. Negative prognostic factors are already fairly well identified.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGMENTS
The authors thank Mai Lindström, SRN, PhD and Karin Lundbäck, SRN, MSc, who performed the lung function measurements in 1986, and further the statisticians Lennarth Nyström, PhD, and Elsy Jönsson, MSc for constructing the OLIN Studies' database, and Ola Bernhoff, MSc, for organizing the computerization of data at all surveys. Swedish Heart-Lung foundation is gratefully acknowledged for financial support over years. Further, the authors thank University of Umeå and Norrbotten's and Northern Sweden's local health authorities for support. Additional funding was received from GlaxoSmithKline World Wide Epidemiology, which is hereby acknowledged.
REFERENCES
- Incalzi R A, Fuso L, De Rosa M, Forastiere F, Rapiti E B, Nardecchia B, Pistelli R. Co-morbidity contributes to predict mortality of patients with chronic obstructive pulmonary disease. Eur Respir J 1997; 10: 2794–2800
- Martinez F J, Foster G, Curtis J L, Criner G, Weinmann G, Fishman A, et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 2006; 173: 1326–1334
- Soriano J B, Vestbo J, Pride N B, Kiri V, Maden C, Maier W C. Survival in COPD patients after regular use of fluticasone propionate and salmeterol in general practice. Eur Respir J 2002; 20: 819–825
- Mannino D M, Gagnon R C, Petty T L, Lydick E. Obstructive lung disease and low lung function in adults in the United States. Data from the National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med 2000; 160: 1683–1689
- Soriano J B, Maier W C, Egger P, Visik G, Thakrar B, Sykes J, Pride N B. Recent trends in physician diagnosed COPD in women and men in the UK. Thorax 2000; 55: 789–795
- Lundbäck B, Jönsson E, Jonsson A-C, Rönmark E, Lindström M, Lindberg A, et al. Not fifteen but fifty percent of smokers develop COPD—report from the Obstructive Lung Disease Studies in Northern Sweden. Respir Med 2003; 97: 115–122
- Mannino D M, Brown C, Giovino G A. Obstructive lung disease deaths in the United States from 1979 through 1993. An analysis using multiple-cause mortality data. Am J Respir Crit Care Med 1997; 156: 814–818
- Halpin D. Mortality in COPD: inevitable or preventable? Insights from the cardiovascular arena. COPD 2008; 5: 187–200
- Schunemann H J, Dorn J, Grant B, Winklestein W, Trevisan M. Pulmonary function is a long term predictor of mortality in the general påopulation. 29-year follow-up of the Buffalo Health Study. Chest 2000; 118: 656–664
- Mannino D M, Buist A S, Petty T L, Enright P L, Redd S C. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax 2003; 58: 388–393
- Lökke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: a 25 year follow up study of the general population. Thorax 2006; 61: 935–939
- Mannino D M, Davis K J. Lung function decline and outcomes in an elderly population. Thorax 2006; 61: 472–477
- Lundbäck B, Nyström L, Rosenhall L, Stjernberg N. Obstructive lung disease in northern Sweden: respiratory symptoms assessed in a postal review. Eur Respir J 1991; 4: 257–266
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995; 152(S)77–120
- Siafakas N M, Vermeire P, Pride N B, Paoletti P, Gibson J, Howard P. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). Eur Respir J 1995; 8: 398–420
- British Thoracic Society. BTS Guidelines for the management of chronic obstructive pulmonary disease. Thorax 1997; 52(S5)1–28
- Lindberg A, Bjerg-Bäcklund A, Rönmark E, Larsson L G, Lundbäck B. Prevalence and under diagnosis of COPD by disease severity and the attributable fraction of smoking – Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med 2006; 100(2)264–272
- Lindberg A, Jonsson A-C, Rönmark E, Lundgren R, Larsson L-G, Lundbäck B. Ten-year cumulative incidence of COPD and risk factors for incident disease in a symptomatic cohort. Chest 2005; 127: 1544–1552
- Lindberg A, Eriksson B, Larsson L G, Rönmark E, Sandström T, Lundbäck B. 7-year cumulative incidence of COPD in an age-stratified general population sample. Chest 2006; 129: 879–885
- Jansson S-A, Lindberg A, Ericsson Å, Borg S, Rönmark E, Andersson F, Lundbäck B. Cost differences for COPD with and without physician-diagnosis. COPD 2005; 3: 427–434
