?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Oil spills remain one of the greatest man-made ecological threats, despite numerous advanced cleanup approaches. They still pose a major challenge in the search for materials and technologies that work as efficiently and sustainably as possible. Promising natural materials include poultry feathers, which are produced in large quantities every day as a byproduct of the meat industry. In this study, the influence of different forms of absorbents (loose feathers, pillows, and sheets) based on chicken feathers and the addition of an inorganic absorbent, sepiolite, on their absorption capacity was investigated. The chemical and physical surface properties, like morphology, chemical composition, zeta potential, surface free energies and oil absorption capacities were analyzed. The Gibbs free energy of immersion wetting with oil and the work of adhesion of the adsorbents, calculated based on contact angle measurements, were confirmed by the tests of adsorption capacities according to the ASTM 726–12 standard. The results showed that pure loose feathers have the highest oil adsorption capacity, while feather pillows have only half, and composite sheets have only a quarter of this capacity. The addition of inorganic adsorbent sepiolite did not increase the absorption capacity of the composites.
摘要
尽管有许多先进的清理方法,但石油泄漏仍然是最大的人为生态威胁之一. 在寻找尽可能高效和可持续工作的材料和技术方面,它们仍然是一个重大挑战. 有前景的天然材料包括家禽羽毛,这些羽毛每天都作为肉类工业的副产品大量生产. 在这项研究中,研究了基于鸡毛的不同形式的吸收剂(松散的羽毛、枕头和床单)以及添加无机吸收剂海泡石对其吸收能力的影响. 分析了表面的化学和物理性质,如形貌、化学成分、ζ电位、表面自由能和吸油能力. 通过根据ASTM 726-12标准的吸附容量测试,证实了基于接触角测量计算的用油浸渍润湿的吉布斯自由能和吸附剂的粘附功. 结果表明,纯松散羽毛的吸油能力最高,而羽毛枕只有一半,复合片材只有四分之一. 无机吸附剂海泡石的加入并没有提高复合材料的吸附能力.
Introduction
Despite the continuous and successful reduction in their numbers due to technological advancements at all levels, oil spills remain one of the most problematic events from an environmental perspective. The most concerning spills in terms of consequences are those occurring in seas and lakes. These spills have severe and significant impacts on the environment, nature, and society (Hoang et al. Citation2021). Despite numerous solutions for managing oil spills, they still pose a significant challenge in the search for materials and technologies that will operate as efficiently and sustainably as possible (Bhardwaj and Bhaskarwar Citation2018). Materials play a pivotal role in managing oil spills. These can be either inorganic (mostly organo-clay materials) or organic, synthetic, or natural, and in current successful solutions, they often appear in various combinations of both inorganic and organic materials (Bhardwaj and Bhaskarwar Citation2018). Currently, three main forms of absorbents are used to manage oil spills in water, namely: 1) enclosed sorbents surrounded by fabrics or nets (booms, pillows), 2) continuous absorbents such as sheets, rolls, mats, and 3) loose fiber absorbents in bundles or strands (Hoang et al. Citation2021). The shape of the absorbent itself has a great influence on the absorption capacity due to the different surface to volume ratio. Synthetic materials such as polypropylene, polyethylene, polyester, polystyrene, or polyurethane in the form of textiles or foam have proven to be highly effective oil absorbers with high sorption capacities (Hoang et al. Citation2021). However, from both an economic and environmental perspective, synthetic materials are not an optimal solution. In line with the green transition, there is a growing effort to find efficient natural and biodegradable materials (Zamparas et al. Citation2020). Research reports focus on the use of cotton and kapok fibers (Cao et al. Citation2017; Thilagavathi, Praba Karan, and Das Citation2018), chicken feathers (Okoya et al. Citation2020), human hair (Ifelebuegu et al. Citation2015), wool (Radetic et al. Citation2008), rice husks (Ali et al. Citation2012), banana fibers, milkweed (Panahi, Moghaddam, and Moezzi Citation2020) and similar (Madasamy, Ramasubbu, and Nambirajan Citation2022). Among all these materials, chicken feathers represent the most promising solution from environmental point of view. Thus, the successful advancement of an oil absorbent utilizing waste chicken feathers holds the potential for a twofold environmental solution: 1. reuse of substantial amounts of problematic waste feathers, and 2. alleviating environmental catastrophes stemming from oil spills.
Poultry feather waste is one of the most complex wastes in the meat industry because it is produced in large quantities. The poultry meat production in EU was 13.6 Mt in 2020 and reached a new high. In comparison to 2019 is this number by about 2.3% higher and compared to 2010 is this a rise of 29.5% (Eurostat Citation2021). The main poultry meat producers in EU are Poland (19.8%), Spain (12.6%), France (12.3%), Germany (11.9%) and Italy (10.2%). Feathers are one of the main wastes in chicken meat industries, as it was estimated, that there is nearly 10 Mt of waste feathers produced in the world per year (Lasekan, Bakar, and Hashim Citation2013). Currently, only about one-third of the discarded feathers are processed into feather meal, which serves as a raw material for producing pet-food. This represents only a partial solution, as it is a relatively inexpensive semi-product that merely only reduces the substantial waste disposal costs in the company’s budget. Approaches to researching the utilization of poultry feathers can be in general categorized into three main groups: 1) studies on accelerated feather biodegradation processes (Jagadeesan et al. Citation2023; Kshetri et al. Citation2022; Li Citation2022; Możejko and Bohacz Citation2022; Shen et al. Citation2021), 2) investigations into environmentally friendlier methods of keratin extraction from feathers and its application in various high-value-added products (Ke et al. Citation2023; Rouse and Van Dyke Citation2010; Wang et al. Citation2017), and 3) exploration of potential uses of feathers in their natural form (Jung, Persi, and Bhattacharyya Citation2019; Reddy and Yang Citation2010; Šafarič et al. Citation2020). And there are three crucial principles in all these types of reuses: 1) maximizing the amounts of recycled waste feathers through a single process or product, 2) developing products with high added value, and, of course, 3) ensuring these new processes and products have a minimal carbon footprint.
It is important to note that majority of the processes of high-value products production are associated with significant additional environmental burdens, including energy consumption, water, and chemicals usage, etc.
