ABSTRACT
We aimed to investigate the impact of pistachio nut consumption on muscle soreness and function following exercise-induced muscle damage. Using a randomised cross-over design, male team-sport players (n = 18) performed a 40-minute downhill treadmill run to induce muscle damage, which was conducted after 2-wks of consuming either control (CON, water), a standard dose of daily pistachios (STD, 42.5 g/d) or a higher dose of daily pistachios (HIGH, 85 g/d). Lower limb muscle soreness (visual analogue scale), muscle function (maximal voluntary isokinetic torque and vertical jump), and blood markers of muscle damage/inflammation (creatine kinase, C-reactive protein, myoglobin, superoxide dismutase) were measured pre (baseline) and post (24, 48, and 72 h) exercise. No trial order effects were observed for any outcome measurement across trials. Mean quadriceps soreness (non-dominant leg) during exercise recovery was reduced (p < 0.05) in HIGH vs. CON (mean difference (95%CI): 13(1–25) mm). Change in soreness in the dominant quadriceps was not different between HIGH vs. CON (p = 0.06; mean difference (95%CI): 13(−1 to 26 mm)). No main effects of time or trial were observed for mean soreness of hamstrings, or on isokinetic torque of knee extensors or knee flexors, during recovery. Serum creatine kinase concentration peaked at 24 h post-damage (mean(SEM): 763(158)µg/L) from baseline (300(87)µg/L), but had returned to baseline by 72 h post (398(80)µg/L) exercise in all trials, with no trial or trial × time interaction evident. These data suggest that high dose pistachio nut ingestion may provide some alleviation of muscle soreness, but no effect on muscle function, following modest muscle damage.
Highlights
Pistachio nuts are considered a rich source of leucine and other essential amino acids, as well as being a good source of antioxidants. These properties suggest that pistachio ingestion could potentially influence recovery from exercise induced muscle damage.
Ingestion of 85 g/d of pistachios, for 2-wks prior to and during recovery from exercise-induced muscle damage, significantly reduced muscle soreness in the non-dominant limb knee extensors, in comparison to 0 g/d control.
No effects of pistachio ingestion were observed on muscle function or blood markers of damage suggesting that a mechanism of action on soreness is likely related to blunting of the inflammation response. However, further work is required to explore these effects in a larger sample when greater damage is induced.
Introduction
Athletes and exercisers routinely embark on high intensity and rigorous training routines to maximise their physiological potential. However, intense exercise training often results in exercise induced muscle damage (EIMD), characterised by muscle soreness, inflammation, the production of reactive oxygen species (ROS), and a temporary decrement in muscle function (Allen, Citation2001; Proske & Morgan, Citation2001). Whilst a degree of EIMD may be considered an important aspect of muscle adaptation (Hughes et al., Citation2018), this response becomes a limiting factor in the short term when muscle soreness and reduced muscle function impairs an athlete’s capacity to train or compete. Thus, considerable interest has been focused on interventions that mitigate muscle soreness and accelerate recovery in athletes, with multiple approaches investigated such as non-steroidal anti-inflammatory drugs, cold water immersion, ergogenic aids and nutritional interventions (Bongiovanni et al., Citation2020; Owens et al., Citation2019).
The International Olympic Committee has recognised creatine monohydrate, beta-hydroxy beta-methylbutyrate, omega-3 polyunsaturated fatty acids, vitamin D, gelatine and vitamin C, and anti-inflammatory supplements such as curcumin as potentially effective nutritional interventions to promote training capacity and exercise recovery (Maughan et al., Citation2018). Recent studies also have explored a food-first approach to promoting exercise recovery (Sousa et al., Citation2014). For instance, milk has been investigated as a cheap and readily available recovery drink with a nutrient profile that includes a rich source of protein, carbohydrates, lipids, vitamins and minerals. Studies have reported that milk ingestion attenuated the decline in peak torque, and reduced muscle soreness during post-exercise recovery in trained individuals (Cockburn et al., Citation2008; Rankin et al., Citation2015). Moreover, tart cherry juice ingestion was shown to attenuate markers of inflammation and oxidative stress following muscle damaging exercise, likely due to the antioxidant and inflammatory effects of vitamins C and E, and high carotenoid content (Bowtell et al., Citation2011; Connolly et al., Citation2006). These data provide promising rationale for investigating alternative functional foods for promoting exercise recovery in athletic populations.
Pistachio nuts are one of the least energy-dense tree nuts (∼165 kcal per 30 g portion), with high antioxidant activity and oxygen radical absorbance capacity (Ojeda-Amador et al., Citation2018; Yuan et al., Citation2022). Pistachios exhibit a unique lipid profile (60% of total fat content is derived from oleic and linoleic acid) and high antioxidant capacity due to their phenolic acid, carotenoid and phytosterol content (Bolling et al., Citation2010). These antioxidant properties are characterised by a series of in-vitro studies (Gentile et al., Citation2007). Raw and roasted pistachio nuts also are protein-rich (∼6 g of protein per 30 g serving) and exhibit high scores for protein quality (1.6 g leucine per 100 g of pistachios) and digestibility (Bailey & Stein, Citation2020; Higgs et al., Citation2021). Moreover, pistachios are rich in magnesium, copper, potassium, and manganese. Hence, this combination of a unique nutritional profile, bioactive compounds, easily digestible nutrients, and high protein content engender pistachio supplementation as a potential novel and contemporary strategy to promote acute muscle recovery following EIMD in athletic populations while supporting adaptations to training (Schoenfeld, Citation2010).
