Abstract
Background/objective
To compare the efficacy of budesonide/formoterol (BF) versus fluticasone/salmeterol (FS) in patients with moderate-to-severe chronic obstructive pulmonary disease (COPD).
Methods
The PubMed, Embase, Cochrane Library, and Web of Science databases were searched for studies comparing BF versus FS in the treatment of COPD from inception to July 17, 2023. Outcomes, including exacerbations, hospitalizations, pneumonia, emergency department (ED) visits for COPD, length of hospitalization, and number of exacerbations, were compared using risk ratio (RR) with corresponding 95% confidence interval (CI) or weighted mean difference (WMD) with 95% CI. All statistical analyses were performed using Stata version 12.0.
Results
Ten studies comprising a total of 136,369 participants were included. Compared with those treated with FS, patients with COPD treated with BF experienced a reduced number of exacerbations (RR 0.91 [95% CI 0.83–1.00]; p = 0.040), hospitalizations (RR 0.77 [95% CI 0.67–0.88]; p < 0.001), and frequency of pneumonia (RR 0.77 [95% CI 0.64–0.92]; p = 0.05). However, no significant difference was observed between BF and FS in terms of ED visits for COPD (RR 0.87 [95% CI 0.69–1.10]; p = 0.243), length of hospitalization (WMD −0.18 [95% CI −0.62–0.27]; p = 0.437), and number of exacerbations (WMD −0.06 [95% CI −0.28–0.16]; p = 0.602). Notably, no significant heterogeneity was noted in length of hospitalization between the two groups, whereas clear heterogeneity was observed in other outcomes (I2 > 50%, p < 0.05).
Conclusion
Compared with FS, BF therapy appears to be a more promising treatment strategy for patients with moderate-to-severe COPD; however, this should be verified in further high-quality studies.
Background
Chronic obstructive pulmonary disease (COPD) is a common respiratory tract disease, accounting for 0.5 billion cases globally, and is the third leading cause of death worldwide due to its fatal pathology [Citation1]. Generally, in patients with COPD, exacerbation is the leading cause of substantial impairment in quality of life and respiratory ability and may even cause death. Therefore, effective prevention and management strategies for COPD are urgently required.
Conventionally, combination therapy involving inhaled long-acting β2-agonist (LABA) and a long-acting muscarinic antagonist (LAMA) or inhaled corticosteroid(s) (ICS) has been the general recommendation for COPD [Citation2]. In fact, according to the 2009 and 2010 GOLD reports, regular treatment with LABAs, ICS, and/or their combination is recommended to improve decline in lung function. Moreover, a report from 2017 reported that patients with a low rate of exacerbation commonly used LABA/LAMA combinations. In these patients, LABA/ICS treatment should be changed to LABA/LAMA therapy if ICS leads to increased side effects; however, the 2020 GOLD report suggests that triple combinations of LABA, ICS, and LAMA may reduce mortality in the most severe stages of COPD [Citation3]. Although the recent 2024 GOLD report discourages the use of LABA/ICS as a first-line treatment for COPD [Citation4], the efficacy of LABA/LAMA versus LABA/ICS still needs to be compared due to the large majority of medications for COPD being LABA/ICS.
In recent years, several different combinations of LABA/ICS and LABA/LAMA have been used clinically, including budesonide/formoterol (BF) and fluticasone/salmeterol (FS). Although the two combination products have different pharmacological properties, they exhibit similar benefits in terms of preventing the symptoms of COPD when combined at a fixed dose [Citation5]. Regarding improvements in COPD, a more rapid onset of action has been shown among patients after treatment using the BF combination compared with that after treatment using the FS combination, which may be due to the faster onset of formoterol than that of salmeterol; however, evidence also supports that fluticasone is 25% more potent than budesonide [Citation6]. Therefore, the effectiveness of these combination therapies for treating COPD exacerbations remains controversial.
To address this gap in knowledge, we conducted a literature search to identify studies comparing the efficacy of BF versus FS in the treatment of COPD. Outcomes, including exacerbations, hospitalization(s), pneumonia, emergency department (ED) visits for COPD, length of hospitalization, and number of exacerbations, were compared using risk ratio (RR) with their corresponding 95% confidence interval (CI) or weighted mean differences (WMD) with 95% CI. BF treatment improved exacerbations, hospitalization, and pneumonia in patients with moderate-to-severe COPD.
