ABSTRACT
The purpose of this study was to investigate the effect of a supplementation with specific collagen peptides (SCP) combined with resistance training (RT) on changes in structural properties of the patellar tendon. Furthermore, tendon stiffness as well as maximal voluntary knee extension strength and cross-sectional area (CSA) of the rectus femoris muscle were assessed. In a randomized, placebo-controlled study, 50 healthy, moderately active male participants completed a 14-week resistance training program with three weekly sessions (70–85% of 1 repetition maximum [1RM]) for the knee extensors. While the SCP group received 5g of specific collagen peptides daily, the other group received the same amount of a placebo (PLA) supplement. The SCP supplementation led to a significant greater (p < 0.05) increase in patellar tendon CSA compared with the PLA group at 60% and 70% of the patellar tendon length starting from the proximal insertion. Both groups increased tendon stiffness (p < 0.01), muscle CSA (p < 0.05) and muscular strength (p < 0.001) throughout the intervention without significant differences between the groups. The current study shows that in healthy, moderately active men, supplementation of SCP in combination with RT leads to greater increase in patellar tendon CSA than RT alone. Since underlying mechanisms of tendon hypertrophy are currently unknown, further studies should investigate potential mechanisms causing the increased morphology adaptions following SCP supplementation.
Trial registration: German Clinical Trials Register identifier: DRKS00029244..
Highlights
A daily supplementation of 5 g of specific collagen peptides during 14 weeks of high-load resistance training increase patellar tendon hypertrophy compared to the same training regimen and placebo.
The resistance training-induced CSA increase, which was most pronounced on proximal and medial patellar tendon sites, is uniformly potentiated along the entire tendon length by supplementation.
Patellar tendon stiffness, CSA of the rectus femoris muscle and maximal voluntary knee extension strength increase due to training independently from supplementation.
Increased tendon CSA as a result of a stimulating effect of the supplementation with specific collagen peptides on collagen synthesis might be able to decrease tendon stress and support tendon healing.
1. Introduction
Similar to the muscle, the tendinous system demonstrates a high level of plasticity when exposed to mechanical loading (Bohm et al., Citation2015). Current research suggests that plastic changes at the macroscopic level of the tendon are supported by anabolic processes in the synthesis of matrix molecules. For example, Miller and colleagues (Miller et al., Citation2005) could demonstrate that collagen synthesis in the patellar tendon was significantly upregulated after a single strenuous exercise session. There is evidence that a repeated high-load resistance training program (>70% of maximum voluntary contraction [MVC]), sufficiently stimulates adaptations in tendon CSA as well as mechanical properties (Arampatzis et al., Citation2007; Kongsgaard et al., Citation2007).
In this context, collagen peptides might also be beneficial to augment these adaptations In vitro studies have shown that specific collagen peptides (SCP) can stimulate primary seeded fibroblasts to synthesize extracellular matrix (ECM) molecules as collagen type I and III (Schunck & Oesser, Citation2013). The low molecular weight and the high proportion of proline and hydroxyproline in collagen peptides lead to a high transport efficiency and resistance to intestinal digestion, which allows a high bioavailability of SCP (Feng & Betti, Citation2017; Taga et al., Citation2014, Citation2016; Wang et al., Citation2015).
In the Achilles tendon, recent data from our laboratory revealed that a daily dosage of 5g SCP during 14 weeks of high load resistance training led to significantly greater increase in cross-sectional area (CSA) compared with a placebo-receiving control group (Jerger et al., Citation2022). Since the study assessed Achilles tendon CSA by ultrasonography, findings about region specific adaptions of tendon morphology were not possible. Furthermore, the morphological features of tendons in various athletes suggest that adaptations are tendon- and load-specific (Ueno et al., Citation2018; Wiesinger et al., Citation2015). Findings of tendon-specific differences indicate that adaptations to training observed in one tendon are not systematically transferable to another one. Yet such findings also highlight the necessity for the Achilles and patellar tendons to adapt to higher loading patterns, and the potential benefit to explore dietary supplements that would facilitate this process for both tendons.
Therefore, the main purpose of the present investigation was to evaluate the effects of the daily supplementation of 5 g SCP for 14 weeks in combination with high load resistance training on morphological adaptions of the patellar tendon across the entire tendon length using magnetic resonance imaging (MRI). The results were compared to a control group with the same training regimen and daily supplementation of a placebo, in a double-blinded manner. In addition, effects of the SCP supplementation on mechanical properties of the patellar tendon (stiffness) as well as maximal dynamic knee extensor strength and morphology of the rectus femoris muscle were assessed. We hypothesized that the group receiving SCP would show higher adaptions of patellar tendon CSA than the placebo group. Additionally, we hypothesize based on the results of previous investigations, that training-related supplementation with specific collagen peptides has a reinforcing effect on mechanical patellar tendon adaptions as well as muscle CSA and maximal voluntary knee extension strength.
2. Methods
2.1. Participants
A total of n = 50 participants were recruited for the study with mean (± SD) anthropometric characteristics of 28.6 ± 5.1 years (age), 182.1 ± 7.9 cm (height), 78.9 ± 10.7 kg (weight) and 24.1 ± 3.7 kg/m² (BMI). In the mean, the participants reported a daily, activity related energy expenditure of 2976.9 ± 1652.9 kcal. The sample size results from an a-priori power analysis (G*Power V 3.1.9.2) based on the outcomes of an interventional study examining patellar tendon adaption after high load resistance training in young man (η² = 0.055; power = 80%; α < 0.05; calculated sample size: n = 38) (Kongsgaard et al., Citation2007) and an approximated dropout rate of 25–30% (Jerger et al., Citation2022). The calculation refers to statistical analysis using a mixed ANOVA (time x group).