To date, exercise-based research into pistachio nuts as a recovery food have yielded mixed results (Nieman et al., Citation2014; Sari et al., Citation2010). Nieman et al. (Citation2014) conducted a randomised cross-over study whereby 2-wks of pistachio supplementation (85.0 g/day) was administered to trained male cyclists. After completing a 75 km cycling exercise, no discernible improvements in oxidative stress markers were reported with pistachio ingestion. Conversely, a randomised controlled trial investigated the effects of dietary substitution with pistachios on oxidative stress in a group of young football players. Twenty-one days of dietary substitution with pistachio nuts (25 g/day) led to an attenuation in biomarkers of oxidative stress compared with the non-substituted group and control (Sari et al., Citation2010). To our knowledge, no study to date has investigated the impact of pistachio supplementation on acute recovery from EIMD. Given the protein content, lipid profile and antioxidant capacity of pistachios, and the potential protective actions of these nutritional components in reducing indices of muscle damage, we aimed to determine whether pistachio nut consumption for 2-wk prior to, and during 3 d of recovery, after a downhill running protocol could impact upon lower limb muscle soreness and function. We also explored whether any effects observed in comparison to a water control trial were dose dependent (42.5, and 85.0 g/d). We hypothesised that feeding of pistachios would mitigate effects of EIMD through a reduction in delayed onset muscle soreness, improved recovery of muscle function, and suppression of markers of muscle damage in comparison to water control, and that these effects would be greater with consumption of a higher than lower dose of pistachios.
Methods
Participants and study design
Twenty-eight participants were recruited and enrolled into this study which had local ethics committee approval (IRAS ethics ID: 239045). However, due to COVID lockdown restrictions 10 participants did not complete the study, leaving a final cohort of n = 18 physically-active male individuals who all competed at a recreationally competitive level in team-sports (n = 10 soccer, n = 4 American football, n = 4 hockey) completing all three experimental trials (mean ± SEM, age (y) 23.1 ± 1.2, stature (cm) 180.7 ± 1.5; body mass (kg) 77.0 ± 3.0; VO2peak (mL/kg/min) 47.5 ± 2.3). Eligible participants were physically active males who performed at least 5 h of intermittent-based exercise per week. Participants also had no known metabolic diseases or eating disorders and were not using any nutritional supplements that could impact antioxidant or inflammatory status within a month preceding the trial. Exclusion criteria also included smoking, musculoskeletal limitations, and use of anti-inflammatory medications.
Trials consisted of a 40-min downhill treadmill run to induce muscle damage, which was conducted after 2-wks of consuming either control (CON, water), a standard dose of daily pistachios (STD, 42.5 g/d) or a higher dose of daily pistachios (HIGH, 85.0 g/d). Pistachios were administrated in three separate portions across the day. Trials were administered in a randomised cross-over fashion with a minimum 3-wk washout period between interventions. Seven participants began the study on the CON trial, six on STD trial and five on the HIGH trial. Pistachio intervention or control ingestion was continued on the day of muscle damaging exercise and during the following 3 days of recovery. Additional energy intakes in STD and HIGH was 256 and 512 kcal/d, respectively. Muscle measurements and blood samples were drawn prior to the downhill run and at 24, 48 and 72 h following completion of the downhill run to assess muscle soreness, function and circulating markers of muscle damage.
Preliminary assessments
At least 1-wk prior to the 2-wk supplementation period, participants were familiarised with the downhill running protocol designed to elicit muscle damage and all outcome measurements. In this regard, an investigator demonstrated the set-up of the treadmill in reverse on a gradient with the safety harness attachment and completed ∼2 min of downhill running. Prior to conducting the familiarisation downhill run, anthropometric measurements of body mass and stature were collected using a set of scales (SECA, UK) and wall mounted stadiometer, respectively. Skinfold measurements were conducted in duplicate across eight body sites (triceps, biceps, subscapular, iliac crest, supraspinal, abdominal, thigh and calf) by a trained ISAK practitioner using Harpenden callipers (HaB, UK). If repeat measurements varied by more than 10%, a third measurement was taken. For each skinfold site, an average value was calculated.
Participants also undertook a VO2peak test to inform the relative exercise intensity of the muscle damage protocol. Participants began the VO2peak test by performing a 10-min self-selected warm up. Following the warm-up, participants began running at 9km/h for 1-min followed by an increase of 1 km/h increments at 1 min intervals until 17 km/h was reached. Once a 1 min period had been completed at 17km/h, treadmill speed remained constant and the treadmill gradient increased by 1% every minute until voluntary exhaustion. Breath-by-breath measurements of VO2 were recorded continuously throughout the exercise duration via a metabolic analyser (Quark CPET, Cosmed, Rome, Italy). HR was recorded continuously via a radiotelemetry HR monitor (POLAR® chest strap) alongside RPE throughout the test. VO2 values of the last 15 s of each stage were averaged. Once the participant reached exhaustion (signalled by the participant raising their hand), the test was stopped and a VO2peak was established.