This is why the reutilization of large quantities of feathers in their native form appears to be the most suitable solution. Due to its specific amino acid composition, feathers have a predominant hydrophobic character, with a 60:40 ratio in favor of non-hydrophilic amino acids and the presence of disulfide bonds of cysteine (Amieva et al. Citation2014; Saravanan and Dhurai Citation2012). Additionally, the unique morphology of feathers, where thicker parts (barbs) branch into thinner ones and further into very fine hooks, and therefore large accessible hydrophobic surface, contributes to their hydrophobic nature. All these properties as well as its low density make feathers a promising material for oil spills management (Kelle and Eboatu Citation2018; Okoya et al. Citation2020). Due to the relevance of the issue, there is currently a wide range of mostly synthetic materials and products for managing oil spills and in the future environmentally friendly solutions have to be investigated to reduce the cost and minimize the environmental burden (Hoang et al. Citation2021). In research for more environmentally friendly solutions and materials, waste chicken feathers are being researched primarily as a source of keratin for manufacturing foams or gels in combination with other biopolymers (Guiza et al. Citation2021) and there are only a few reports on waste feather application in their original form (Ifelebuegu and Chinonyere Citation2016; Kelle and Eboatu Citation2018).
In our current study, we aimed to investigate how different compositions and structures of oil absorbers based on waste chicken feathers affect their oil absorption capacity. To this end, we analyzed loose chicken feathers and those prepared in the form of pillows and flat sheets in combination with the inorganic absorbent sepiolite. Chemical properties as well as the surface morphology and zeta potential of the absorbents were studied thoroughly. In addition, the surface free energies and Gibbs free energies of immersion wetting with oil and their influence on the oil absorption capacity were investigated.
Materials and methods
Materials
Grid-washed chicken feathers were obtained from the Perutnina Ptuj d.d. poultry meat production plant, Slovenia. Sepiolite powder with particle size distribution: 88.2% 200–450 mm, 3.2% 150–200 mm and 7.9% < 150 mm was provided by the Department of Materials Science, Faculty of Technology and Metallurgy, University of Belgrade. Polylactide fibers (PLA) Ingeo (NatureWorks LLC) with the average fiber diameter 19 ± 1 m, tenacity 2.5 ± 0.5 cN/dtex and melting temperature 170°C were used as thermoplastic adhesive component to produce the composite sheets. Polyethylene terephthalate (PET) fabric from Bema Trade d.o.o. was used to manufacture the composite pillows. The fabric was plain weave with a weave density of 40 threads/cm. The thickness of the fabric was 0.35 ± 0.1 mm and weight per unit area of 120 ± 1.4 g/m2. The light mineral hydraulic oil Kiperol 20 (INA Maziva d.o.o.) with a density of 0.86 g/cm3 and a kinematic viscosity at 40°C of 10 mm2/s was used for the oil adsorption tests.
Methods
Waste feather cleaning
Prior to the preparation of the composite, the waste feathers were washed thoroughly at 60°C with a nonionic detergent (Sandoclean PC, Sandoz) and then rinsed with warm and cold water. The cleaned feathers () were dried in a ventilated dryer at 40°C for 72 h (Strnad et al. Citation2019).
Composite samples preparation
Pillows. To allow the largest accessible active surface area in the second approach of the composite preparation, we tried to keep the feathers as loose as possible. Therefore, feathers (F-Pillow) and/or feathers and sepiolite (FS-Pillow) were distributed between two layers of PET fabric and composite pillows were prepared ().
Sheets. The composite sheet specimens were prepared by thermal compression, using small quantities of PLA fibers as spot adhesives. A device with two electronically controlled heating plates (Roaches Ltd.) was used for thermal compression. The base composite (F-sheet) was prepared from layers of feathers (0.5 g) bonded by thermal compression between two hot plates at 170°C for 30 s. PLA fibers were used in small quantities as a thermoplastic point bonding agent. In the next step, sepiolite particles were uniformly distributed between two base composite layers and thermally compressed again under the same conditions. The sample was designated as FS-sheet ().
The samples’ designations and descriptions are represented in .
Table 1. Designations and composition of the samples.
Microscopy
The surface morphology of feathers and sepiolite samples was studied using a Zeiss Supra 35 VP and Quanta 200 3D field emission scanning electron microscopes.
FTIR spectroscopy
FT-IR spectra were recorded on a Perkin Elmer spectrum GX FT-IR spectrometer with a Golden Gate ATR attachment and diamond crystal. The absorbance measurements were carried out within the range of 400–4000 cm−1, with 16 scans and a resolution of 4 cm−1.
Zeta potential determination
The Zeta Potential (ZP) measurements of sepiolite samples were performed with Litesizer 500 from Anton Paar via electrophoretic light scattering (ELS), using patented cmPALS technology. A unique Ω-shaped cuvette, which creates a stable electric field exactly at the measurement position, was used for the prepared dispersions in aqueous media. The change of ZP as a function of pH was measured with an accessory titration system from Metrohm. The pH was automatically adjusted with 0.05 M NaOH and 0.05 M HCl in the range of 9–2. During the measurements the data were recorded using the manufacturer’s Anton Paar Kalliope software.
The Zeta Potential measurements of the feather samples were performed with SurPASS 3 (Anton Paar GmbH, Austria) using the cylindrical cell. The sample was fixed by placing the support discs and filters. The permeability index of the sample, by rotating the micrometer, was set to around 100. 1 mM KCl electrolyte solution was used, and the pH was automatically adjusted with 0.05 M NaOH and 0.05 M HCl. The pH dependence of the Zeta Potential was determined in the range of pH 3–9. A pressure gradient of 200–600 mbar was applied to generate the streaming potential.