Male participants aged between 18 and 40 years with a BMI between 18.9 and 29.9 and low to moderate physical activity (maximum of 120 min per week) were included in the study. Furthermore, injuries of the lower extremities, cardiovascular diseases as well as contraindications to physical activity or to the intake of the investigational products (collagen peptides or maltodextrine) were defined as exclusion criteria. In addition, participants who reported an intake of supplemental products (i.e. any forms of proteins, creatine) within the 6 months prior to the intervention were excluded and all participants were instructed to abstain from any supplementation apart from the assigned supplemental product throughout the course of the study.
Before the experimental testings, participants were informed about the study procedures and any potential risks. In accordance with the Declaration of Helsinki, all participants gave their written informed consent. The study was approved by the local ethics committee (F-2020-091) and the trial was prospectively registered at the German Clinical Trials Register (DRKS00029244).
2.2. Study design
A monocentric, prospective, placebo-controlled double-blinded trial was implemented to investigate the effects of collagen peptide supplementation on training induced adaptions of the patellar tendon. All measurements and trainings were performed at the Department of Sport and Sport Science of the University of Freiburg, Germany. Therefore, all participants were equally allocated into either a specific collagen peptide group (SCP) or a placebo group (PLA) in double-blinded fashion and block randomized for equal distribution of the main parameter patellar tendon cross sectional area (CSA) between the groups.
One week before the start of the intervention, participants underwent a screening procedure with medical anamnesis to check the inclusion criteria. Before and after the intervention, all participants completed measurements assessing patellar tendon morphology and mechanical properties as well as CSA and dynamic muscle strength (one repetition maximum [1RM]) of the quadriceps muscle. Between baseline and post-intervention, 14 weeks (three times per week) of high-load resistance training for the lower extremities was performed. Evidence suggests, that 14 weeks are sufficient to induce tendon adaptive responses (Bohm et al., Citation2015). All training sessions and measurements were supervised by experienced sport scientists. All experimenters of the study were blinded to treatment allocation throughout the entire course of the study including outcome assessment, training and statistical analyses. Blinding was conducted by a staff member not related to the study and unveiled after final statistical analyses. Participants were also blinded by providing supplements with similar taste and appearance.
2.3. Exercise protocol
Three weekly exercise sessions were conducted for 14 weeks. To ensure optimal recovery, two training days were separated by at least one day of rest. The participants started each session with a standardized ten minutes warm-up on a cycle ergometer (50-80 Watt).
The exercise protocol included leg press, knee extensions as well as calf raises in a standing and sitting position with three sets each. The training load was progressively increased every four weeks from 70% to 85% of the individual 1RM (Centner et al., Citation2019). In order to adjust the load to the individual training progress, 1RM was assessed before the loading was increased. Standardized resting periods between two sets were one min and three mins between two exercises. Leg press and knee extension exercises were performed from 90° knee angle to nearly complete extension. Calf raising exercises were performed in full range of motion from complete dorsal to plantar flexion. Additionally, latissimus pull and bench press exercises were added to the exercise protocol to increase compliance to the training and performed following the same regimen as the lower body exercises.
2.4. Supplementation
Participants in the SCP group received a daily dose of 5g of specific collagen peptides (TENDOFORTE, GELITA AG, Eberbach, Germany) and the PLA group supplemented the same dose of maltodextrin. The supplements were always dissolved in ∼250 ml water. According to recent research, tendon metabolism is mostly active during and immediately after loading (Baar, Citation2017) and amino acid absorption peaks at 30 min after supplementation (Shaw et al., Citation2017). The participants were, therefore, advised to ingest half of the supplement 30 min before and the other half immediately after the exercise sessions to ensure that the amino acids and peptides were provided to the tendon tissue at these periods of increased activity. On non-training days, the participants were advised to supplement at the same time of day as their last exercise session. Both water-soluble preparations could not be distinguished by taste or optical appearance.
2.5. Patellar tendon cross-sectional area
Patellar tendon CSA was measured using magnetic resonance imaging (MRI; Magnetom, Aera 1.5T, Siemens, Berlin, Germany) with a T1-weighted, turbo spin-echo, axial plane sequence (repetition time: 540ms; echo time: 12.0ms) in perpendicular direction to the patellar tendon alignment (Seynnes et al., Citation2009). Slice thickness of the contiguous axial MRI scans was set to 4mm without inter-slice gap. FOV was set to 200 × 200 and Matrix to 448 × 358. During the scans, the participants were placed in a supine position with the knee fixed in full extension. The contour of the patellar tendon CSA was manually outlined with the image processing software ImageJ (vers. 1.51, NIH, Maryland, USA) in each scan along the entire tendon length, from the patellar tendon apex to the tibial tuberosity. For that purpose, the mean of three manual CSA measurements was calculated and interpolated at each 10% interval of the tendon length (0–100% from patellar insertion to tibia insertion). Tendon length was assessed via sagittal MRI scans.
2.6. Patellar tendon mechanical properties
B-mode ultrasound of the patellar tendon deformation [60 mm, 10 MHz; ArtUs EXT-1H, Telemed, Vilnius, Lithuania] was recorded with a gel pad between ultrasonic probe and skin in order to enhance image quality. To check for potential probe shifts in relation to the joint, a hypoechoic marker was placed on the skin.