Habitual dietary intake was assessed over 3 days during the preliminary period using Nutritics software, and this analysis revealed that participant’s diets contained a total dietary carbohydrate of 423.1 ± 46.2 g/day, dietary protein of 113.6 ± 14.2 g/day, and dietary fat of 98.7 ± 10.1 g/day. Participants replicated dietary intake prior to trials and when questioned did not report any major deviations in dietary macronutrient content or total calorie intake.
Muscle damage protocol
The muscle damage protocol consisted of a 40-min downhill treadmill run, as described previously (Malm et al., Citation2004; Schwane et al., Citation1983). Participants maintained a steady-state HR throughout the 40-min downhill run at a −10% gradient. Target HR during the downhill run was set at predicted 70% VO2peak as calculated via regression analysis by plotting the individualised HR-VO2 relationship recorded during the preliminary VO2peak test. Accordingly, treadmill speed was selected to maintain a constant HR throughout the three trials. Heart rate was monitored to ensure a target exercise intensity was achieved. Over the 48 h period prior to the downhill run, participants abstained from exercise, and were prohibited from alcohol and caffeine intake. Water was consumed ad libitum and RPE was measured using the modified Borg scale at 5-min intervals during exercise (Borg, Citation1973).
Blood markers of muscle damage
Blood samples were drawn in the overnight fasted state from a medial cubital vein before the downhill run (0 h) and at 24, 48, and 72 h thereafter and dispensed into potassium EDTA and plain serum vacutainer tubes. The sample in the EDTA tube was kept on ice and then spun (3000 rpm) at 4°C and plasma was stored at –70°C until analysis. Plasma samples were assayed for creatine kinase (CK) and glucose concentration at each time point using enzymatic methods from commercially available kits on an iLAB Aries automated analyzer. The second sample tube was allowed to clot at room temperature, spun, and serum stored at –70°C until analysis. Serum samples were measured for high sensitivity C-reaction protein, myoglobin, superoxide dismutase (% inhibition) at each time point using ILab automated blood analyser, and insulin concentration using commercially available ELISA assay kits on a Biotek synergy plate reader. Insulin concentrations were converted from µIU/mL to pmol/L using a correction factor of 1 µIU/mL = 6 pmol/L (Knopp et al., Citation2019).
Muscle soreness and muscle function
A validated visual analogue scale (VAS) was used to measure muscle soreness of the quadriceps, hamstrings, gluteal, gastrocnemius and tibialis anterior muscle groups of both the dominant and non-dominant legs (Lau et al., Citation2015). In brief, participants marked their perceived feeling of soreness on a 100 mm scale between two anchor points that represented no pain (far left) or most pain ever experienced (far right). Measurements of soreness were conducted with participants in the following positions: the knee joint flexed at 90° (knee flexion), extended to 0° (knee extension) and general soreness of specific muscle groups without manipulation (quadriceps, hamstrings, gluteus maximus, gastrocnemius and tibialis anterior).
Muscle function was assessed via peak isokinetic torque performed using an isokinetic dynamometer (KinCom) at two contraction speeds (60 and 120 °/sec) for knee extension and knee flexion of both the dominant and non-dominant legs. Three maximal contractions were separated by a 30 sec rest interval and assessed at each contraction speed for each leg, with the best effort recorded. Peak (best of three) torque was recorded at each timepoint.
Vertical jump (best of 3 attempts) was assessed prior to the exercise bout and at each recovery time point using a Takei Jump Meter with hands placed on hips at all times during a countermovement jump (Takei Scientific Instruments Co., Ltd, Tokyo, Japan). A 30-sec rest period was provided between repetitions.
Statistical analysis
Data were analysed using a two factor (time and trial) repeated measures ANOVA with post-hoc Paired T-tests with Bonferroni correction for multiple comparisons when appropriate. Initial analysis of raw data for muscle soreness, muscle damage and muscle function examined for trial order effects in the model to determine if any learning effect or repeated bout effects occurred. When no order effect was observed this variable was then removed from the model and main effects of trial and time and their interaction were examined (IBM, SPSS Inc v26). The trial variable consisted of three levels (CON, STD and HIGH) and time consisted of four levels (0, 24, 48, 72 h) in a within-subjects model. Post hoc tests were used to analyse where any significant interactions and main effects occurred. Mean muscle soreness ratings were calculated during the 24-72 h recovery period following damage to determine overall effects across the whole recovery period for this variable. Statistical significance was set at P ≤ 0.05 and all data were reported as mean ± SD, unless otherwise stated.
Results
Downhill treadmill run
No differences in treadmill speed (CON: 15.2 ± 0.6 km/h; STD: 15.0 ± 0.7 km/h; HIGH: 15.3 ± 0.7 km/h, p > 0.05), average HR (CON: 150 ± 12 bpm; STD: 147 ± 9 bpm; HIGH: 151 ± 10 bpm, p > 0.05) and average RPE (CON: 16 ± 2; STD: 15 ± 3; HIGH: 16 ± 3, p > 0.05) were observed between trials over the 40-min treadmill run. No trial order effects were observed for any of the key outcome measurements (muscle soreness, blood parameters, vertical jump height, or MVC) across trials.