Contact angles and surface free energies determination
Powder contact angle method. To determine the contact angles between solid samples and solvents (test liquids), the Powder Contact Angle method was employed. The solid samples in a form of powders (ground chicken feathers and sepiolite) were placed in a glass tube with a perforated lower surface on a Kruss K12 tensiometer (Kruss GmbH, Hamburg). Upon contact of the sample’s lower surface with the test liquid, the sample wets, which leads to an increase in its mass over time (m2/t). The evaluation of the measured data is based on modifications of the Washburn equation for a single capillary (Washburn Citation1921), obtained by combining the expression for Laplace pressure and the Hagen-Poiseuille equation for steady-state flow conditions (Grundke, Boerner, and Jacobasch Citation1991). If the wetting height of a single capillary is replaced with the change in mass due to the penetration of liquid through n capillaries, the modified Washburn equation is as follows:
where θ = contact angle between solid and liquid phases/°, m = sample mass/kg, t = time/s, η = liquid viscosity/mPa s, ρ = liquid density/gcm−3, γ = surface tension of the liquid/mN m−1
Three different test liquids, n-heptane, water and diiodomethane were applied for contact angles determination. The n-heptane (η = 0,4/mPas, ρ = 0,6836/gcm−3, γL = 20,4) was used to calculate the constant c to be used in Equationequation 1(1)
(1) , as:
where nk = number of capillaries and r = capillary radius/m.
Determination of the surface tensions of test liquids and oil. A Krüss K12 tensiometer was applied using a standard Du Nouy ring (for water) and Wilhelmy Pt plate method (for n-heptane, diiodomethane and oil) for surface tensions (SFT - γl) and their dispersive (γld) and polar (γlp) components determination of liquid samples. The standard PTFE plate was applied for the determination of dispersive (γld) components of surface tensions of the test liquids and oil. The PTFE surface is assumed to have a surface energy of 18.0 mJ/m2 and does not form polar interactions with the liquid test sample. From the above, it is assumed that the polar contribution for PTFE is zero. The known values are taken into account in the following equation (Rulison Citation2000):
where is dispersive component of a liquid (mN/m),
is the surface tension of liquid sample (mN/m) and θ is the angle between PTFE plate and sample liquid (o).
Based on the result obtained and the measured contact angle between the PTFE plate and the measured liquid sample, one can calculate the missing polar contribution to the SFT in the relation:
where is the polar contribution (mN/m) to the surface tension.
The total surface free energy (SFE) with associated dispersive and polar components for the solid (feather and sepiolite) samples (γs) were calculated from the measured contact angles using a two-component Owens, Wendt, Rabel and Kaelble (OWRK) model. The model assumes that the intermolecular interactions between the solid and liquid phases consist of two main components – the dispersive (due to London forces) and the polar component (due to Keesom and Debye forces). Therefore, the SFE of the solid phase (γs) is according to (Gindl et al. Citation2001; Wu Citation1971; Zhao, Liu, and Abel Citation2004) derived from the contributions of the interactions as the sum of the two components, the dispersion (γd) and the polar (γp) component as:
where i stands for material (solid (s) or liquid (l) phase)
According to (Owens and Wendt Citation1969) in the geometric mean model, the energy between the solid/liquid phases can be evaluated as the following relation:
Considering Young’s equation:
the relationship changes to:
Work of adhesion (Wa). The driving force for the adsorption is a reduction in surface energy in the case of a solid surface and/or surface tension in the case of a liquid (Van Oss, Chaudhury, and Good Citation1988):
where: γ - surface tension (usually for liquids and gases) or surface free energy (usually for solid surfaces); Gσ - interfacial Gibbs free energy; A – interfacial area; T – absolute temperature; n – number of moles
Based on that, the Gibbs free energy of immersion wetting can be calculated (Rulison Citation2000):
Accordingly, the work of adhesion is defined as the work required for the separation of two phases and is described by the Young-Dupré equation:
Oil adsorption testing
Adsorption tests were conducted in accordance with ASTM F726–12 Standard Test Method for Sorbent Performance of Adsorbents (ASTM Citation2012). This test method covers laboratory tests of the performance of adsorbents for removing non-emulsified oils and other floating, immiscible liquids from the water surface. According to the definitions in this standard test method, our samples were categorized as follows: loose feathers and sepiolite were the Type II (unconsolidated particulate material without sufficient form and strength), composite pillows were Type III (pillows, adsorbent material contained in an outer fabric or netting that is permeable to oil but whose openings are small enough to retain the adsorbent material within the fabric or mesh), and thermally bonded composites were Type I (sheet, pad, a material whose length and width are much greater than its thickness). Based on this categorization, oil adsorption tests performance were chosen. The objective of adsorption tests is to determine the optimum adsorbent without the competing presence of water and can be used to compare the oil capacities of adsorbents with each other. The data refer only to oil layer thicknesses equal to or greater than the thickness of the adsorbent.
Prior to testing, all specimens were conditioned for at least 24 h at 23 ± 4°C and 70 ± 2% relative humidity. All samples were first tested with the so-called Dynamic Degradation Test, in which the buoyancy of the samples on water is tested after a defined shaking process. Pure sepiolite, of course, did not pass this test, but the composite samples containing sepiolite did. Thus, the standard test method was appropriate for chicken feathers as well as for all prepared composite samples. In this study, sepiolite was tested as an additional oil adsorbing component that could improve the adsorption capacity of the chicken feather composites. Samples with the defined dimensions and weights were allowed to float freely in a defined initial layer (minimum thickness 2.5 cm) of the test liquid (light oil) for a short (15 ± 2 min) or long (24 h ±30 min) period. After this time, the adsorbent samples were taken and allowed to drain for 30 ± 3 s. The tared weighing pan was then placed under the adsorbent to collect any additional drops, the sample was then placed in a pan and its weight was determined. For the loose feathers (adsorbent type II), a basket with an appropriate perforation was used to lower the sample into the test liquid and allow it to collect and drain. All tests were performed in triplicates and the average of the three runs was used for the calculations. Oil adsorption capacity was calculated as the ratio of weight of oil adsorbed to dry adsorbent weight:
Where:
S0 – initial dry adsorbent weight [g]
SST – weight of adsorbent sample at the end of oil test (Short Test – ST or Long Test – LT) [g].
Results and discussion
Microstructure and surface morphology
The electron microscopy images of the feathers and sepiolite used in this investigation are represented in .
Figure 4. Scanning electron micrographs of chicken feathers: whole feather (a), rachis and barb (b), barbules (c and d).