During the testing, participants were positioned in an isokinetic dynamometer [ISOMED 2000, Ferstl, Germany] in sitting position with a 60° hip angle. The right leg was fixed to the arm of the dynamometer with a knee flexion angle of 90°. All participants started with five ramped, isometric contractions until 80% of MVC for familiarization and tendon conditioning (Maganaris, Citation2003). Subsequently, five maximal, isometric ramp contractions were recorded with a standardized loading rate of 50 Nm/s. Visual feedback was provided to ensure a constant loading. All participants reached their MVC within three to five seconds (Werkhausen et al., Citation2018). During the time of the contraction, movement of tibia and patellar were recorded by ultrasound with a sampling frequency of 100 Hz and torque data were sampled at a frequency of 2000 Hz. Electromyography-based corrections for antagonist torque used in certain previous studies were not implemented in the present trial. This methodological choice was made in order to limit measurement noise, as recent data indicates an unclear connection between myoelectric activity and co-activation of hamstring muscles during voluntary knee extensions (Avrillon et al., Citation2018).
Patellar tendon elongation was measured by semi-automatic tracking (Tracker, V 4.95), as the distance from the tibial plateau to the inferior tip of the patellar during the isometric MVC. Patellar tendon force was calculated by dividing knee extension torque by the patellar tendon moment arm. The latter was assessed by sagittal MRI images and defined as the perpendicular distance between the midpoint of the tibio-femoral contact points in the medial and lateral femoral condyles to the tendon centre (Seynnes et al., Citation2009). For all collected data a second order low-pass Butterworth filter was used (cut-off frequency 15 Hz).
In accordance with earlier studies, tendon stiffness was defined as the linear slope of the force-elongation curve between 50 and 80% of MVC whereby for both measurement time points, the maximal individual contraction from baseline testing was set as 100% MVC (Werkhausen et al., Citation2018).
2.7. Muscle cross-sectional area
Rectus femoris muscle CSA was assessed by T1-weighted, turbo spin-echo, axial plane sequence MRI scans [Magnetom, Aera 1.5T, Siemens, Berlin, Germany] perpendicular to the thigh with the following parameters: repetition time = 544 ms, echo time = 9.9 ms, slice thickness = 1.0 cm, interslice gap = 0 mm. Muscle CSA was measured as described for patellar tendon CSA.
2.8. One-repetition maximum assessment
At the beginning of training weeks one, five, nine and thirteen as well as after the fourteenth week the participants conducted dynamic 1RM measurements for all exercises. The results of the individual 1RM testings were used to determine the training load. After an exercise-specific warm up (sets 1–2: 10 repetitions, ∼50% of estimated 1RM; sets 3–4: 3–5 repetitions, ∼80% of estimated 1RM), single repetitions with increased weight were performed (Baechle & Earle, Citation2000). In case of a successful execution (through whole range of motion and with proper technique), the participants rested for 4 min before the weight was progressively increased by 5–10% for the next trial. This procedure was repeated until the participants failed to execute the exercise properly (Patterson & Ferguson, Citation2011). The last successful execution was set as 1RM. All final 1RMs were achieved within five attempts.
2.9. Statistics
SPSS version 26.0 [IBM, Armonk, USA] was used for the statistical analysis of the data. All parameters were tested for significant group differences using t-test comparisons of baseline values. A Kolmogorov–Smirnov test was used on all variables to test their normality of distribution. Interaction effects were tested by mixed ANOVA (within-group factor: time; between-group factor: group) after confirming normal distribution and homogeneity of variances. Additionally, paired t-tests were calculated to test for significant changes from pre to post within both groups. Effect sizes are calculated using partial eta-squared (ηp²). Grubb’s test was used to identify outliers in main outcome criteria (Grubbs, Citation1950). All values identified as outliers were truncated according to (Steyerberg, Citation2009). If not stated otherwise, all data is presented as mean ± standard deviation. Additionally, the ηp² was calculated as a measure of effect size (small effect: ηp2 > 0.01, medium effect: ηp2 > 0.06, large effect: ηp2 > 0.14). The alpha level of significance was set to p < 0.05.
3. Results
A total of n = 31 participants successfully completed the study with n = 9 dropouts in the SCP group and n = 10 dropouts in the PLA group. All dropouts had voluntarily withdrawn from participation after the initial examination and none of the reasons for dropping out were related to the study (11x non-attendance due to lack of time, 4x non-attendance due to lack of enjoyment, 3x illness/injury outside the study, 1x change in personal circumstances). No adverse events were noted, and no pathological findings were observed in the routine anamnesis.
Comparison of anthropometric baseline characteristics of both groups showed no significant differences for age (SCP: 29.5.6 ± 5.7 years; PLA: 27.6 ± 4.1 years), height (SCP: 182.3 ± 8.8 cm; PLA: 181.9 ± 7.0 cm), weight (SCP: 79.4 ± 11.4 kg; PLA: 78.3 ± 9.8 kg) or BMI (SCP: 24.1 ± 3.7 kg/m2; PLA: 24.2 ± 3.8 kg/m2). In addition, none of the baseline values of the efficacy variables differed significantly between groups.
3.1. Patellar tendon cross-sectional area
Analyzing patellar tendon CSA in 10% intervals (0–100%), significant time effects (p ≤ 0.001) were identified at all locations except at 90% and 100% (p90% = 0.063, p100% = 0.498) in the ANOVA. Within group analyzation with paired t-tests showed significant CSA increases at all sites from 0% to 90% (SCP) and 0% to 70% (PLA), respectively (p < 0.05; (A/B)). Furthermore, there were significant time*group interactions at 60% and 70% of patellar tendon length in favour of the SCP group (p < 0.05; (C); ).