Muscle soreness
Based on analysis of raw soreness data, significant time and trial effects for subjective measures of muscle soreness were observed in the non-dominant quadriceps (p < 0.05), but no trial × time interaction effect was observed ((b)). Peak soreness was reported at 48 h of recovery. However, analysis of the mean non-dominant quadriceps soreness response over the entire 72 h recovery period revealed significantly reduced soreness in HIGH than CON (p < 0.05). No difference in mean dominant quadriceps soreness was observed between trials (p > 0.05; (a)). No main effects (trial, time, or trial × time interaction) were observed in soreness ratings for dominant and non-dominant hamstrings between trials ((c,d)), or in mean soreness of hamstrings across the recovery period despite soreness being highest during the CON trial. No main effects (trial, time, or trial × time interaction) were observed for soreness ratings of the dominant and non-dominant gastrocnemius ((e,f)). No main effects were observed for soreness ratings or mean soreness for any other muscle group, assessed during the passive state or under knee extension and flexion ().
Figure 1. Dominant (Dom, panels a, c and e) and non-dominant (Non-dom, panels b, d and f) mean muscle soreness (0-100 mm scale) measured in a passive state at baseline (0 h) and during the 72 h recovery period following downhill running in quadriceps, hamstring and gastrocnemius (Gastroc) muscles. Mean soreness over the 72 h recovery period is also presented as inset figures for the dominant (Dom, panels a, c and e) and non-dominant (Non-dom, panels b, d and f) quadriceps. Data are expressed as means ± SD. CON, Control (water) trial; STD, standard dose of 42.5 g/d pistachio supplementation; HIGH, a high dose 85 g/d pistachio supplementation. *indicates significant difference between HIGH and CON (p < 0.05).
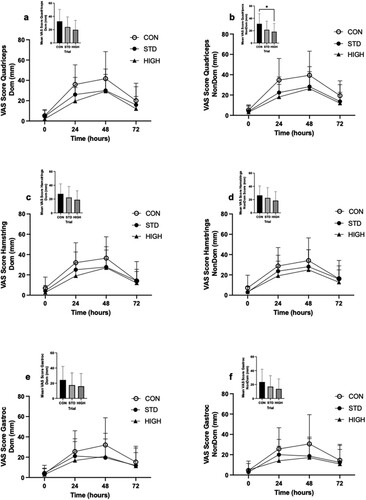
Table 1. Perceived soreness (0–100 mm scale) in muscle groups assessed before and during the recovery period, and mean soreness score over the whole 72 h recovery period, following downhill running.
Muscle function
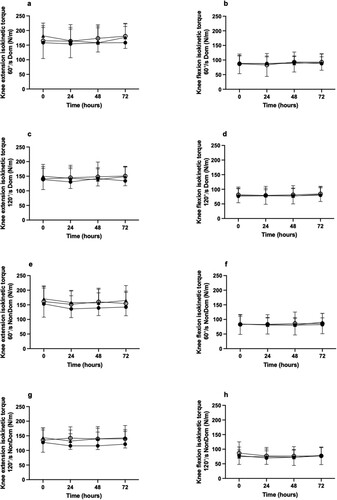
A time effect was observed for vertical jump height (p < 0.01) but no trial or trial × time interactions were detected. Bonferroni corrected pairwise comparisons did not reveal any specific time points at which jump height significantly differed from time 0 h. However, jump height declined from 51.7 ± 1.8 cm prior to damage, to 50.0 ± 1.8 cm and 50.1 ± 1.8 cm at 24 and 48 hr post damage respectively, and was then restored to 52.7 ± 1.7 cm at 72 hr of recovery.
No effects of time, trial, or trial × time interaction effects were observed for peak isokinetic torque of knee extensors or knee flexors of the dominant and non-dominant legs at either 60° or 120° / sec contraction velocities ().
Figure 2. Knee extension (panels a, c, e, & g) and knee flexion (panels b, d, f, & h) peak isokinetic torque at 60°/sec and 120°/sec in the dominant (Dom) and non-dominant (Non-dom) legs at baseline (0 h) and during the 72 h recovery period following downhill running in the control trial (CON), standard pistachio dose trial (1.5 oz/d STD) and high pistachio dose trial (3.0 oz/d, HIGH). Data are expressed as means ± SD.
Blood markers of muscle damage
A time effect for serum creatine kinase concentration was observed, but no trial effect or trial × time interaction was detected (). Creatine kinase concentration peaked at 24 h post-damage and remained elevated above baseline at 48 h post but had returned to baseline by 72 h post. No effect of time, trial, or trial × time interaction was observed for myoglobin, hsCRP, SOD, glucose and insulin concentration responses ().
Table 2. Plasma glucose, and serum insulin, high sensitivity C-reactive protein (hsCRP), creatine kinase, myoglobin, and superoxide dismutase (SOD) measured before and over the 72 h recovery period following downhill running.