Methods
Literature search
The current meta-analysis comparing BF and FS combination treatments for COPD was conducted in accordance with the principles of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (i.e. “PRISMA”) statement.
The PubMed, Embase, Cochrane Library, and Web of Science databases were searched for studies comparing BF and FS combination treatments for COPD from inception to July 17, 2023. The search was performed without language restrictions using the following key words: (“budesonide formoterol fumarate drug combination” OR “Budesonide” OR “formoterol” OR “fluticasone salmeterol drug combination” OR “fluticasone” OR “salmeterol”) AND “chronic obstructive pulmonary disease.” The reference lists of the retrieved articles were manually searched for additional relevant and potentially eligible studies.
Eligibility criteria
Studies fulfilling the following inclusion criteria were selected: patients diagnosed with COPD were included; a comparison of outcomes between the BF and FS groups was conducted; and data regarding exacerbations, hospitalizations, pneumonia, ED visits for COPD, length of hospitalization, and number of exacerbations were reported.
Articles, including reviews, conference abstracts, comments, and self-controlled studies were excluded. Moreover, for duplicate publications, only those with the most complete research information were included, while excluding all others.
Data selection and quality assessment
The following data were extracted from the included studies using a predesigned table: first author, year of publication, number and age of enrolled subjects, diagnostic criteria, study type, and outcomes of COPD. Data extraction was performed by two investigators who independently reviewed the full texts. A third investigator checked all the data and resolved any disagreements. Full-text studies fulfilling the inclusion criteria were selected for further analysis.
The methodological quality of the non-randomized clinical studies was evaluated using the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) tool [Citation7], whereas the quality of the randomized controlled studies was evaluated using the Cochrane Risk of Bias Assessment Tool [Citation8].
Statistical analysis
For categorical variables, the RR and corresponding 95% CI were calculated to assess the risk of BF compared to that of FS. In contrast, for continuous variables, WMD with corresponding 95% CI was calculated. Heterogeneity among the included studies was assessed using the chi-square test and I2 statistic [Citation9]. Studies with p < 0.1 or I2 > 50% were defined as having clear heterogeneity, and the random-effects model was adopted [Citation10]; in contrast, a p > 0.1 or I2 < 50% indicated non-significant heterogeneity among studies, for which the fixed-effects model was used.
Egger’s test was used to evaluate publication bias between studies. Additionally, sensitivity analysis was performed by omitting one study at a time to evaluate the impact of individual studies on the merged results. All statistical analyses were performed using Stata version 12.0 (StataCorp LLC, College Station, TX, USA).
Results
Studies included in the meta-analysis
The initial literature search retrieved 670 articles, of which 185 were duplicates and excluded, leaving 485 articles (). After reviewing titles and abstracts, 471 additional articles were excluded. Moreover, after reading the full texts, four articles were removed, including three without control groups and one review. Ultimately, 10 studies were included in the final analysis [Citation11–20]. Details of the search strategy and study retrieval from the PubMed, Embase, Web of Science, and Cochrane Library databases are summarized in Supplementary Tables 1–4.
Table 1. Characteristics of the included studies in this meta-analysis.
Table 2. Outcomes of the sensitivity analysis and test of publication bias.
Characteristics of the included studies
In total, 10 studies were included in the meta-analysis, including one RCT [Citation13], one prospective cohort study [Citation15], and eight retrospective cohort studies [Citation11,Citation12,Citation14,Citation16–20]. These studies were conducted in Asia, Europe, and North America, with sample sizes ranging from 60 to 86,224. In total, 136,369 subjects were enrolled in these studies, including 46,780 in the BF group and 89,589 in the SF group, with most included studies having a follow-up of ≥1 year. Notably, no significant differences were noted in age or sex between the two groups. The characteristics of the included studies are summarized in .
