Abstract
Introduction. It is well known that vapor emitted from metallic mercury is a potent neurotoxic agent, but little toxicological information on liquid metallic mercury itself is available, especially with respect to developmental neurotoxicity. We were unable to find any cases of parental metallic mercury administration that reveal its effect on embryogenesis and human development. Case report. Young woman who, following a suicide attempt through the intravenous injection of liquid mercury, developed extremely high blood and urine mercury levels but was able to become pregnant and deliver an apparently mature and healthy infant. Analysis. To our knowledge, there were no similar cases reported in the literature. Therefore, we chose to perform a detailed analysis of this unique clinical case. We report levels of mercury in maternal, umbilical cord, and infant blood in samples of breast milk and placental tissues. Despite high levels of mercury in blood and urine samples, no immediate adverse heath effects were observed in either mother or infant. This case may shed a new light and information on the toxicology of mercury.
Introduction
It is well known that the vapor emitted from metallic mercury is a potent neurotoxic agent, but little toxicological information on liquid metallic mercury itself is available, especially with respect to developmental neurotoxicity.Citation1–3 There are several studies described in the literature that pregnant women are chronically exposed to high levels of inorganic mercury vapor with different reproductive outcomes and follow-ups.Citation4,Citation5 We were unable to find any cases of parenteral metallic mercury administration that reveal its effect on embryogenesis and human development.
We handled a unique case of a young woman who, following a suicide attempt through the intravenous injection of liquid mercury, developed extremely high blood and urine mercury levels but was able to become pregnant and deliver an apparently mature, healthy infant.
To our knowledge, there were no similar cases reported in the literature; therefore, we had performed detailed analysis of this prenatal mercury exposure and postnatal outcome. We were able to follow up the infant's neurological status and age of achievement of developmental milestones during first 8 months of life.
Maternal case report
A 27-year-old pregnant woman, at 8 weeks gestational age, with extremely high blood and urine mercury levels, presented for consultation with a toxicologist.
She had attempted suicide 10 years previously, injecting approximately 3 mL of liquid metallic mercury into her left antecubital vein. Surgical removal of this vein for severe phlebitis and recovery of residual mercury was performed at that time. She had several hospitalizations in 1993–1995 after the suicide attempt and underwent seven reconstructive surgeries to the left arm and axilla. She had been healthy as a child and never had any significant illnesses. She was a lifelong nonsmoker and did not drink alcohol or use illicit drugs.
A toxicologist treated her with repeated chelations for a period of about 1 year following the incident. Nonetheless, blood and urine levels remained high. For example, in 2003, the blood level was 209 ngHg/mL and the urine level was 413 ngHg/mL well above background levels (). Metallic mercury remnants were still visible on X-ray in the left arm, axilla, and supraclavicular region. Because she was asymptomatic, chelations were discontinued and she was advised that her body would rid itself of the remaining mercury over the next several years.
Table 1. Biomarkers of mercury exposure at the beginning of pregnancy
During the 10 years following the incident she was in good health. She remained neurologically intact and did not have any depression or other suicide attempts during this period. In 2002, she had a spontaneous abortion at approximately 8 weeks gestation by dates which required dilation and curettage. No testing for mercury was performed at that time. In 1 year the young woman became pregnant again and sought medical care, at which time she was noted to have continued elevated blood and urine mercury levels.
Her blood mercury level at that time was 174 ngHg/mL, as reported by a commercial clinical laboratory. That lab also reported the acceptable levels as less than 20 ngHg/mL. She denied heavy consumption of fish or sea food and any environmental mercury exposure or accidents other than the suicide attempt with mercury in 1993.
The patient's weight was 52 kg and height 152 cm, and her vital signs were normal. The general physical examination revealed no abnormality except for numerous postoperative scars on the left arm mostly on the medial surface from wrist to shoulder. Neurological examination did not reveal any deviation.
Laboratory studies, including complete blood count, comprehensive metabolic panel with serum creatinine, urinalysis, and all routine prenatal labs, were normal, except for extremely high levels of mercury biomarkers. The level of mercury in serum, whole blood, and urine was assayed by Cold Vapor Atomic Absorption Spectroscopy by the procedure of Magos and Clarkson.Citation6 The results, all produced by the University of Rochester Mercury laboratory, and corresponding normal mercury levelsCitation7 are presented in .