Discussion
The overarching aim of this study was to investigate the impact of standard dose (STD) and higher dose (HIGH) pistachio consumption vs. control (CON) on muscle recovery from damaging exercise in physically-active young men. Our ecologically valid eccentric exercise protocol consisted of a downhill treadmill run and was successful in eliciting a moderate level of muscle damage, as evidenced by (i) an increase in muscle soreness in muscle groups assessed over the acute (72 h) recovery period, (ii) a small but consistent decrement in vertical jump performance, and (iii) an increase in serum CK concentration. Our principal finding was that muscle soreness of the non-dominant leg was attenuated in HIGH compared to CON during acute eccentric exercise recovery. However, this reduction in muscle soreness was not mediated by between-trial differences in putative blood biomarkers of muscle damage, inflammation or antioxidant status. Taken together, these data provide preliminary evidence, based on data averaged over the entire recovery period for the quadriceps muscles of the non-dominant leg, that daily snacking with a high dose of pistachios may mitigate against the perceived feelings of muscle soreness associated with eccentric-based muscle damaging exercise in physically-active individuals. However, the physiological relevance of this protective effect of pistachio ingestion on functional outcomes remains unclear. Moreover, in practical terms, daily snacking on such a high dose of pistachio nuts may not be feasible for all individuals.
The observation of highest muscle soreness ratings (according to mean values) in CON highlights the potential protective impact of pistachio consumption on perceived feelings of damage and recovery. A statistical difference in muscle soreness between pistachio ingestion trials and the placebo trial was detected in the quadriceps muscle group (non-dominant limb). Interestingly, the quadriceps muscle group was damaged to the greatest extent during the downhill running task, as evidenced by the highest soreness ratings at 48 h into recovery. This observation may be attributed to the dominant role of the quadriceps in controlling the downward motion of the body during downhill running, in addition to the higher proportion of fast twitch fibres compared to other (gastrocnemius, gluteus maximus and hamstring) muscle groups (Quindry et al., Citation2011), although this intuitive idea is not exclusively supported (Giandolini et al., Citation2017). A soreness rating of ∼40 mm was observed in CON for quadriceps compared with ratings of ∼20-30 mm in other muscle groups. Therefore, pistachio consumption appears to reduce muscle soreness during exercise recovery in muscles that have experienced the most damage. Accordingly, the non-dominant quadriceps muscle group demonstrated a larger effect than the dominant quadriceps muscle group. Statistical significance was reached between CON and HIGH trials for mean non dominant quadriceps muscle soreness rating. In the dominant quadriceps, this effect did not reach statistical significance although the magnitude of difference was very similar (∼13 points lower soreness score for CON than HIGH for both dominant and non-dominant quadriceps). The absence of statistical differences between trials in mean soreness ratings for the dominant quadriceps likely reflects slightly larger variance in response in this leg. Moreover, the absence of differences between trials for most other muscle groups assessed is likely attributed to the lesser damage incurred in these muscles, and therefore any potential effects of the pistachio intervention on muscle soreness likely fall within typical day-to-day variation of the measurement, or variance induced in a relatively small sample size. Future studies using a more potent eccentric loading stress on the muscle groups concerned, or recruitment of less active participants to exacerbate the muscle damage response, may yield significant outcomes from high dose pistachio snacking on muscle soreness during the acute recovery period.
The stimulus intensity and/or duration of the exercise protocol did not induce a large muscle damage response. Instead, a moderate level of damage is evidenced by a lack of change in serum myoglobin concentration, and soreness ratings that peaked at ∼30-40 mm on a scale of 0-100 mm across the muscle groups examined. Moreover, a relatively modest increase in plasma CK concentration was observed after 24 and 48 h in the current study in all treatment groups, with a peak increase (∼2.5-fold change) at 24 h post exercise. The CK response over the entire 72 h recovery period was lower in comparison to several similarly designed muscle damage studies that target the lower body (Eston et al., Citation1996; Philpott et al., Citation2018). Hence, coupled with the lack of change in muscle function, the observed CK response further supports the notion that our downhill running protocol in active young male team sport players induced only modest damage to lower limb muscles. As previously reported using a similar study design (Cooke et al., Citation2010), there was considerable variability between individual participants in the CK response to exercise. Although other blood markers of muscle damage and inflammation, i.e. lactate dehydrogenase and hsCRP are often measured in studies of this kind, they typically display a similar trend to CK. While our damage marker data suggest that a more intense damage protocol is required to markedly elevate these damage related responses, we were still able to detect a statistically significant amelioration of muscle soreness following HIGH dose pistachio consumption.