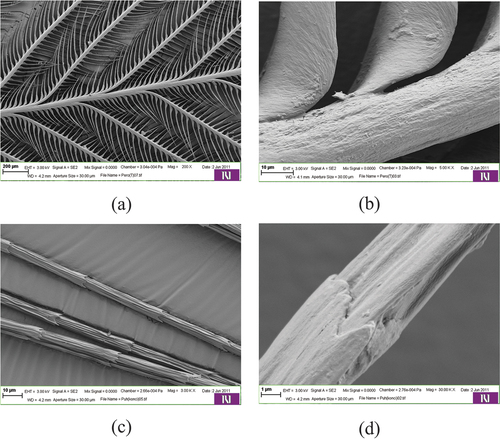
Figure 5. Scanning electron micrographs of cross-sections of a barb (a) and barbule (b) showing the details of the walls of the internal “honeycomb” structure (c and d).
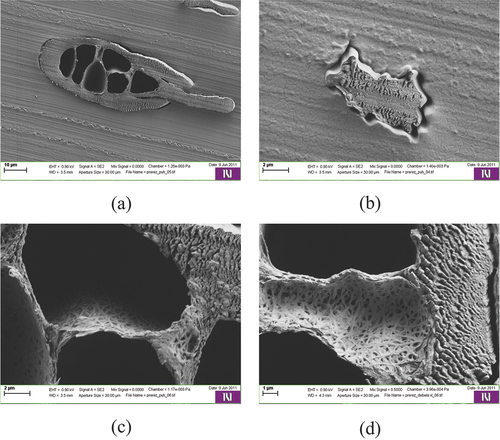
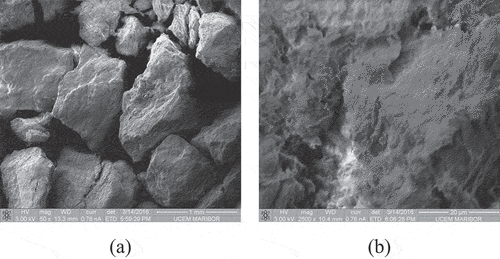
Figure 6. Scanning electron micrographs of the sepiolite specimen at 50x (a) and 2500x (b) magnification.
shows all three main structural levels of chicken feathers. The main stem of a feather is called the rachis, from which the barbs branch off. The barbs consist of a central shaft, the ramus, and tiny branches, the barbules. The barbules consist of a series of cells, starting with the short cells at the base and ending with a series of longer, distal cells called the pennulum (Prum Citation1999). The thicknesses of barbules were about 4 to 7 m.
Feathers cross sections () show the porous internal structure of barbes and barbules. The interior of the cross-sections of feather barbs consists of honeycomb-shaped hollow cells. The cell walls are thin and consist of a meshwork of randomly arranged fibrils.
The sepiolite particles are shown in . The layered microfibrous surface morphology is clearly visible.
Surface chemical structure
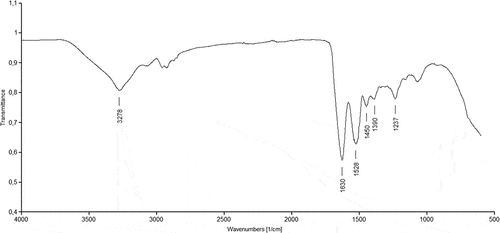
The FTIR spectrum of feathers () shows typical peaks for keratin. The peak at 3278 cm−1 is typical of the N-H stretching vibration of amides and depends not on the conformation of the backbone but on the strength of the hydrogen bonds. The strongest adsorption band in proteins is amide I, whose transmission band is found between 1600 and 1700 cm−1. In feathers, this band appears at 1628 cm−1 and is dominated mainly by C=O and C-N bonds. The second characteristic band for proteins is the amide II at 1528 cm−1 and the band typical of C-N vibrations appears at 1235 cm−1.
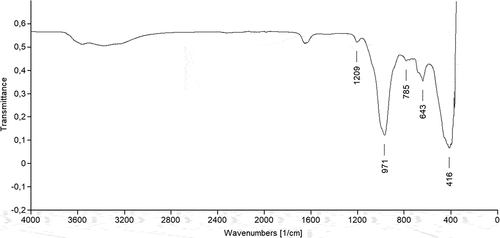
In the FTIR spectrum of the sepiolite sample () the bands in the region from ~3200 to ~3700 cm−1 and the band at ~1661 cm−1 are attributed to the presence of different kinds of water molecules in the sepiolite structure, such as OH-groups in the octahedral Mg ions and OH stretching vibrations in the external surface as well as the zeolitic or interior or channel water (Alkan, Tekin, and Namli Citation2005; Perraki and Orfanoudaki Citation2008). However, in the applied sepiolite sample the week peaks at that wavenumbers indicate low amounts of hydrophilic OH groups as well as in the inner structure kept water molecules (Sanguanwong et al. Citation2021). On the other hand, the distinct bands at 1209 cm−1 and 785 cm−1 are characteristic for Si-O-Si. The bands at ~971 and ~416 cm−1 are also assigned to Si-O-Si and the band at ~370 cm−1 is attributed to Si-O-Mg bonds (Perraki and Orfanoudaki Citation2008).
Zeta potential
The zeta potential of cleaned chicken feathers shows a typical amphoteric character of feathers with the point of zero charge at pH 4.3 (). Below this point feathers have positive zeta potential owing to protonated surface amino groups of the keratin and above the isoelectric point of the main protein feathers show negative zeta potential, which at about pH 6.1 riches the plateau value of −31.2 mV. The negative charge is likely a result of the presence of weak acids, most probably caused by deprotonated carboxyl groups that are also found in keratin. It can be observed that at pH below 3 and pH higher than 5, the zeta potential is greater than ±20 mV, which theoretically indicates the stability of colloidal solutions due to strong repulsive forces among same ionizing groups.
Figure 9. Zeta potential as a function of pH for feather marked as x) and sepiolite (marked as o) samples.