Figure 1. A/B: Patellar tendon CSA from pre to post in SCP (A) and PLA (B) group along entire tendon length from 0% (proximal) to 100% (distal). C: Percent change in patellar tendon CSA at 60% and 70% of tendon length. All data are mean and SEM. * indicates significant effects of time within group. # indicates significant time*group interaction effects.
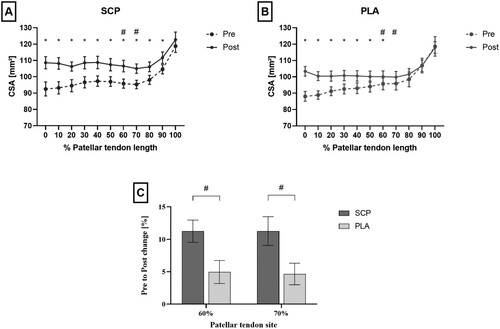
Table 1. ANOVA time*group interaction values for all patellar tendon sites.
3.2. Patellar tendon mechanical properties
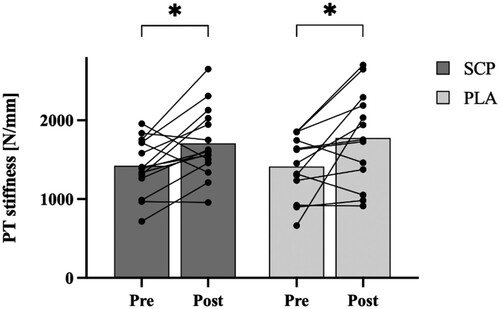
Patellar tendon stiffness increased from 1422.0 ± 336.9 N/mm to 1708.9 ± 410.2 N/mm and from 1459.3 ± 501.3 N/mm to 1774.9 ± 554.6 N/mm in the SCP and PLA group, respectively. The statistical analysis revealed a significant time effect (p < 0.01, ηp2 = 0.280) as both groups significantly increased their patellar tendon stiffness (SCP: + 20.2%, p < 0.05; PLA: + 21.6%, p < 0.01; ). There was, however, no time*group interaction (p = 0.97, ηp2 = 0.000).
3.3. Muscle cross-sectional area
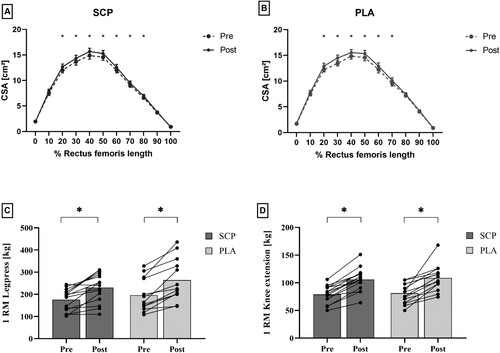
Location specific analyzes (0–100%) showed significant effects of time at all sites between 20 and 80% (p20-70% < 0.01; p80% < 0.05) but not at 0%, 10%, 90% and 100% (p0% = 0.68; p10% = 0.11; p90% = 0.09; p100% = 0.86; (A/B)). Within group analyzation with paired t-tests showed significant CSA increases at all sites from 20% to 80% (SCP) and 20% to 70% (PLA), respectively (p < 0.05; (A/B)). No significant time*group interaction effects were found.
Figure 3. A/B: Rectus femoris CSA from pre to post in SCP (A) and PLA (B) group along entire muscle length from 0% (proximal) to 100% (distal) shown in mean and SEM. C/D: Pre to post analysis of 1RM measurement of SCP and PLA group for leg press (C) and knee extension (D) presented as mean with individual pre to post values. * indicates significant within-group differences between pre and post.
3.4. Maximal muscular strength
Leg press 1RM changed from 175.9 ± 47.0 kg to 230.3 ± 62.7 kg in the SCP group and in the PLA group from 192.0 ± 68.6 kg to 263.8 ± 91.6 kg. Mixed ANOVA results revealed a significant time effect (p < 0.001, ηp2 = 0.701) but no interaction effect (p = 0.396, ηp2 = 0.028). Significant within-group differences were observed in both groups (p < 0.001).
Knee extension 1RM in the SCP group changed from 79.0 ± 15.1 kg to 105.9 ± 20.0 kg and in the PLA group from 81.2 ± 17.0 kg to 108.6 ± 22.6 kg. Mixed ANOVA results revealed a significant time effect (p = 0.001, ηp2 = 0.526) but no interaction effect (p = 0.805, ηp2 = 0.004). Significant within-group differences were observed in both groups (p < 0.001) ((C/D)).
4. Discussion
The present findings provide novel evidence that oral supplementation of specific collagen peptides (5 g per day) can potentiate patellar tendon hypertrophy after 14 weeks of high load resistance training (three sessions per week). Tendon stiffness, muscular strength and muscle CSA increased as a result of resistance training.