- Rabe K F, Hurd S, Anzueto A, Barnes P J, Buist S A, Calverley P, Fukuchi Y, Jenkins C, Rodriquez-Roisin R, van Weel C, Zielinski J. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. GOLD Executive Summary. Am J Respir Crit Care Med 2007; 176: 532–555
- Celli B, MacNee W, committee members. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23: 932–946
- Lundbäck B, Stjernberg N, Nyström L, Forsberg B, Lindström M, Lundbäck K, et al. Epidemiology of respiratory symptoms, lung function and important determinants. Report from the Northern Sweden Obstructive Lung Disease Project. Tuber Lung Dis 1994; 75: 116–126
- Lindberg A, Larsson L-G, Rönmark E, Jonsson A-C, Larsson K, Lundbäck B. Decline in FEV1 in relation to incident chronic obstructive pulmonary disease in a cohort with respiratory symptoms. COPD 2007; 4: 5–13
- Rönmark E, Lundbäck B, Jönsson E, Jonsson A-C, Lindström M, Sandström T. Incidence of asthma in adults—report from the Obstructive Lung Disease in Northern Sweden study. Allergy 1997; 52: 1071–1078
- Rönmark E, Jönsson E, Lundbäck B. Remission of asthma in middle-aged and elderly - report from the Obstructive Lung Disease in Northern Sweden Study. Thorax 1999; 54: 611–613
- American Thoracic Society. Snowbird workshop on standardization of spirometry. Am Rev Respir Dis 1979; 119: 831–838
- Berglund E, Birath G, Grimby G. Spirometric studies in normal subjects, forced expirograms in subjects between 7 and 70 years of age. Acta Med Scand 1963; 173: 185–192
- Pallasaho P, Lundbäck B, Läspä S-L, Jönsson E, Sovijärvi A, Laitinen L. Increasing prevalence of asthma but not chronic bronchitis in Finland—Report from the FinEsS-Helsinki study. Respir Med 1999; 93: 798–809
- Soler-Cataluña J J, Martínez-García M A, Sánchez P R, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60: 925–931
- Statistics Sweden, 1995, 1996, 1997, 1998, 1999, 2000, 2001, 2002, 2003, 2004, 2005. Causes of death, www.scb.se
- Fuhrman C, Jougla E, Nicolau D, Eilstein D, Delmas M C. Deaths from chronic obstructive pulmonary disease in France, 1979–2002: a multiple cause analysis. Thorax 2006; 61: 930–934
- Hansell A L, Walk J A, Soriano J B. What do chronic obstructive pulmonary disease patients die from? A multiple cause coding analysis. Eur Respir J 2003; 22: 809–814
- Mannino D M, Homa D M, Akinbami L J, Ford E S, Redd S C. Chronic obstructive pulmonary disease surveillance – United States, 1971–2000. Respir Care 2002; 47: 1184–1199
- Stang P, Lydick E, Silberman C, Kempel A, Keating E. The prevalence of COPD. Using smoking rates to estimate disease frequency in the general population. Chest 2000; 117(S)354–359
- Calverley P M, Anderson J A, Celli B, Ferguson G T, Jenkins C, TORCH investigators, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356: 777–789
- Bale G, Martinez-Camblor P, Burge P S, Soriano J B. Long-term mortality follow-up of the ISOLDE participants: Causes of death during 13 years after trial completion. Respir Med 2008; 102: 1468–1472
- Anthonisen N R, Skeans M A, Wise R A, Manfreda J, Kanner R E, Connett J E. The effects of a smoking cessation intervention on 14.5-year mortality. A Randomized Clinical Trial. Ann Intern Med 2005; 142: 233–239
- Kauffmann F, Annesi I, Chwalow J. Validity of subjective assessment of changes in respiratory health status: a 30 year epidemiological study of workers in Paris. Eur Respir J 1997; 10: 2508–2514
- Bakke P S, Hanoa R, Gulsvik A. Educational level and obstructive lung disease given smoking habits and occupational airborne exposure: a Norwegian community study. Am J Epidemiol 1995; 141: 1080–1088
- Bergdahl I A, Torén K, Eriksson K, Hedlund U, Nilsson T, Flodin R, Järvholm B. Increased mortality in COPD among construction workers exposed to inorganic dust. Eur Respir J 2004; 23: 402–406
- Pope C A, Ezzati M, Dockery D W. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med 2009; 360: 376–386
- Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest 2005; 128: 2640–2646
- Ekberg-Aronsson M, Pehrsson K, Nilsson JÅ, Nilsson P M, Löfdahl C G. Mortality in GOLD stages of COPD and its dependence on symptoms of chronic bronchitis. Respir Res 2005; 6: 98, doi:10.1186/1465-9921-6-98
- Kotaniemi J T, Sovijärvi A, Lundbäck B. Chronic Obstructive Pulmonary Disease in Finland: Prevalence and risk factors. COPD 2005; 3: 331–339