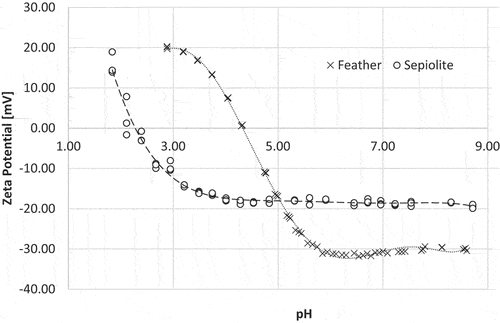
The pH dependence of zeta potential function of sepiolite shows an acidic characteristic of the sepiolite surface, as the isoelectric point is very low (at the pH 2.2.) and above it the surface has negative potential in the whole measured pH region (until pH 9). Above IEP sepiolite is negatively charged reaching a plateau at pH 4.3 with ZP of −18.9 mV. Most clay minerals have IEP around 2 (Padilla et al. Citation2011; Sabah et al. Citation2007). Negative charge of sepiolite could be the consequence of crystal lattice imperfections and substitutions of Si4+ with Al3+ and Al3+ with Mg2+ in the crystal lattice, which can lead to an excess of negative charge in the layers (Padilla et al. Citation2011). On the other hand, breaking of siloxane group (Si-O-Si) bonds during grinding leads to negative surface charge (Alkan, Tekin, and Namli Citation2005; Chen et al. Citation2012) due to forming of siloxide (-SiO-) groups. It was also found (Padilla et al. Citation2011) that sepiolite is stable at large pH range from 2 to 12 and that at pH below 2 mostly Mg2+ ions are released.
Below the isoelectric point (IEP), sepiolite becomes positively charged due to the adsorption of specific ions, such as H3O+, which are relatively abundant in such an acidic environment. These ions can interact with the surface and specifically attach to surface functional groups, particularly the silanol groups, turning the surface charge into a positive one.
In comparison to feather dispersions, sepiolite exhibits lower stability and a greater tendency to agglomeration.
Surface free energy
Before measuring the contact angles (CA) and determining the surface free energies (SFE) of solid surfaces, the surface tensions, the polar and dispersive components, and the polarity of the test liquids had to be determined. The density and viscosity data of the test liquids were taken from the Krüss database. The results are summarized in .
Table 2. Viscosity (η), Density (ρ), Surface tension (SFT) of test liquids and their corresponding dispersive (Dis) and polar (Pol) contributions, and polarity.
The same technique was used to determine the surface tension and its dispersive and polar components for the test oil. Results are represented in .
Table 3. The surface tension ( and its dispersive (
and polar (
contributions of sample diesel oil.
The SFE of solid samples was evaluated upon the results of CA obtained by capillary rise method. OWRK mathematical model was used to evaluate total SFE and corresponding dispersive and polar contributions. The results are presented in .
Table 4. The total surface free energies (SFE) and their dispersive and polar contributions estimated by OWRK model for sepiolite (S), feathers (F) and polyester fabric (PET).
Based on surface tension of oil sample and surface energies of solid samples determination and by knowing their polar contributions, the Gibbs free energy of immersion wetting (ΔG) was calculated according to Equationequation (10)(10)
(10) . It is well known that a negative ΔG means the wetting is thermodynamically favorable or spontaneous. It is clear from the results in , that this is the case for oil and feathers. Thus, the work of adhesion (Wsl) in the case of oil and feathers is about 35% higher than that of sepiolite and oil. The same analyses were performed on polyester fabric used as a barrier material for the pillow samples with feathers or feathers and sepiolite. The system PET/oil has the highest positive Gibbs free energy of immersion wetting with oil and the lowest work of adhesion among all three samples (). It is therefore to be expected that the wrapping with polyester fabric reduces the oil adhesion capacity.
Table 5. Gibbs free energy of wetting (G) and work of adhesion (Wsl) for the solid/liquid systems (feathers/oil (F/oil) and sepiolite/oil (S/oil)).
Oil adsorption capacity
The results of the test method for the oil sorption performance of the adsorbents are shown in . The oil adsorption capacity is shown as the weight ratio between the adsorbed oil and the dry adsorbent (calculated according to EquationEquation 6(6)
(6) - Oil adsorption capacityw). Loose chicken feather fibers exhibited the highest oil adsorption capacityw, as they adsorbed about 18.9 ± 0.5 g of oil per g of feathers in the 15-minute test and about 20.5 ± 1.9 g/g in the 24-h test. There is no significant difference between the results of the short-term and long-term tests, which is consistent with the results of the oil adsorption kinetics of chicken feathers obtained by (Kelle and Eboatu Citation2015). When feathers were processed into a pillow composite using PET fabric, the oil adsorption capacityw decreased to 9.9 g/g and 10 g/g (after 15 min and 24 h). When sepiolite was added to the pillow composite, the oil adsorption capacityw decreased to about 5.3 g/g. Similar results (about 6 g/g) were obtained for thermally compressed feather sheet composite (F-sheet), and for the sheet composite with sepiolite addition (FS-sheet), the oil adsorption capacityw dropped to 2.5 g/g.
Figure 10. The oil adsorption capacity as mass ratio of oil adsorbed to dry adsorbent weight of loose feathers (F), pillow composites (F-pillow, FS-pillow) and sheet composites (F-sheet, FS-sheet).
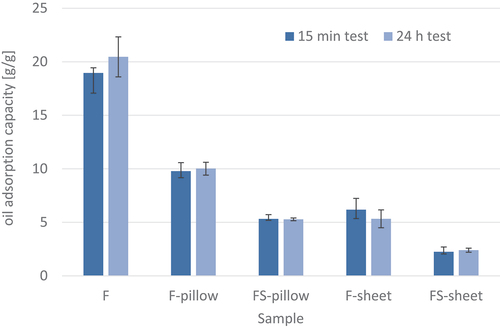
Different structures and/or the addition of sepiolite to the feathers in the composites had a significant effect on the weight of the sorbents, but at the same time did not significantly increase the adsorption capacity of the composites. Thus, the oil adsorption capacity weight ratio (oil adsorption capacityw) of the sample F-pillow was about 50% lower than that of the loose feathers and that with added sepiolite (FS-pillow) was even about 70% lower. The oil adsorption capacity of sepiolite alone determined for these tests was 0.9 g/g, which is consistent with the studies of (Zadaka-Amir, Bleiman, and Mishael Citation2013). In addition, the density of sepiolite is 2.5 g/cm3, while the density of feathers is 1.5 g/cm3. Thermal bonding of the feathers in the F-sheet samples reduced the accessible oleophilic surface area of the feathers, resulting in an approximately 70% decrease in oil adsorption capacity compared to lose feathers (F).