The reduction in non-dominant quadriceps muscle soreness during exercise recovery in HIGH did not translate to functional improvements in muscle performance, as determined by MVC and vertical jump measurements. The apparent disconnect between muscle soreness and muscle function outcomes has been reported in similar previous studies where fish oils (Philpott et al., Citation2018) or branched chain amino acids (Jackman et al., Citation2010) have been administered as the nutritional intervention. This lack of functional effect on muscle performance reinforces the notion that only a moderate degree of muscle damage was induced by the downhill running protocol in this cohort of physically-active, young men. The surprising observation of no change in peak isokinetic torque over time may be attributed, at least in part, to study design, given that we likely missed the nadir in muscle dysfunction typically observed within 1 h of exercise. This lack of time effect also may be attributed to a methodological constraint with the measurement of isokinetic peak torque rather than also measuring peak isometric torque that is considered a more sensitive measurement of muscle function. We decided to conduct measurements of isokinetic MVC rather than isometric MVC in order to more closely simulate muscle function which has a kinetic component. Nonetheless, given that mean values for peak isokinetic torque in all trials returned to baseline by 24 h of recovery, it is unlikely that our intervention modulated muscle function during exercise recovery. Moreover, a reduced soreness response in the non-dominant quadriceps muscle following pistachio ingestion, may reflect a reduction in inflammatory response to the muscle damage stimulus. A reduction in the general inflammatory response to tissue disruption may act to reduce nociceptor stimulation, and thus perceived feelings of muscle soreness (Basbaum et al., Citation2009). Although there was no effect of pistachio ingestion on the selected blood marker of inflammation (hsCRP) in the present study, an exploration of the impact of pistachio consumption on other inflammatory mediators such as eicosanoids, prostaglandins, thromboxanes, and leukotrienes may be warranted. This potential anti-inflammatory action of pistachio ingestion could be of particular interest to novice exercisers who undertake intense or unaccustomed physical activity sessions leading to a greater inflammatory response and muscle damage than in the present group of trained team sport players.
A key factor in detecting effects on primary outcome variables in any study is the population sample recruited in the investigation. We aimed to recruit healthy, active male participants who regularly participated in exercise training. In the present study, our participant sample regularly trained for team sport activities. Team sports are characterised by periods of intermittent high-intensity exercise, with several rapid accelerations and decelerations required during a typical game or training situation (Nedelec et al., Citation2013). Regular exposure to these types of activities likely predisposes participants to being more resistant to muscle damage during a downhill running task, through training induced pre-conditioning. This concept is analogous to the repeated bout effect and predisposes participants as less likely to suffer from a large muscle damage response to the downhill running task. These factors potentially explain the modest effect of downhill running on muscle soreness noted in our study cohort. However, despite this modest effect of the damaging exercise we still observed a partial reduction in muscle soreness, at least for the quadriceps muscles of the non-dominant leg, with the highest pistachio dose when compared with placebo.
The main strength of this study relates to the investigation of a standard functional food, rather than isolated supplement, that could be easily incorporated as a snack into the habitual diet. Moreover, the muscle damage stimulus of downhill running was more ecologically valid than many previously employed models such as isokinetic dynamometry induced damage (Jackman et al., Citation2010) or box drop jumps (Hohenauer et al., Citation2020). However, one potential limitation of the study was the randomised cross-over design. A main issue with muscle damage protocols designed in a cross-over fashion is the potential for a repeated bout effect (McHugh, Citation2003). This effect is where participants may adapt to a single bout of intense muscle-damaging eccentric exercise and therefore may gain some protection against damage in subsequent bouts of eccentric exercise. However, this repeated bout effect is likely to be greater when the severity of muscle damage is increased such as eccentric damage induced using a dynamometer. The more modest damage induced by downhill running may reduce the impact of repeated bout effects being evident, although the repeated bout effect has previously been observed after only 10 repetitions of maximal isometric contractions (Lima et al., Citation2018). Moreover, we implemented a 3 wk washout period between trials in order to reduce the chance of a repeated bout effect. To explore the potential for a repeated bout effect impacting on our findings, we examined whether there was an order effect in the trial responses for all key outcome variables. We observed no statistically significant order effects for these key outcomes in our analysis. Therefore, we can conclude that the order in which participants undertook the trials did not influence results. This observation indicates that any significant outcomes observed are not due to the order of trials but are more likely due to the intervention itself.
To conclude, we report that high dose consumption of pistachios leads to a modest but significant reduction in muscle soreness in the non-dominant quadriceps muscle group compared with placebo. This effect was of a similar magnitude in the dominant quadriceps but did not reach statistical significance. These data suggest that high dose pistachio consumption may provide some alleviation of soreness, particularly in muscles that are damaged to a greater extent. The impact of pistachio consumption on muscle soreness over 72 h of recovery implies that the mechanism of action is most likely related to an effect on degeneration and/or inflammation responses that occur during this time period (Allen, Citation2001; Proske & Morgan, Citation2001). Further work is warranted to explore whether continued ingestion of pistachios beyond the initial 72 h post-damage period could promote exercise recovery in exercisers of all ages. During this extended recovery period, the muscle enters the regeneration / remodelling phase (Urso, Citation2013). During these phases of recovery, the potential advantages of the amino acid profile and leucine content of pistachio nuts could support greater remodelling of muscle tissue.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Allen, D. G. (2001). Eccentric muscle damage: Mechanisms of early reduction of force. Acta Physiologica Scandinavica, 171(3), 311–319. https://doi.org/10.1046/j.1365-201x.2001.00833.x
- Bailey, H. M., & Stein, H. H. (2020). Raw and roasted pistachio nuts (Pistacia vera L.) are ‘good’ sources of protein based on their digestible indispensable amino acid score as determined in pigs. Journal of the Science of Food and Agriculture, 100(10), 3878–3885. https://doi.org/10.1002/jsfa.10429
- Basbaum, A. I., Bautista, D. M., Scherrer, G., & Julius, D. (2009). Cellular and molecular mechanisms of pain. Cell, 139(2), 267–284. https://doi.org/10.1016/j.cell.2009.09.028
- Bolling, B. W., McKay, D. L., & Blumberg, J. B. (2010). The phytochemical composition and antioxidant actions of tree nuts. Asian Pacific Jornal of Clinical Nutrition, 19(1), 117–123.