4.1. Tendinous adaptions
Mean patellar tendon CSA increased by +6.5% in the PLA group and by +10.7% in the SCP group. The present results of the control group are in accordance with other studies examining effects of exercise interventions of comparable duration (Wiesinger et al., Citation2015). For instance, in a study by Kongsgaard et al. (Citation2007), 12 weeks of unilateral high load resistance training led to a patellar tendon CSA increase of 6% in the proximal and 4% in the distal portion, respectively (Kongsgaard et al., Citation2007). There is little evidence coming from studies that have focused on structural tendon adaptations by collagen peptides. To our best knowledge, the only other study examining effects of protein supplementation on exercise related adaptions of patellar tendon CSA reported a similar tendon hypertrophy of 14.9% after 12 weeks of high load resistance training and supplementation (Farup et al., Citation2014). Furthermore, the results are in line with a very recent study, in which the oral administration the same dose of SCP lead to a significantly greater increase of Achilles Tendon CSA compared to placebo by 11.0% and 4.7%, respectively (Jerger et al., Citation2022).
Interestingly, even though the results show CSA increase over the entire tendon, hypertrophy was unevenly distributed along the length of the patellar tendon. Proximal and medial sites (0% to 70% SCP group, 60% PLA group; (A/B)) showed greater morphological increase than the distal end of the tendon. This is in line with previous studies reporting region specific tendon hypertrophy with tendency of greater changes in proximal regions (Kongsgaard et al., Citation2007; Seynnes et al., Citation2009). Some authors have attributed this to greater compressive loads of the tendon during movement, which could be responsible for increased stimulation of synthesis of matrix molecules (Kongsgaard et al., Citation2007).
Looking at group differences in the local distribution of the tendon hypertrophy, we find that, descriptively, the SCP group shows increased hypertrophy alongside the entire Patellar tendon. However, the group differences are most pronounced and reach statistical significance in more distal regions where the hypertrophy induced exclusively by training is lower and not in proximal regions with expectedly higher metabolic activity. This suggests that hypertrophy is uniformly potentiated by SCP supplementation in all tendon regions. In the present study, the additional effect of SCP supplementation on tendon CSA may be related to a stimulating effect of SCP on collagen synthesis and collagen fibril diameter as indicated by in vitro experiments (Schunck & Oesser, Citation2013) and in Achilles tendons of rodents (Minaguchi et al., Citation2005). Furthermore, in a study by Shaw et al. (Citation2017), supplementation with 15g of gelatin in combination with rope skipping exercises doubled the concentration of amino-terminal propeptide of collagen I (PINP) in blood samples compared to rope skipping and a placebo. Since PINP is known as a sensitive indicator of collagen I synthesis (Koivula et al., Citation2012), the authors concluded an increased collagen synthesis rate after gelatin intake and exercise in humans (Shaw et al., Citation2017). However, direct measurement of collagen turnover and analyzation of the deposited tendon tissue would be necessary to clarify the underlying mechanisms and nature of tendon hypertrophy. Both interventional groups equally increased tendon stiffness throughout the 14 weeks of resistance training. With a relative increase of +20.2% (SCP group) and +21.6% (PLA group) the results are in line with previous findings showing patellar tendon stiffness increases between +13.9% and +22.7% (Carroll et al., Citation2011; Kongsgaard et al., Citation2007; Seynnes et al., Citation2009).
The increased tendon hypertrophy is not accompanied by a similar increase in tendon stiffness. At the first glance, this finding may seem surprising,but previous research indicates that distinct patterns of loading may induce different relations between tendon morphological and mechanical properties (Wiesinger et al., Citation2017), suggesting that the structure and composition of the tendons influence mechanical properties in different ways. Optimal mechanical properties are highly depending on the individual task of the tendon (Thorpe et al., Citation2016) and alterations in non-collagenous components such as cross links, elastin fibres and glycoproteins within the extracellular matrix (Birch, Citation2007; Millesi et al., Citation1995; Thorpe et al., Citation2016) seem to be accountable for adapting the mechanical properties for optimal energy storage and positional control (Wiesinger et al., Citation2017). Physically, the level of tendon stress during loading is depending on the size of tendon CSA. Tendons with increased CSA should, therefore, be able to tolerate greater forces without suffering from pathological tissue damage. On clinical view, this might play a role in the prevention and treatment of tendon ruptures. Furthermore, it could potentially help to prevent overuse tendon injuries. Especially injuries occurring as a consequence of accumulated micro tears after frequent or excessive loading (Fung et al., Citation2010) are a common problem in running or resistance training (Kvist, Citation1994; Mersmann et al., Citation2017) and could potentially be improved by the intake of SCP. This assumption is supported by results from a pilot study by Praet et al. (Citation2019), in which symptoms of Achilles tendinopathic patients decreased by concomitant rehabilitation training and collagen peptide supplementation (Praet et al., Citation2019). On a functional level, an increase in tendon hypertrophy without alteration of tendon stiffness could therefore be beneficial to prevent or improve tendon injuries but still enable optimal energy transmission at the same time.
4.2. Muscular adaptations
Mean rectus femoris CSA increased by +4.6% in the SCP group and +5.5% in the PLA group. Both groups showed significant muscle hypertrophy in the middle region of the muscle (SCP: at 20–80% of muscle length; PLA: at 20–70% of muscle length; ), with no group differences at any location. Other studies report an increase in fat free mass after supplementation with SCP, indicating that SCPs might have an effect on muscle hypertrophy (Jendricke et al., Citation2019; Zdzieblik et al., Citation2015, Citation2021). Different results for different muscle groups seem to be indicative of a muscle-specific effect. For the gastrocnemius muscle, we found larger increases in muscle thickness with SCP supplementation in a recent study with comparable design (Jerger et al., Citation2022). One possible reason for this could relate to the different relative involvement of the muscles in the respective training exercises. The gastrocnemius muscle is the predominant contributor to plantar flexion torque. In addition to the rectus femoris muscle, the other three heads of the quadriceps are strongly involved in knee extension, with the rectus femoris muscle having the smallest physiological CSA. Muscle hypertrophy could be distributed over the entire muscle volume and could theoretically be underestimated in this measurement. Following numerous studies (Centner et al., Citation2022; Seynnes et al., Citation2009), we nevertheless decided to analyze the morphology of the rectus femoris muscle representative of the quadriceps muscle for reasons of better visibility by MRI.