Conclusion
The objective of this study was to determine how different combinations of chicken feathers with inorganic solid adsorbent, sepiolite, and various forms of composites based on chicken feathers affect their oil adsorption capacity. The surface chemical structure of the feathers indicates the presence of amides I and II as well as C=O, C-N and hydrogen bonds, while the chemical structure of the sepiolite contains OH groups, typical Si-O-Si and Si-O-Mg bonds. The functional dependence of the zeta potential on pH shows the typical amphoteric character of feathers with an isoelectric point at pH 4.3, while the zeta potential of sepiolite is mostly negative in the pH range, since its isoelectric point is at pH 2.2. Moreover, above pH 6, the zeta potential of feathers reaches a value of about −30 mV, which theoretically indicates the stability of colloidal solutions due to strong repulsive forces among same ionizing groups, while that of sepiolite is about −20 mV.
To analyze the surface properties of the samples and their hydrophobic (oleophilic) properties, the contact angles of solid feathers and sepiolite samples were measured with test solvents and the surface free energies were calculated. Based on the determination of the surface tension and its polar/dispersive components of the test oil, the Gibbs free energy of wetting and the work of adhesion were determined for the solid/liquid feather/oil and sepiolite/oil systems. The results showed that the Gibbs free energy of wetting of feathers with oil was −6.08 mJ, which indicates a spontaneous wetting process of feathers and oil, while the Gibbs free energy for the sepiolite/oil system was 4.13 mJ. Therefore, also the work of adhesion of the system feathers/oil was 35% greater than for the sepiolite/oil system.
To determine the behavior of the adsorbents and their oil absorption capacity in the real conditions, an absorption test was performed in accordance with the ASTM F726–12 standard. The results proved all the surface and interface analyses because they showed that loose feathers in their natural form had the highest absorption capacity, absorbing about 20 g of oil per g of feathers. When the feathers were formed into pads using PET fabric, their absorption capacity decreased by half compared to lose feathers, while thermal compression of the feathers into sheets further reduced their absorption capacity to one-quarter. The addition of sepiolite in small amounts, which does not affect buoyancy, reduced the absorption capacity of the samples to about 5 g/g for pads and even to about 2.5 g/g for sheets.
This study has clearly shown that the maximum adsorption capacity for oil can only be achieved with loose feathers. In this form, feathers absorb 100% more oil than the next most effective form of absorption, feather-filled PET pillows. This is most likely attributed to the greater accessibility of the active hydrophobic surface in the case of loose feathers. Contrary to expectations, the addition of sepiolite did not improve the absorption performance of any of feather composite forms investigated.
Highlights
Waste poultry feathers are among the most promising natural materials for oil adsorption.
The form and composition of feather-based oil adsorbents have a significant influence on their oil absorption capacity.
The addition of inorganic sepiolite did not improve the absorption performance of any of feather composite forms investigated.
The surface free energies and Gibbs free energies of wetting of feather-based adsorbents with oil are excellent indicators of oil adsorption ability.
Chicken feathers in loose form are excellent oil adsorbents with more than 100% higher oil adsorption capacity compared to other adsorbents based on feather fibers.
Acknowledgments
The authors are grateful to Tanja Kos and Tjaša Glaser from the Laboratory for Characterization and Processing of Polymers at the University of Maribor, Slovenia for help and support at laboratory processing and characterization of feather fibers and sepiolite samples.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ali, N., M. El-Harbawi, A. Abo Jabal, and C.-Y. Yin. 2012. “Characteristics and Oil Sorption Effectiveness of Kapok Fibre, Sugarcane Bagasse and Rice Husks: Oil Removal Suitability Matrix.” Environmental Technology 33 (4): 481–18. https://doi.org/10.1080/09593330.2011.579185.
- Alkan, M., G. Tekin, and H. Namli. 2005. “FTIR and Zeta Potential Measurements of Sepiolite Treated with Some Organosilanes.” Microporous and Mesoporous Materials 84 (1): 75–83. https://doi.org/10.1016/j.micromeso.2005.05.016.
- Amieva, E. J.-C., C. Velasco-Santos, A. L. Martínez-Hernández, J. L. Rivera-Armenta, A. M. Mendoza-Martínez, and V. M. Castaño. 2014. “Composites from Chicken Feathers Quill and Recycled Polypropylene.” Journal of Composite Materials 49 (3): 275–283. https://doi.org/10.1177/0021998313518359.
- ASTM. 2012. ASTM F726-12, Standard Test Method for Sorbent Performance of Adsorbents. West Conshohocken, PA: ASTM International.
- Bhardwaj, N., and A. N. Bhaskarwar. 2018. “A Review on Sorbent Devices for Oil-Spill Control.” Environmental pollution (1987) 243 (Pt B): 1758–1771. https://doi.org/10.1016/j.envpol.2018.09.141.
- Cao, S., T. Dong, G. Xu, and F.-M. Wang. 2017. “Oil Spill Cleanup by Hydrophobic Natural Fibers.” Journal of Natural Fibers 14 (5): 727–735. https://doi.org/10.1080/15440478.2016.1277820.
- Chen, C., F. Wang, J. Liang, Q. Tang, and Y. Chen. 2012. “Zeta Potential of Sepiolite in Aqueous System.” Advanced Materials Research 427:208–211. https://doi.org/10.4028/www.scientific.net/AMR.427.208.
- Eurostat. 2021. “Agricultural production - livestock and meat.” Eurostat, Accessed August 9, 2022. https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agricultural_production_-_livestock_and_meat&oldid=549389#Poultrymeat.
- Gindl, M., G. Sinn, W. Gindl, A. Reiterer, and S. Tschegg. 2001. “A Comparison of Different Methods to Calculate the Surface Free Energy of Wood Using Contact Angle Measurements.” Colloids and Surfaces A, Physicochemical and Engineering Aspects 181 (1): 279–287. https://doi.org/10.1016/S0927-7757(00)00795-0.