Quality evaluation results of the RCT are summarized in Supplementary Table 5. The included study did not describe relevant information hidden in the allocation and was conducted in a single-blind manner. Therefore, there was a moderate risk of selection, performance, and detection biases. Low-quality data was defined based on published data and random sequence generation.
The quality evaluation results of the cohort studies are summarized in Supplemental Table 6. The results revealed that these included studies had moderate bias in confounding factors, intervention, and selective reporting, and low quality was observed for selection bias, classification of interventions, missing data, and outcome(s) measurement. However, overall, moderate methodological quality was observed in the included studies.
Comparison of BF vs. FS in terms of exacerbations, hospitalizations, pneumonia, and ED visits for COPD
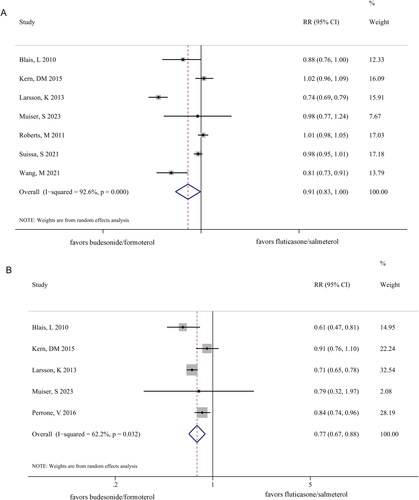
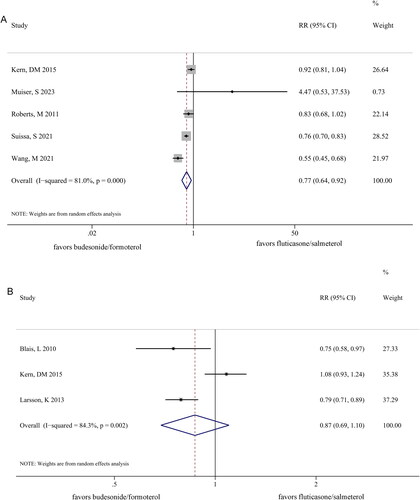
After treatment with BF, patients with COPD exhibited greater improvement in exacerbations (RR 0.91 [95% CI 0.83–1.00]; p = 0.040) (), hospitalizations (RR 0.77 [95% CI 0.67–0.88]; p < 0.001) (), and pneumonia (RR 0.77 [95% CI 0.64–0.92]; p = 0.05) () than those treated with FS. However, no significant differences were observed between the BF and FS groups in terms of ED visits for COPD (RR 0.87 [95% CI 0.69–1.10]; p = 0.243) (). Notably, a clear heterogeneity was observed between the two groups in terms of exacerbations (I2 = 92.6%, p = 0.000), hospitalization (I2 = 62.2%, p = 0.032), pneumonia (I2 = 81.0%, p = 0.000), and ED visits for COPD (I2 = 84.3%, p = 0.002).
Comparison of BF vs. FS in terms of length of hospitalization and number of exacerbations
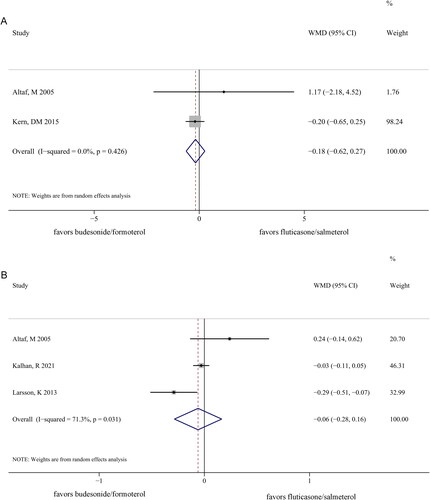
No significant difference was observed in length of hospitalization (WMD −0.18 [95% CI −0.62–0.27]; p = 0.437) () and number of exacerbations (WMD −0.06 [95% CI −0.28–0.16]; p = 0.602) between the two groups (). Additionally, no significant heterogeneity existed between the two groups in terms of the length of hospitalization (I2 = 0%, p = 0.426). However, clear heterogeneity was noted between the two groups in terms of the number of exacerbations (I2 = 71.3%, p = 0.031).