Parallel to muscular hypertrophy, both groups equally increased their maximal muscular strength in leg press and knee extension. With an increase of +30.9% (leg press) and +34.1% (knee extension) in the SCP group and +37.0 (leg press) and +33.7 (knee extension) in the PLA group, both groups showed significant improvements in both exercises due to the exercise intervention, but no further effect of supplementation was detected ((C/D)).
4.3. Limitations
First evidence suggests that the composition of collagen peptide preparations is heterogeneous with disparate pharmacological effects and that the efficacy of a collagen peptide mixture cannot extrapolated to other formulations (Schadow et al., Citation2017). Therefore, it must be emphasized that the presented results are only reliable for the collagen peptide product that was investigated in this study and cannot be transferred to other collagen products.
Although it was not within the scope of the present trial, it is currently unknown to what extent the daily dosage of supplemented SCP or the intervention duration might influence myo-tendinous adaptations. Evidence indicates that the effect of collagen peptides is dose-dependent on body composition (Zdzieblik et al., Citation2017) but information is lacking for tendon adaptations. The available studies looking at tendon-specific adaptions with SCP show an effect with a daily supplementation of 5 g or less after several months (Jerger et al., Citation2022; Praet et al., Citation2019). Due to the effectiveness of the 5 g dosage observed by Jerger et al. (Citation2022), the similarity of the target parameters and study design, the present study was based on 5 g supplementation. Another point which needs to be considered when interpreting the findings from the present study is, that only young and moderately active males were included. Reasons for this decision were based on differences in the response to mechanical loading between men and women (Magnusson et al., Citation2007). The selection of the study population in the present study was therefore made to minimize variability in measurements and to increase statistical power. Future studies should include both sexes to represent the entire population. Lastly, the results could possibly indicate a positive influence of a supplementation with collagen peptides in the context of rehabilitation training after tendon injuries. However, studies in clinical settings with patients in the rehabilitation of tendon ruptures or tendinopathies are warranted to provide evidence in this regard.
5. Conclusion
The findings of this study demonstrated a potentiating effect of a daily supplementation of 5g SCP in combination with 14 weeks of high load resistance training on structural adaptions of the patellar tendon in men.
The data revealed a significantly greater raise in patellar tendon cross-sectional area at 60% and 70% of tendon length compared to the placebo group. Tendon mechanical properties, muscle strength and muscle morphology similarly increased in both groups after the training intervention.
The results might be relevant for clinical application, since increased tendon CSA induced by collagen deposition is accompanied by lower tendon stress levels during loading. Furthermore, a potential stimulating effect of SCP supplementation on collagen synthesis might as well be supportive during structural tendon healing processes.
Acknowledgements
We thank all participants who volunteered for this study. SJ designed research, performed experiments, analyzed data, interpreted results of experiments, prepared figures, and drafted and edited the manuscript. CC designed research, performed experiments, analyzed data, interpreted results of experiments, prepared figures, drafted and revised manuscript. BL designed research, analyzed data, interpreted results of experiments, edited and revised manuscript. ORS designed research, interpreted results of experiments, edited and revised manuscript. TF designed research, performed experiments and analyzed data. DL designed research, performed experiments and analyzed data. AG designed research, edited and revised manuscript. DK designed research, interpreted results of experiments, edited and revised manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Arampatzis, A., Karamanidis, K., & Albracht, K. (2007). Adaptational responses of the human Achilles tendon by modulation of the applied cyclic strain magnitude. Journal of Experimental Biology, 210(15), 2743–2753. https://doi.org/10.1242/jeb.003814
- Avrillon, S., Hug, F., & Guilhem, G. (2018). Between-muscle differences in coactivation assessed using elastography. Journal of Electromyography and Kinesiology, 43, 88–94. https://doi.org/10.1016/j.jelekin.2018.09.007
- Baar, K. (2017). Minimizing injury and maximizing return to play: Lessons from engineered ligaments. Sports Medicine, 47(S1), 5–11. https://doi.org/10.1007/s40279-017-0719-x
- Baechle, T. R., & Earle, R. W. (2000). Essentials of strength training and conditioning. Human Kinetics.