- Grundke, K., M. Boerner, and H. J. Jacobasch. 1991. “Characterization of Fillers and Fibres by Wetting and Electrokinetic Measurements.” Colloids and Surfaces 58 (1–2): 47–59. https://doi.org/10.1016/0166-6622(91)80197-V.
- Guiza, K., R. B. Arfi, K. Mougin, C. Vaulot, L. Michelin, L. Josien, G. Schrodj, and A. Ghorbal. 2021. “Development of Novel and Ecological Keratin/cellulose-Based Composites for Absorption of Oils and Organic Solvents.” Environmental Science and Pollution Research International 28 (34): 46655–46668. https://doi.org/10.1007/s11356-020-11260-7.
- Hoang, A. T., X. P. Nguyen, X. Q. Duong, and T. T. Huynh. 2021. “Sorbent-Based Devices for the Removal of Spilled Oil from Water: A Review.” Environmental Science and Pollution Research 28 (23): 28876–28910. https://doi.org/10.1007/s11356-021-13775-z.
- Ifelebuegu, A. O., and P. Chinonyere. 2016. “Oil Spill Clean-Up from Sea Water Using Waste Chicken Feathers.” Proceedings of the 4th international conference on advances in applied science and environmental technology (ASET’16), Bangkok, Thailand: IRED.
- Ifelebuegu, A. O., T. V. A. Nguyen, P. Ukotije-Ikwut, and Z. Momoh. 2015. “Liquid-Phase Sorption Characteristics of Human Hair As a Natural Oil Spill Sorbent.” Journal of Environmental Chemical Engineering 3 (2): 938–943. https://doi.org/10.1016/j.jece.2015.02.015.
- Jagadeesan, Y., S. Meenakshisundaram, K. Raja, and A. Balaiah. 2023. “Sustainable and Efficient-Recycling Approach of Chicken Feather Waste into Liquid Protein Hydrolysate with Biostimulant Efficacy on Plant, Soil Fertility and Soil Microbial Consortium: A Perspective to Promote the Circular Economy.” Process Safety and Environmental Protection 170:573–583. https://doi.org/10.1016/j.psep.2022.12.029.
- Jung, D., I. Persi, and D. Bhattacharyya. 2019. “Synergistic Effects of Feather Fibers and Phosphorus Compound on Chemically Modified Chicken Feather/Polypropylene Composites.” ACS Sustainable Chemistry & Engineering 7 (23): 19072–19080. https://doi.org/10.1021/acssuschemeng.9b04894.
- Kelle, H. I., and A. N. Eboatu. 2015. “Determination of Adsorption of Diesel Onto a Poultry Waste: Chicken Feather.” Pakistan Journal of Chemistry 5 (2): 80. https://doi.org/10.15228/2015.v05.i02.p06201508012830.
- Kelle, H. I., and A. N. Eboatu. 2018. “Determination of the Viability of Chicken Feather As Oil Spill Clean-Up Sorbent for Crude Oil and Its Lower Fractions.” Journal of Applied Sciences and Environmental Management 22 (2): 267–273. https://doi.org/10.4314/jasem.v22i2.19.
- Ke, Y., J. Wu, Y. Ye, X. Zhang, T. Gu, Y. Wang, F. Jiang, and J. Yu. 2023. “Feather Keratin-Montmorillonite Nanocomposite Hydrogel Promotes Bone Regeneration by Stimulating the Osteogenic Differentiation of Endogenous Stem Cells.” International Journal of Biological Macromolecules 243:125330–125330. https://doi.org/10.1016/j.ijbiomac.2023.125330.
- Kshetri, P., P. L. Singh, S. B. Chanu, T. S. Singh, R. Chongtham, K. Tamreihao, H. N. Singh, et al. 2022. “Biological Activity of Peptides Isolated from Feather Keratin Waste Through Microbial and Enzymatic Hydrolysis.” Electronic Journal of Biotechnology 60:11–18. https://doi.org/10.1016/j.ejbt.2022.08.001.
- Lasekan, A., F. A. Bakar, and D. Hashim. 2013. “Potential of Chicken By-Products As Sources of Useful Biological Resources.” Waste management (Elmsford) 33 (3): 552–565. https://doi.org/10.1016/j.wasman.2012.08.001.
- Li, Q. 2022. “Perspectives on Converting Keratin-Containing Wastes into Biofertilizers for Sustainable Agriculture.” Frontiers in Microbiology 13:918262–918262. https://doi.org/10.3389/fmicb.2022.918262.
- Madasamy, S., R. Ramasubbu, and N. Nambirajan. 2022. “Utilization of Reformed Coconut Fiber and Banana Stem Fibre As Green Oil Sorbent.” Journal of Natural Fibers 19 (15): 12339–12346. https://doi.org/10.1080/15440478.2022.2057385.
- Możejko, M., and J. Bohacz. 2022. “Optimization of Conditions for Feather Waste Biodegradation by Geophilic Trichophyton Ajelloi Fungal Strains Towards Further Agricultural Use.” International Journal of Environmental Research and Public Health 19 (17): 10858–10888. https://doi.org/10.3390/ijerph191710858.
- Okoya, A. A., N. O. Ochor, A. B. Akinyele, and O. O. Olaiya. 2020. “The Use of Chicken Feather Waste As an Adsorbent for Crude Oil Clean Up from Polluted Water.” Journal of Agriculture and Ecology Research International 43–53. https://doi.org/10.9734/jaeri/2020/v21i330136.
- Owens, D. K., and R. C. Wendt. 1969. “Estimation of the Surface Free Energy of Polymers.” Journal of Applied Polymer Science 13 (8): 1741–1747. https://doi.org/10.1002/app.1969.070130815.
- Padilla, E., R. Ramos, J. Mendoza-Barron, R. Guerrero-Coronado, A. Jacobo-Azuara, and A. Aragon-Piña. 2011. “Adsorption of Heavy Metal Ions from Aqueous Solution Onto Sepiolite.” Adsorption Science & Technology 29 (6): 569–584. https://doi.org/10.1260/0263-6174.29.6.569.