- Bongiovanni, T., Genovesi, F., Nemmer, M., Carling, C., Alberti, G., & Howatson, G. (2020). Nutritional interventions for reducing the signs and symptoms of exercise-induced muscle damage and accelerate recovery in athletes: Current knowledge, practical application and future perspectives. European Journal of Applied Physiology, 120(9), 1965–1996. https://doi.org/10.1007/s00421-020-04432-3
- Borg, G. A. (1973). Perceived exertion: A note on “history” and methods. Medicine & Science in Sports, 5(2), 90–93.
- Bowtell, J. L., Sumners, D. P., Dyer, A., Fox, P., & Mileva, K. N. (2011). Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Medicine & Science in Sports & Exercise, 43(8), 1544–1551. https://doi.org/10.1249/MSS.0b013e31820e5adc
- Cockburn, E., Hayes, P. R., French, D. N., Stevenson, E., & St Clair, G. A. (2008). Acute milk-based protein-CHO supplementation attenuates exercise-induced muscle damage. Applied Physiology, Nutrition, and Metabolism, 33(4), 775–783. doi:10.1139/H08-057
- Connolly, D. A., McHugh, M. P., Padilla-Zakour, O. I., Carlson, L., & Sayers, S. P. (2006). Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. British Journal of Sports Medicine, 40(8), 679–683. https://doi.org/10.1136/bjsm.2005.025429
- Cooke, M. B., Rybalka, E., Stathis, C. G., Cribb, P. J., & Hayes, A. (2010). Whey protein isolate attenuates strength decline after eccentrically-induced muscle damage in healthy individuals. Journal of the International Society of Sports Nutrition, 7(1), 30–30. doi:10.1186/1550-2783-7-30
- Eston, R. G., Finney, S., Baker, S., & Baltzopoulos, V. (1996). Muscle tenderness and peak torque changes after downhill running following a prior bout of isokinetic eccentric exercise. Journal of Sports Sciences, 14(4), 291–299. https://doi.org/10.1080/02640419608727714
- Gentile, C., Tesoriere, L., Butera, D., Fazzari, M., Monastero, M., Allegra, M., & Livrea, M. A. (2007). Antioxidant activity of Sicilian pistachio (Pistacia vera L. var. Bronte) nut extract and its bioactive components. Journal of Agricultural and Food Chemistry, 55(3), 643–648. https://doi.org/10.1021/jf062533i
- Giandolini, M., Horvais, N., Rossi, J., Millet, G. Y., Morin, J. B., & Samozino, P. (2017). Effects of the foot strike pattern on muscle activity and neuromuscular fatigue in downhill trail running. Scandinavian Journal of Medicine & Science in Sports, 27(8), 808–819. https://doi.org/10.1111/sms.12692
- Higgs, J., Styles, K., Carughi, A., Roussell, M. A., Bellisle, F., Elsner, W., & Li, Z. (2021). Plant-based snacking: Research and practical applications of pistachios for health benefits. Journal of Nutritional Science, 10, e87. doi:10.1017/jns.2021.77
- Hohenauer, E., Costello, J. T., Deliens, T., Clarys, P., Stoop, R., & Clijsen, R. (2020). Partial-body cryotherapy (-135 degrees C) and cold-water immersion (10 degrees C) after muscle damage in females. Scandinavian Journal of Medicine & Science in Sports, 30(3), 485–495. https://doi.org/10.1111/sms.13593
- Hughes, D. C., Ellefsen, S., & Baar, K. (2018). Adaptations to endurance and strength training. Cold Spring Harbor Perspectives in Medicine, 8(6), https://doi.org/10.1101/cshperspect.a029769
- Jackman, S. R., Witard, O. C., Jeukendrup, A. E., & Tipton, K. D. (2010). Branched-chain amino acid ingestion can ameliorate soreness from eccentric exercise. Medicine & Science in Sports & Exercise, 42(5), 962–970. https://doi.org/10.1249/MSS.0b013e3181c1b798
- Knopp, J. L., Holder-Pearson, L., & Chase, J. G. (2019). Insulin units and conversion factors: A story of truth, boots, and faster half-truths. Journal of Diabetes Science and Technology, 13(3), 597–600. https://doi.org/10.1177/1932296818805074
- Lau, W. Y., Blazevich, A. J., Newton, M. J., Wu, S. S., & Nosaka, K. (2015). Assessment of muscle pain induced by elbow-flexor eccentric exercise. Journal of Athletic Training, 50(11), 1140–1148. https://doi.org/10.4085/1062-6050-50.11.05
- Lima, L. C. R., Bassan, N. M., Cardozo, A. C., Gonçalves, M., Greco, C. C., & Denadai, B. S. (2018). Isometric pre-conditioning blunts exercise-induced muscle damage but does not attenuate changes in running economy following downhill running. Human Movement Science, 60, 1–9. https://doi.org/10.1016/j.humov.2018.05.002
- Malm, C., Sjodin, B., Sjoberg, B., Lenkei, R., Renstrom, P., Lundberg, I. E., & Ekblom, B. (2004). Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. The Journal of Physiology, 1(556), 983–1000. doi:10.1113/jphysiol.2003.056598