- Birch, H. L. (2007). Tendon matrix composition and turnover in relation to functional requirements. International Journal of Experimental Pathology, 88(4), 241–248. https://doi.org/10.1111/j.1365-2613.2007.00552.x
- Bohm, S., Mersmann, F., & Arampatzis, A. (2015). Human tendon adaptation in response to mechanical loading: A systematic review and meta-analysis of exercise intervention studies on healthy adults. Sports Medicine – Open, 1(1), 7. https://doi.org/10.1186/s40798-015-0009-9
- Carroll, C. C., Dickinson, J. M., LeMoine, J. K., Haus, J. M., Weinheimer, E. M., Hollon, C. J., & Trappe, T. A. (2011). Influence of Acetaminophen and ibuprofen on in vivo patellar tendon adaptations to knee extensor resistance exercise in older adults. Journal of Applied Physiology, 111(2), 508–515. https://doi.org/10.1152/japplphysiol.01348.2010
- Centner, C., Jerger, S., Lauber, B., Seynnes, O., Friedrich, T., Lolli, D., … König, D. (2022). Low-load blood flow restriction and high-load resistance training induce comparable changes in patellar tendon properties. Medicine & Science in Sports & Exercise, 54(4), 582–589. https://doi.org/10.1249/mss.0000000000002824
- Centner, C., Lauber, B., Seynnes, O. R., Jerger, S., Sohnius, T., Gollhofer, A., … König, D. (2019). Low-load blood flow restriction training induces similar morphological and mechanical Achilles tendon adaptations compared to high-load resistance training. Journal of Applied Physiology, 127(6), 1660–1667. https://doi.org/10.1152/japplphysiol.00602.2019
- Farup, J., Rahbek, S. K., Vendelbo, M. H., Matzon, A., Hindhede, J., Bejder, A., & Vissing, K. (2014). Whey protein hydrolysate augments tendon and muscle hypertrophy independent of resistance exercise contraction mode. Scandinavian Journal of Medicine & Science in Sports, 24(5), 788–798. https://doi.org/10.1111/sms.12083
- Feng, M., & Betti, M. (2017). Transepithelial transport efficiency of bovine collagen hydrolysates in a human CaCo-2 cell line model. Food Chemistry, 224, 242–250. https://doi.org/10.1016/j.foodchem.2016.12.044
- Fung, D. T., Wang, V. M., Andarawis-Puri, N., Basta-Pljakic, J., Li, Y., Laudier, D. M., & Flatow, E. L. (2010). Early response to tendon fatigue damage accumulation in a novel in vivo model. Journal of Biomechanics, 43(2), 274–279. https://doi.org/10.1016/j.jbiomech.2009.08.039
- Grubbs, F. E. (1950). Sample criteria for testing outlying observations. The Annals of Mathematical Statistics, 21(1), 27–58. https://doi.org/10.1214/aoms/1177729885
- Jendricke, P., Centner, C., Zdzieblik, D., Gollhofer, A., & König, D. (2019). Specific collagen peptides in combination with resistance training improve body composition and regional muscle strength in premenopausal women: A randomized controlled trial. Nutrients, 11(4), 892. https://doi.org/10.3390/nu11040892
- Jerger, S., Centner, C., Lauber, B., Seynnes, O., Sohnius, T., Jendricke, P., … König, D. (2022). Effects of specific collagen peptide supplementation combined with resistance training on Achilles tendon properties. Scandinavian Journal of Medicine & Science in Sports, 32(7), 1131–1141. https://doi.org/10.1111/sms.14164
- Koivula, M. K., Risteli, L., & Risteli, J. (2012). Measurement of aminoterminal propeptide of type I procollagen (PINP) in serum. Clinical Biochemistry, 45(12), 920–927. https://doi.org/10.1016/j.clinbiochem.2012.03.023
- Kongsgaard, M., Reitelseder, S., Pedersen, T. G., Holm, L., Aagaard, P., Kjaer, M., & Magnusson, S. P. (2007). Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiologica, 191(2), 111–121. https://doi.org/10.1111/j.1748-1716.2007.01714.x
- Kvist, M. (1994). Achilles tendon injuries in athletes. Sports Medicine, 18(3), 173–201. https://doi.org/10.2165/00007256-199418030-00004
- Maganaris, C. N. (2003). Tendon conditioning: Artefact or property? Proceedings of the Royal Society of London. Series B: Biological Sciences, 270(1), S39–S42. https://doi.org/10.1098/rsbl.2003.0004
- Magnusson, S. P., Hansen, M., Langberg, H., Miller, B., Haraldsson, B., Westh, E. K., & Kjaer, M. (2007). The adaptability of tendon to loading differs in men and women. International Journal of Experimental Pathology, 88(4), 237–240. https://doi.org/10.1111/j.1365-2613.2007.00551.x
- Mersmann, F., Bohm, S., & Arampatzis, A. (2017). Imbalances in the development of muscle and tendon as risk factor for tendinopathies in youth athletes: A review of current evidence and concepts of prevention. Frontiers in Physiology, 8, 987. https://doi.org/10.3389/fphys.2017.00987
- Miller, B. F., Olesen, J. L., Hansen, M., Døssing, S., Crameri, R. M., Welling, R. J., & Rennie, M. J. (2005). Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. The Journal of Physiology, 567(3), 1021–1033. https://doi.org/10.1113/jphysiol.2005.093690
- Millesi, H., Reihsner, R., Hamilton, G., Mallinger, R., & Menzel, E. J. (1995). Biomechanical properties of normal tendons, normal palmar aponeuroses, and tissues from patients with Dupuytren’s disease subjected to elastase and chondroitinase treatment. Clinical Biomechanics, 10(1), 29–35. https://doi.org/10.1016/0268-0033(95)90434-b