- Panahi, S., M. K. Moghaddam, and M. Moezzi. 2020. “Assessment of Milkweed Floss As a Natural Hollow Oleophilic Fibrous Sorbent for Oil Spill Cleanup.” Journal of Environmental Management 268:110688. https://doi.org/10.1016/j.jenvman.2020.110688.
- Perraki, T., and A. Orfanoudaki. 2008. “Study of Raw and Thermally Treated Sepiolite from the Mantoudi Area, Euboea, Greece.” Journal of Thermal Analysis and Calorimetry 91 (2): 589–593. https://doi.org/10.1007/s10973-007-8329-8.
- Prum R O. (1999). Development and evolutionary origin of feathers. J Exp Zool, 285(4), 291–306.
- Radetic, M., V. Ilic, D. Radojevic, R. Miladinovic, D. Jocic, and P. Jovancic. 2008. “Efficiency of Recycled Wool-Based Nonwoven Material for the Removal of Oils from Water.” Chemosphere 70 (3): 525–530. https://doi.org/10.1016/j.chemosphere.2007.07.005.
- Reddy, N., and Y. Yang. 2010. “Light-Weight Polypropylene Composites Reinforced with Whole Chicken Feathers.” Journal of Applied Polymer Science 116 (6): 3668–3675. https://doi.org/10.1002/app.31931.
- Rouse, J. G., and M. E. Van Dyke. 2010. “A Review of Keratin-Based Biomaterials for Biomedical Applications.” Materials 3 (2): 999–1014. https://doi.org/10.3390/ma3020999.
- Rulison, C. 2000. “Two-Component Surface Energy Characterization as a Predictor of Wettability and Dispersability.” KRUSS Application Note AN213 1–22. https://scholar.google.com/scholar_lookup?title=Two-Component+Surface+Energy+Characterization+as+a+Predictor+of+Wettability+and+Dispersability&author=Rulison,+C.&publication_year=2000.
- Sabah, E., U. Mart, M. Çınar, and M. S. Çelik. 2007. “Zeta Potentials of Sepiolite Suspensions in Concentrated Monovalent Electrolytes.” Separation Science and Technology 42 (10): 2275–2288. https://doi.org/10.1080/15275920701313616.
- Šafarič, R., L. F. Zemljič, M. Novak, B. Dugonik, B. Bratina, N. Gubeljak, S. Bolka, and S. Strnad. 2020. “Preparation and Characterisation of Waste Poultry Feathers Composite Fibreboards.” Materials 13 (21): 1–17. https://doi.org/10.3390/ma13214964.
- Sanguanwong, A., A. E. Flood, M. Ogawa, R. Martín-Sampedro, M. Darder, B. Wicklein, P. Aranda, and E. Ruiz-Hitzky. 2021. “Hydrophobic Composite Foams Based on Nanocellulose-Sepiolite for Oil Sorption Applications.” Journal of Hazardous Materials 417:126068. https://doi.org/10.1016/j.jhazmat.2021.126068.
- Saravanan, K., and B. Dhurai. 2012. “Exploration on the Amino Acid Content and Morphological Structure in Chicken Feather Fiber.” Journal of Textile and Apparel, Technology and Management 7 (3): 1–6.
- Shen, R., H. Wang, K. Wu, J. Gao, and J. Li. 2021. “Characterization and Antimicrobial Properties of Ferulic Acid Grafted Self‐Assembled Bacterial Cellulose‐Chitosan Membranes.” Journal of Applied Polymer Science 138 (33): 50824–50837. https://doi.org/10.1002/app.50824.
- Strnad, S., Z. Oberholentzer, O. Šauperl, T. Kreže, and L. F. Zemljič. 2019. “Modifying Properties of Feather Keratin Bioplastic Films Using Konjac Glucomannan.” Cellulose Chemistry and Technology 53 (9–10): 1017–1027. https://doi.org/10.35812/CelluloseChemTechnol.2019.53.100.
- Thilagavathi, G., C. Praba Karan, and D. Das. 2018. “Oil Sorption and Retention Capacities of Thermally-Bonded Hybrid Nonwovens Prepared from Cotton, Kapok, Milkweed and Polypropylene Fibers.” Journal of Environmental Management 219:340–349. https://doi.org/10.1016/j.jenvman.2018.04.107.
- Van Oss, C. J., M. K. Chaudhury, and R. J. Good. 1988. “Interfacial Lifshitz-van der Waals and Polar Interactions in Macroscopic Systems.” Chemical Reviews 88 (6): 927–941. https://doi.org/10.1021/cr00088a006.
- Wang, J., S. Hao, T. Luo, Z. Cheng, W. Li, F. Gao, T. Guo, Y. Gong, and B. Wang. 2017. “Feather Keratin Hydrogel for Wound Repair: Preparation, Healing Effect and Biocompatibility Evaluation.” Colloids and Surfaces B, Biointerfaces 149:341–350. https://doi.org/10.1016/j.colsurfb.2016.10.038.
- Washburn, E. W. 1921. “The Dynamics of Capillary Flow.” Physical Review 17 (3): 273–283. https://doi.org/10.1103/PhysRev.17.273.
- Wu, S. 1971. “Calculation of Interfacial Tension in Polymer Systems.” Journal of Polymer Science Part C: Polymer Symposia 34 (1): 19–30. https://doi.org/10.1002/polc.5070340105.
- Zadaka-Amir, D., N. Bleiman, and Y. G. Mishael. 2013. “Sepiolite As an Effective Natural Porous Adsorbent for Surface Oil-Spill.” Microporous and Mesoporous Materials 169:153–159. https://doi.org/10.1016/j.micromeso.2012.11.002.
- Zamparas, M., D. Tzivras, V. Dracopoulos, and T. Ioannides. 2020. “Application of Sorbents for Oil Spill Cleanup Focusing on Natural-Based Modified Materials: A Review.” Molecules 25 (19): 4522. https://doi.org/10.3390/molecules25194522.
- Zhao, Q., Y. Liu, and E. W. Abel. 2004. “Effect of Temperature on the Surface Free Energy of Amorphous Carbon Films.” Journal of Colloid and Interface Science 280 (1): 174–183. https://doi.org/10.1016/j.jcis.2004.07.004.