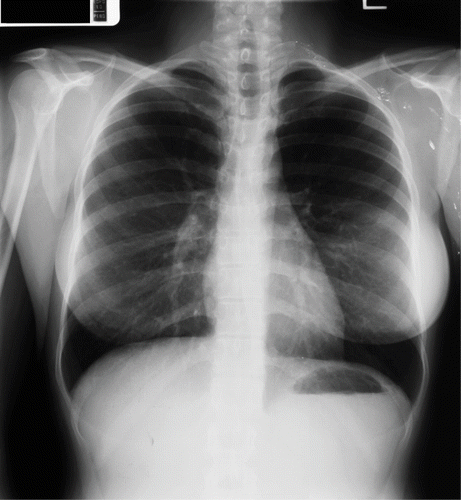
Prior to her pregnancy, a PA and lateral chest radiograph was obtained for this patient. The chest PA image is displayed in . The chest radiograph was normal, except for the presence of punctate radiopaque material (mercury) in the soft tissue of the left supraclavicular region and left axilla.
The patient was thoroughly evaluated by several physicians and different specialists. Obstetric ultrasound was initially performed at 8 weeks confirming gestational age. Other than the elevated mercury levels, the pregnancy was considered normal. In addition, a neurologist concluded that this woman had not developed any apparent neurological sequelae or long-term toxic damage from this exposure. Electromyogram and nerve conduction studies were normal.
We explained in detail to the patient and her husband the risks of prenatal mercury exposure to the fetus. In the case of methyl mercury exposure, adverse effects on the offspring include microcephaly; mental and neurological disturbances; ataxia; tremor; impairment of speech, gait, and vision development; cerebral palsy; as well as mental retardation and delay in achievement of developmental milestones.Citation8 Less information is available on inhaled mercury vapor during pregnancyCitation1 and virtually nothing is known about systemic exposure to metallic liquid mercury.
The risk to the developing child depended upon whether mercury present in the mother's circulation could cross the placenta and enter the embryo or the fetus. Factors possibly important to the outcome included the degree of protein binding of mercury in the mother and the valence state of the mercury reaching the placental barrier. It is theorized that a high degree of protein binding or a valence state different from zero might limit the amount of mercury available for transfer by the placenta. On the other hand, organic species such as methyl mercury easily cross the placental barrier, and at high doses can cause a devastating effect on the fetus and especially on the developing fetal brain.Citation8
The clinical team attempted to answer all of the patient's questions, with the understanding that a certain degree of uncertainty existed regarding the risk because this was an extremely unusual case. Despite her history of mercury exposure, her extremely high blood and urine mercury levels and potential risk of mercury-related developmental toxicity to the offspring, the parents decided to continue the pregnancy under close physicians’ monitoring.
Amniocentesis and fetal cord blood sampling to assess mercury levels were offered to the patient and were declined. Serial ultrasounds were completed throughout the pregnancy and revealed normal appearing anatomy and appropriate growth. The pregnancy developed without significant complications.
Biophysical testing remained reassuring until 37 weeks gestation at which time oligohydramnios and fetal breech presentation were noted. Due to these findings the patient was admitted for scheduled cesarean delivery. A primary low segment transverse cesarean delivery was performed.
Outcome
The delivered female infant was vigorous, weighing 2,910 g and 47 cm in length (15th percentile for these two variables). Apgar scores were 8 at 1 and 9 at 5 min, respectively. A little clear amniotic fluid and 2.0 cm fundal uterine septum were noted.
Specimens of maternal blood, urine, hair, cord blood, amniotic fluid, and placental tissue were obtained at the time of delivery and sent for determination of mercury levels. Colostrum and breast milk were collected over the first 3 weeks following the delivery and similarly sent for mercury determination ().
Table 2. Biomarkers of mercury exposure at time of delivery
The pathology report on the placenta noted a third trimester placenta with increased syncytial knots consistent with hypoperfusion. The trivascular umbilical cord, extra placental membranes, and fetal surface of the placenta were free of inflammation. The postoperative period was uneventful and newborn was fed by formula only.
Pediatric case report
The child, a 37-week term Cesarean birth, was evaluated at the age of 2 weeks. The baby was doing well, being bottle-fed and tolerating formula without any problems. There were no dysmorphic futures. Neonatal course was uncomplicated. The parents were of differing ethnic backgrounds with no history of consanguinity.
At the age of 2 weeks, the baby's weight was 3,050 g and length was 49 cm, head circumference 35 cm, and her vital signs were normal. The general physical examination revealed no abnormality except for a few newborn acne marks on the face. Her reflexes and neurological status were normal.