- Minaguchi, J., Koyama, Y., Meguri, N., Hosaka, Y., Ueda, H., Kusubata, M., & Takehana, K. (2005). Effects of ingestion of collagen peptide on collagen fibrils and glycosaminoglycans in Achilles tendon. Journal of Nutritional Science and Vitaminology, 51(3), 169–174. https://doi.org/10.3177/jnsv.51.169
- Patterson, S. D., & Ferguson, R. A. (2011). Enhancing strength and postocclusive calf blood flow in older people with training with blood-flow restriction. Journal of Aging and Physical Activity, 19(3), 201–213. https://doi.org/10.1123/japa.19.3.201
- Praet, S. F. E., Purdam, C. R., Welvaert, M., Vlahovich, N., Lovell, G., Burke, L. M., … Waddington, G. (2019). Oral supplementation of specific collagen peptides combined with calf-strengthening exercises enhances function and reduces pain in Achilles tendinopathy patients. Nutrients, 11(1), 76. https://doi.org/10.3390/nu11010076
- Schadow, S., Simons, V. S., Lochnit, G., Kordelle, J., Gazova, Z., Siebert, H. C., … Steinmeyer, J. (2017). Metabolic response of human osteoarthritic cartilage to biochemically characterized collagen hydrolysates. International Journal of Molecular Sciences, 18(1), 207. https://doi.org/10.3390/ijms18010207
- Schunck, M., & Oesser, S. (2013). Specific collagen peptides benefit the biosynthesis of matrix molecules of tendons and ligaments. Journal of the International Society of Sports Nutrition, 10(Suppl. 1), P23–P23. https://doi.org/10.1186/1550-2783-10-S1-P23
- Seynnes, O. R., Erskine, R. M., Maganaris, C. N., Longo, S., Simoneau, E. M., Grosset, J. F., & Narici, M. V. (2009). Training-induced changes in structural and mechanical properties of the patellar tendon are related to muscle hypertrophy but not to strength gains. Journal of Applied Physiology, 107(2), 523–530. https://doi.org/10.1152/japplphysiol.00213.2009
- Shaw, G., Lee-Barthel, A., Ross, M. L., Wang, B., & Baar, K. (2017). Vitamin C-enriched gelatin supplementation before intermittent activity augments collagen synthesis. The American Journal of Clinical Nutrition, 105(1), 136–143. https://doi.org/10.3945/ajcn.116.138594
- Steyerberg, E. W. (2009). Clinical prediction models – A practical approach to development, validation, and updating. Springer.
- Taga, Y., Kusubata, M., Ogawa-Goto, K., & Hattori, S. (2014). Highly accurate quantification of hydroxyproline-containing peptides in blood using a protease digest of stable isotope-labeled collagen. Journal of Agricultural and Food Chemistry, 62(50), 12096–12102. https://doi.org/10.1021/jf5039597
- Taga, Y., Kusubata, M., Ogawa-Goto, K., & Hattori, S. (2016). Efficient absorption of X-hydroxyproline (Hyp)-Gly after oral administration of a novel gelatin hydrolysate prepared using ginger protease. Journal of Agricultural and Food Chemistry, 64(14), 2962–2970. https://doi.org/10.1021/acs.jafc.6b00609
- Thorpe, C. T., Karunaseelan, K. J., Ng Chieng Hin, J., Riley, G. P., Birch, H. L., Clegg, P. D., & Screen, H. R. (2016). Distribution of proteins within different compartments of tendon varies according to tendon type. Journal of Anatomy, 229(3), 450–458. https://doi.org/10.1111/joa.12485
- Ueno, H., Suga, T., Miyake, Y., Takao, K., Tanaka, T., & Misaki Isaka, T. (2018). Specific adaptations of patellar and Achilles tendons in male sprinters and endurance runners. Translational Sports Medicine, 1(3), 104–109. https://doi.org/10.1002/tsm2.21
- Wang, L., Wang, Q., Liang, Q., He, Y., Wang, Z., He, S., & Ma, H. (2015). Determination of bioavailability and identification of collagen peptide in blood after oral ingestion of gelatin. Journal of the Science of Food and Agriculture, 95(13), 2712–2717. https://doi.org/10.1002/jsfa.7008
- Werkhausen, A., Albracht, K., Cronin, N. J., Paulsen, G., Bojsen-Moller, J., & Seynnes, O. R. (2018). Effect of training-induced changes in Achilles tendon stiffness on muscle-tendon behavior during landing. Frontiers in Physiology, 9, 794. https://doi.org/10.3389/fphys.2018.00794
- Wiesinger, H. P., Kosters, A., Muller, E., & Seynnes, O. R. (2015). Effects of increased loading on in vivo tendon properties: A systematic review. Medicine & Science in Sports & Exercise, 47(9), 1885–1895. https://doi.org/10.1249/MSS.0000000000000603
- Wiesinger, H. P., Rieder, F., Kösters, A., Müller, E., & Seynnes, O. R. (2017). Sport-specific capacity to use elastic energy in the patellar and Achilles tendons of elite athletes. Frontiers in Physiology, 8, 132. https://doi.org/10.3389/fphys.2017.00132
- Zdzieblik, D., Jendricke, P., Oesser, S., Gollhofer, A., & König, D. (2021). The influence of specific bioactive collagen peptides on body composition and muscle strength in middle-aged, untrained men: A randomized controlled trial. International Journal of Environmental Research and Public Health, 18(9), 4837. https://doi.org/10.3390/ijerph18094837
- Zdzieblik, D., Oesser, S., Baumstark, M. W., Gollhofer, A., & Konig, D. (2015). Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: A randomised controlled trial. British Journal of Nutrition, 114(8), 1237–1245. https://doi.org/10.1017/S0007114515002810
- Zdzieblik, D., Oesser, S., Jendricke, P., Gollhofer, A., & König, D. (2017). Effect of specific collagen peptides with various dosages on body composition in untrained men. Proceedings of the Nutrition Society, 76(OCE4). https://doi.org/10.1017/S0029665117003731