Sensitivity analysis and publication bias
In total, only two studies addressed the length of hospitalization; therefore, sensitivity analysis and Egger’s test could not be performed for this outcome. The results of the analysis of other outcome indicators are summarized in and Supplemental Figure 1A–E. Sensitivity analysis revealed that the pooled results for the risk of COPD exacerbation, ED visits for COPD, and pneumonia were unstable. After excluding individual studies, the combined results were reversed, and the risk of hospitalization for COPD and number of examinations were stable. Notably, Egger’s test revealed no significant publication bias when pooling the included studies (p > 0.05).
Discussion
Evaluation of human health in patients with COPD remains challenging due to the high morbidity and mortality associated with COPD. In the present analysis, the 10 studies including 136,369 patients with COPD were only of a moderate quality. When comparing patients treated with BF and those treated with FS, BF treatment resulted in improvement in exacerbations, hospitalizations, and pneumonia, whereas no significant differences were observed between the BF and FS groups in terms of ED visits for COPD, length of hospitalization, and number of exacerbations. Notably, clear heterogeneity was observed between the BF and FS groups in terms of all outcomes, except for length of hospitalization.
Exacerbation is the primary cause of morbidity and mortality in patients with COPD. Therefore, improvements in exacerbations, hospitalizations, and pneumonia are the main goals of preventive management of COPD. In patients with COPD, increased sputum production, dyspnea, and chest tightness would occur over several years, and the considerable impact of acute exacerbations on well-being and activities of daily living therefore cannot be ignored [Citation21]. Conventionally, ICSs combined with LABAs are recommended for preventing COPD exacerbation. In fact, previous studies have demonstrated that salmeterol and LABAs such as formoterol can significantly improve lung function [Citation22]. Moreover, when BF is used for treatment, a faster onset of the effects can be induced by formoterol [Citation23]. Therefore, the combination of steroids and β2-agonists may be the main components of BF treatment that are accountable for the improvement of lung function and severe exacerbations [Citation24]. Formoterol is a β2-agonist with long-acting and fast-acting bronchodilators, while salmeterol is a type of short-acting β2-agonist with long-acting roles. Notably, our data showed that treatment with BF was only slightly inferior to that with FS in terms of preventing exacerbations (RR 0.91 [95% CI 0.83–1.00]; p = 0.040). However, of the seven included studies, only two showed improved exacerbations in patients after BF treatment compared with that after FS treatment, whereas five studies showed no significant differences in exacerbations between the two groups. However, this might have been due to differences in the severity of COPD, medication adherence, and follow-up periods between the studies. Therefore, future multicenter prospective studies are warranted to better evaluate the effects of these factors.
One disadvantage of ICSs is that they are usually associated with side effects such as pneumonia in patients with COPD [Citation25]. However, Tricco et al. confirmed that 24 treatments for COPD, including BF and FS, were more effective than the placebo, although fluticasone is associated with a higher risk of pneumonia than is placebo [Citation26]. In contrast, a previous meta-analysis by Halpin et al. demonstrated that BF was associated with fewer pneumonia events [Citation27]. Similarly, we demonstrated a reduced risk of pneumonia with BF than with FS.
Observational studies have led to controversial conclusions regarding the comparative efficacy of BF versus FS. Previously, Muiser et al. demonstrated that fixed-dose therapy with BF and FS had similar efficacies in patients with COPD [Citation13]. However, in the present study, the doses of BF and FS varied across different medical centers, which may explain these controversial conclusions. However, the present meta-analysis included a relatively large sample size and wide region. Moreover, the methodological quality of the included studies was moderate and the risk of bias—such as loss to follow-up and reporting bias—was low. Therefore, collectively, our data clearly showed that BF treatment yielded greater benefits in improving the risk of exacerbations, hospitalizations, and pneumonia than FS treatment.