Over the next 8 months the baby's monthly weight, length, head circumference, and neurological status were monitored for any early sign of developmental delay and achievement of neurobehavioral milestones. During this time the baby girl had no problems. She was eating, sleeping, voiding and stooling well, continued to tolerate formula, and grew and developed appropriately. At the age of 6 months she weighed 8,500 g (90th percentile for age). Length was 68 cm (90th percentile) and head circumference was 43 cm (80th percentile). She received immunizations on a regular schedule.
Her developmental neurobehavioral milestones and growth were appropriate for her age.
At 1–2 months: She was able to hold her head erect and lift her head, became alert and responsive to voice, and recognized both parents and smiled spontaneously.
At 3–5 months: She was able to grasp toys, reach for and bring objects to the mouth, sit with support, laugh and babble, anticipate food on sight, and turn from back to side.
At 6–8 months: She was able to sit alone for short period, reached with one hand, passed object from hand to hand, rolled from back to stomach, started babbling, imitated “bye-bye.”
These are all characteristics of a well baby with no symptoms or signs of developmental delay.
Analysis
It is well known that target organs for inhaled elemental mercury are primarily the brain and the kidney but damage to the lung may also occur.Citation1 Usually, inhaled elemental mercury vapor readily crosses the blood–brain barrier and then is oxidized to mercuric mercury, believed to be the proximate toxic agent. Limited data from animal studies indicate that mercury vapor inhaled during pregnancy can be harmful to brain development in the fetus.Citation9
At the beginning of pregnancy (), the blood mercury level was 216 ngHg/mL or about 600-fold higher than mean Hg blood concentration for the U.S. population (0.34 ngHg/mL). A urine mercury level was 413 ngHg/mL or about 500-fold higher than normal urine Hg range (0.6–0.8 ngHg/mL).Citation7
Based on our findings (), metallic mercury crosses the placental barrier and reaches the fetus. The mercury level in maternal serum and cord serum is almost the same (179 and 145 ngHg/mL, respectively). Therefore, we can assume that even though the placenta had extremely high content of mercury (1,111 ngHg/g), it is not an absolute barrier which can prevent transmission of mercury from maternal blood to fetal blood (). This conclusion is consistent with the findings of a previous report on females exposed to inhaled mercury vapor.Citation10
In addition, confirming our hypothesis that the placental barrier is permeable to elemental mercury, we found that the mercury level in amniotic fluid is relatively high (8 ngHg/mL). This suggests that the fetus has received prenatal mercury exposure and has excreted mercury through its kidney into the amniotic fluid.
Maternal breast milk mercury levels were also high, ranging from 281 to 318 ngHg/mL (), much higher than the corresponding levels in maternal blood and more than 100-fold higher than median mercury concentration for the general population (1.59 ngHg/mL).Citation11
Table 3. Maternal, fetal, infant's biomarkers of mercury exposure during beginning pregnancy to postpartum (4 months)
Based on , there is evidence that maternal biomarkers of mercury exposure (whole blood, serum, urine) tend to decrease during pregnancy. The concentration in the placenta, 1,111 ngHg/g wet weight, indicates a high level of accumulation of mercury in this tissue. The maternal biomarkers of mercury exposure (serum, urine) at the beginning of the pregnancy, at delivery, and at 4 months postpartum show this trend but still remain extremely high. The increase in blood volume that takes place during pregnancy may be responsible for some or all of the declining mercury levels.
The half-life of mercury in the body, whether in the organic or inorganic form, is about 2–3 months.Citation1,Citation8 Therefore, we can expect that blood and urine mercury levels will decrease over time in the infant because mercury exposure has ceased. In addition, this baby girl was bottle fed and, therefore, she did not receive any additional mercury exposure from breast milk.
The infant's mercury levels fell from about 190 ngHg/mL cord blood to about 17 ngHg/mL infant blood or a factor of 10 (at the age of 4 months). The infant weighed 2.9 kg at birth and 7.4 kg at 4 months. This change is in part due to the growth-related increase in tissue and circulating blood volume, but the remainder must be explained by elimination. Despite the finding in rodents that mercury is not well excreted during the suckling period,Citation12 the findings in this report and those on infants exposed to a phenyl mercury compoundCitation13 indicate that excretion does occur in human infants.
The equal distribution between red cells and plasma and accumulation in the placenta are compatible with what is seen after exposure to divalent inorganic mercury, Hg2+.Citation1 The easy passage across the placenta is typical of dissolved metallic mercury in blood after exposure to mercury vapor. Thus our findings on the disposition of mercury in maternal blood, placenta, and cord blood suggest that both dissolved metallic mercury and its oxidation product, Hg2+, are present in the maternal blood.