- Maughan, R. J., Burke, L. M., Dvorak, J., Larson-Meyer, D. E., Peeling, P., Phillips, S.M., ..., Engebretson, L., (2018). Ioc consensus statement: Dietary supplements and the high-performance athlete. International Journal of Sport Nutrition and Exercise Metabolism, 28(2), 104–125. https://doi.org/10.1123/ijsnem.2018-0020
- McHugh, M. P. (2003). Recent advances in the understanding of the repeated bout effect: the protective effect against muscle damage from a single bout of eccentric exercise. Scandinavian Journal of Medicine & Science in Sports, 13(2), 88–97. https://doi.org/10.1034/j.1600-0838.2003.02477.x
- Nedelec, M., McCall, A., Carling, C., Le Gall, F., Berthoin, S., & Dupont, G. (2013). Physical performance and subjective ratings after a soccer-specific exercise simulation: Comparison of natural grass versus artificial turf. Journal of Sports Sciences, 31(5), 529–536. https://doi.org/10.1080/02640414.2012.738923
- Nieman, D. C., Scherr, J., Luo, B., Meaney, M. P., Dreau, D., Sha, W., Dew, D. A., Henson, D. A., & Pappan, K. L. (2014). Influence of pistachios on performance and exercise-induced inflammation, oxidative stress, immune dysfunction, and metabolite shifts in cyclists: A randomized, crossover trial. PLoS One, 9(11), e113725. https://doi.org/10.1371/journal.pone.0113725
- Ojeda-Amador, R. M., Fregapane, G., & Salvador, M. D. (2018). Composition and properties of virgin pistachio oils and their by-products from different cultivars. Food Chemistry, 240, 123–130. doi:10.1016/j.foodchem.2017.07.087
- Owens, D. J., Twist, C., Cobley, J. N., Howatson, G., & Close, G. L. (2019). Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? European Journal of Sport Science, 19(1), 75–85. https://doi.org/10.1080/17461391.2018.1505957
- Philpott, J. D., Donnelly, C., Walshe, I. H., MacKinley, E. E., Dick, J., Galloway, S. D. R., Tipton, K. D., & Witard, O. C. (2018). Adding fish oil to whey protein, leucine, and carbohydrate over a six-week supplementation period attenuates muscle soreness following eccentric exercise in competitive soccer players. International Journal of Sport Nutrition and Exercise Metabolism, 28(1), 26–36. https://doi.org/10.1123/ijsnem.2017-0161
- Proske, U., & Morgan, D. L. (2001). Muscle damage from eccentric exercise: Mechanism, mechanical signs, adaptation and clinical applications. The Journal of Physiology, 537(2), 333–345. https://doi.org/10.1111/j.1469-7793.2001.00333.x
- Quindry, J., Miller, L., McGinnis, G., Irwin, M., Dumke, C., Magal, M., Triplett, N. T., McBride, J., & Urbiztondo, Z. (2011). Muscle-fiber type and blood oxidative stress after eccentric exercise. International Journal of Sport Nutrition and Exercise Metabolism, 21(6), 462–470. https://doi.org/10.1123/ijsnem.21.6.462
- Rankin, P., Stevenson, E., & Cockburn, E. (2015). The effect of milk on the attenuation of exercise-induced muscle damage in males and females. European Journal of Applied Physiology, 115(6), 1245–1261. https://doi.org/10.1007/s00421-015-3121-0
- Sari, I., Baltaci, Y., Bagci, C., Davutoglu, V., Erel, O., Celik, H., Ozer, O., Aksoy, N., & Aksoy, M. (2010). Effect of pistachio diet on lipid parameters, endothelial function, inflammation, and oxidative status: A prospective study. Nutrition, 26(4), 399–404. https://doi.org/10.1016/j.nut.2009.05.023
- Schoenfeld, B. J. (2010). The mechanisms of muscle hypertrophy and their application to resistance training. Journal of Strength and Conditioning Research, 24(10), 2857–2872. https://doi.org/10.1519/JSC.0b013e3181e840f3
- Schwane, J. A., Johnson, S. R., Vandenakker, C. B., & Armstrong, R. B. (1983). Delayed-onset muscular soreness and plasma CPK and LDH activities after downhill running. Medicine & Science in Sports & Exercise, 15(1), 51–56. https://doi.org/10.1249/00005768-198315010-00010
- Sousa, M., Teixeira, V. H., & Soares, J. (2014). Dietary strategies to recover from exercise-induced muscle damage. International Journal of Food Sciences and Nutrition, 65(2), 151–163. https://doi.org/10.3109/09637486.2013.849662
- Urso, M. L. (2013). Anti-inflammatory interventions and skeletal muscle injury: benefit or detriment? Journal of Applied Physiology, 115(6), 920–928. https://doi.org/10.1152/japplphysiol.00036.2013
- Yuan, W., Zheng, B., Li, T., & Liu, R. H. (2022). Quantification of phytochemicals, cellular antioxidant activities and antiproliferative activities of raw and roasted American pistachios (Pistacia vera L.). Nutrients, 14(15), https://doi.org/10.3390/nu14153002