However, the present meta-analysis has several limitations. First, clear heterogeneity was observed between the two groups in the evaluation of exacerbations, hospitalizations, pneumonia, ED visits for COPD, and number of exacerbations. The effectiveness of these drugs can be affected by treatment length and dosing [Citation28]. Moreover, other factors such as the background of the enrolled patients, region, and research type may affect the efficacy of BF or FS. However, owing to the lack of effective information and significant differences in covariates, it was not possible to evaluate the impact of factors such as region and research type on the results through subgroup analysis and meta-regression. Second, most of the included studies were retrospective. Therefore, although confounding factors were controlled for in sample matching and multi-factor correction, the merged results may still be influenced by confounding factors such as disease course and medication dosage. Third, the combined results of some outcomes were unstable. Therefore, it is crucial to design and conduct higher-quality, large-scale, randomized controlled trials to verify the conclusions obtained in the present study.
In conclusion, our results suggest that BF is more effective than FS in the treatment of patients with moderate-to-severe COPD. However, further high-quality studies are required to confirm this conclusion.
Authors’ contributions
Yueping Jin designed the study. Nan Shang and Yang Liu performed literature searching and collected the literature. Nan Shang performed the statistical analysis. Nan Shang was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
ABBREVIATIONS:
| COPD | = | chronic obstructive pulmonary disease |
| BF | = | budesonide/formoterol |
| CI | = | confidence interval |
| ED | = | emergency department |
| FS | = | fluticasone/salmeterol |
| ICS | = | inhaled corticosteroid(s) |
| LABA | = | long-acting β2-agonist |
| LAMA | = | long-acting muscarinic antagonist |
| RCT | = | randomized controlled trial |
| RR | = | risk ratio |
| WMD | = | weighted mean difference |
Supplemental Material
Download TIFF Image (36.9 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data analyzed in this study was available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Szalontai K, Gémes N, Furák J, et al. Chronic obstructive pulmonary disease: epidemiology, biomarkers, and paving the way to lung cancer. J Clin Med. 2021;10(13):1. doi: 10.3390/jcm10132889.
- Umeda A, Shimada H, Yamane T, et al. Real-world effects of once-daily inhaled steroid (fluticasone furoate) combined with long-acting beta-2 agonist (vilanterol) and long-acting muscarinic antagonist (umeclidinium) on lung function tests of asthma patients in Japan. Front Physiol. 2023;14:1131949. doi: 10.3389/fphys.2023.1131949.
- Terry PD, Dhand R. Inhalation therapy for stable COPD: 20 years of GOLD reports. Adv Ther. 2020;37(5):1812–9. doi: 10.1007/s12325-020-01289-y.
- Venkatesan P. GOLD COPD report: 2024 update. Lancet Respir Med. 2024;12(1):15–16. doi: 10.1016/S2213-2600(23)00461-7.
- Dahl R, Chuchalin A, Gor D, et al. EXCEL: a randomised trial comparing salmeterol/fluticasone propionate and formoterol/budesonide combinations in adults with persistent asthma. Respir Med. 2006;100(7):1152–1162. doi: 10.1016/j.rmed.2006.03.001.
- Kelly HW. Establishing a therapeutic index for the inhaled corticosteroids: part I. Pharmacokinetic/pharmacodynamic comparison of the inhaled corticosteroids. J Allergy Clin Immunol. 1998;102(4 Pt 2):S36–S51. doi: 10.1016/s0091-6749(98)70004-1.
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919.
- Tarsilla M. Cochrane handbook for systematic reviews of interventions. J Multidiscip Eval. 2010;6(14):142–148. doi: 10.56645/jmde.v6i14.284.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557.
- Gu WJ, Wu XD, Wang F, et al. Ultrasound guidance facilitates radial artery catheterization: a meta-analysis with trial sequential analysis of randomized controlled trials. Chest. 2016;149(1):166–179. doi: 10.1378/chest.15-1784.
- Blais L, Forget A, Ramachandran S. Relative effectiveness of budesonide/formoterol and fluticasone propionate/salmeterol in a 1-year, population-based, matched cohort study of patients with chronic obstructive pulmonary disease (COPD): effect on COPD-related exacerbations, emergency department visits and hospitalizations, medication utilization, and treatment adherence. Clin Ther. 2010;32(7):1320–1328. doi: 10.1016/j.clinthera.2010.06.022.
- Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273(6):584–594. doi: 10.1111/joim.12067.