Even though this infant did not have any signs or symptoms of developmental delay and her neurological status was normal, it is too early to make the assumption that subtle neurobehavioral problems such as learning disability or low IQ score will not someday develop. It is known, at least in the case of methyl mercury, that there may be a long latent period between exposure and onset of symptoms. Also prenatal exposure to mercury can affect the fetal kidney. While there are no abnormalities of this baby's kidney function at this point, such an occurrence cannot be precluded in the future.
Although it is beyond the scope of this report, the mother and daughter will be followed over the next 6–8 years to see whether the child develops any evidence of delay in achievement of neurobehavioral milestones or learning disability.
This case failed to show expected developmental effects associated with mercury from this type of exposure. For many reasons caution should be taken before extrapolating this result to other cases. Susceptibility to the effects of mercury may be predetermined by many factors, some of which may yet be unknown, and this case may shed new light and information on the toxicology of mercury.
Acknowledgments
The authors appreciate valuable contributions and advice provided by Dr. George Delclos at the University of Texas Health Science Center at Houston, School of Public Health. We thank Dr. Irwin Horwitz and Dr. Arch Carson at The University of Texas Health Science Center at Houston, School of Public Health and Dr. Grant Fowler at University of Texas Medical School at Houston for their assistance.
Authors appreciate collaboration and support during our study by staff of Department of Obstetrics, Gynecology and Reproductive Medicine at University of Texas Health Science Center at Houston and Mercury Laboratory at Medical School, University of Rochester, at Rochester, NY. We are thankful to Dr. Gary Myers at Rochester University, NY, for his help and encouragement and Dr. Susan Ramin at The University of Texas Medical School at Houston for clinical consultation on this case.
References
- WHO. Inorganic Mercury. Environmental Health Criteria 118. Geneva:International Program on Chemical Safety. World Health Organization; 1991.
- Givica-Pérez A, Santana-Montesdeoca JM, Díaz-Sánchez M, Martínez-Lagares FJ, Castaneda WR. Deliberate, repeated self-administration of metallic mercury injection: case report and review of the literature. Eur Radiol 2001; 11(8):1351–1354.
- Winker R, Schaffer AW, Konnaris C, Barth A, Giovanoli P, Osterode W, Rüdiger HW, Wolf C. Health consequences of an intravenous injection of metallic mercury. Int Arch Occup Environ Health 2002; 75(8):581–586.
- Schuurs AH. Working with mercury: cause of infertility in women?. Ned Tijdschr Tandheelkd 1998; 105(11):401–403.
- Olfert SM. Reproductive outcomes among dental personnel: a review of selected exposures. J Can Dent Assoc 2006; 72(9):821–825.
- Magos L, Clarkson TW. Atomic absorption determination of total, inorganic and organic mercury in blood. J Assoc off Anal Chem 1972; 55:966–971.
- Clarkson TW, Magos L, Myers G. Current concepts: the toxicology of mercury – current exposure and clinical manifestations. N Engl J Med 2003; 349(18):1731–1737.
- WHO. Environmental Health Criteria 101: Methylmercury. Geneva:International Program on Chemical safety, World Health Organization; 1990.
- Newland MC, Warfinge K, Berlin M. Behavioral consequences of in‐utero exposure to mercury vapor: alterations in lever-press durations and learning in squirrel monkeys. Toxicol Appl Pharmacol 1996; 139:374–386.
- Yang J, Jiang Z, Wang Y, Qureshi IA, Wu XD. Maternal–fetal transfer of metallic mercury via the placenta and milk. Ann Clin Lab Sci 1997; 27(2):135–141.
- Gundacker C, Pietschnig B, Wittmann KJ, Lischka A, Salzer H, Hohenauer L, Schuster E. Lead and mercury in breast milk. Pediatrics 2002; 110(5):873–878..
- Ballatori N, Clarkson TW. Biliary secretion of glutathione and of glutathione–metal complexes. Fundam Appl Toxicol 1985; 5(5):816–831..
- Pichichero ME, Gentile A, Giglio N, Umido V, Clarkson T, Cernichiari E, Zareba G, Gotelli C, Gotelli M, Yan L, Treanor J. Mercury levels in newborns and infants after receipt of thimerosal-containing vaccines. Pediatrics 2008; 121(2):e208–e214.