- Muiser S, Imkamp K, Seigers D, et al. Budesonide/formoterol maintenance and reliever therapy versus fluticasone/salmeterol fixed-dose treatment in patients with COPD. Thorax. 2023;78(5):451–458. doi: 10.1136/thorax-2022-219620.
- Wang MT, Lai JH, Huang YL, et al. Comparative effectiveness and safety of different types of inhaled long-acting β(2)-agonist plus inhaled long-acting muscarinic antagonist vs inhaled long-acting β(2)-agonist plus inhaled corticosteroid fixed-dose combinations in COPD a propensity Score-Inverse probability of treatment weighting cohort study. Chest. 2021;160(4):1255–1270. doi: 10.1016/j.chest.2021.05.025.
- Altaf M, Zubedi AM, Nazneen F, et al. Cost-effectiveness analysis of three different combinations of inhalers for severe and very severe chronic obstructive pulmonary disease patients at a tertiary care teaching hospital of South India. Perspect Clin Res. 2015;6(3):150–158. doi: 10.4103/2229-3485.159940.
- Kalhan R, Slade D, Ray R, et al. Umeclidinium/vilanterol compared with fluticasone propionate/salmeterol, budesonide/formoterol, and tiotropium as initial maintenance therapy in patients with COPD who have high costs and comorbidities. Int J Chron Obstruct Pulmon Dis. 2021;16:1149–1161. doi: 10.2147/COPD.S298032.
- Kern DM, Davis J, Williams SA, et al. Comparative effectiveness of budesonide/formoterol combination and fluticasone/salmeterol combination among chronic obstructive pulmonary disease patients new to controller treatment: a US administrative claims database study. Respir Res. 2015;16(1):52. doi: 10.1186/s12931-015-0210-x.
- Perrone V, Sangiorgi D, Buda S, et al. Comparative analysis of budesonide/formoterol and fluticasone/salmeterol combinations in COPD patients: findings from a real-world analysis in an Italian setting. Int J Chron Obstruct Pulmon Dis. 2016;11:2749–2755. doi: 10.2147/COPD.S114554.
- Roberts M, Mapel D, Petersen H, et al. Comparative effectiveness of budesonide/formoterol and fluticasone/salmeterol for COPD management. J Med Econ. 2011;14(6):769–776. doi: 10.3111/13696998.2011.622817.
- Suissa S, Dell’Aniello S, Ernst P. Comparing initial LABA-ICS inhalers in COPD: real-world effectiveness and safety. Respir Med. 2021;189:106645. doi: 10.1016/j.rmed.2021.106645.
- Seemungal TA, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. doi: 10.1164/ajrccm.157.5.9709032.
- Cazzola M, Donner CF. Long-acting beta2 agonists in the management of stable chronic obstructive pulmonary disease. Drugs. 2000;60(2):307–320. doi: 10.2165/00003495-200060020-00005.
- Celik G, Kayacan O, Beder S, et al. Formoterol and salmeterol in partially reversible chronic obstructive pulmonary disease: a crossover, placebo-controlled comparison of onset and duration of action. Respiration. 1999;66(5):434–439. doi: 10.1159/000029427.
- Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(1):74–81. doi: 10.1183/09031936.03.00031402.
- Yang IA, Fong KM, Sim EH, et al. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2007;18(2):CD002991.
- Tricco AC, Strifler L, Veroniki AA, et al. Comparative safety and effectiveness of long-acting inhaled agents for treating chronic obstructive pulmonary disease: a systematic review and network meta-analysis. BMJ Open. 2015;5(10):e009183. doi: 10.1136/bmjopen-2015-009183.
- Halpin DM, Gray J, Edwards SJ, et al. Budesonide/formoterol vs. salmeterol/fluticasone in COPD: a systematic review and adjusted indirect comparison of pneumonia in randomised controlled trials. Int J Clin Pract. 2011;65(7):764–774. doi: 10.1111/j.1742-1241.2011.02685.x.
- Rennard SI, Tashkin DP, McElhattan J, et al. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trial. Drugs. 2009;69(5):549–565. doi: 10.2165/00003495-200969050-00004.