ABSTRACT
A simple and efficient synthesis of cuprous oxide and cupric oxide nanoparticles was carried out with controlled surface properties via soft chemistry approach. The monodisperse and versatile Cu2O and CuO nanoparticles were characterized with the help of FT-IR, UV-Vis, TGA/DSC, XRD, SEM-EDX, TEM, and BET. From the XRD analysis, the size of Cu2O and CuO nanoparticles were calculated as 31 nm and 32 nm, respectively, which is calculated with the help of Scherer equation, as supported by TEM. The morphology of the nanoparticles can be controlled by tuning the amount of KOH and nature of solvent. Chemie douce approach is a novel, cheap, and convenient technique suitable for industrial production.
INTRODUCTION
Metal oxide nanoparticles are a versatile material with many scientific and industrial applications.[ Citation 1 ] Synthesis of high-quality nanopowders with respect to chemical purity, phase selectivity, crystallinity, and homogeneity in particle size with controlled state of agglomeration in a cost-effective procedure is still a challenge to material chemists.[ Citation 2 ] The nanostructure materials have attracted considerable interest in synthetic methodology and its sensor device technology. Copper Oxide is a p-type semiconductor with direct band gap and high absorption properties that make it a promising material for low-cost photovoltaic cells. Copper oxide nanoparticles are used in a wide range of applications such as gas sensors, magnetic storage media, solar energy transformation, semiconductors, and organic catalysis.[ Citation 3 , Citation 4 , Citation 5 , Citation 6 ] For synthesizing copper oxide nanoparticles, many efficient approaches has been carried out such as Sonochemical preparation,[ Citation 7 ]alkoxide-based preparation,[ Citation 8 ] microwave irradiation,[ Citation 9 ] precipitation-pyrolysis,[ Citation 10 ] and thermal decomposition.[ Citation 11 ] So far these methods were not able to control the particle properties and gives low yield with poor crystallinity.
The present work is focused on the cost-effective synthesis and characterization of nanometer-sized ultrafine particles with monodispersity, carried out via soft-chemical approach with controlled properties. Single-source molecular precursor undergoes hydrolysis and polycondensation reactions to the preparation of green functional nanoparticles.
The conversion of the precursor into its respective metal oxides is considered to be topotactic in nature with favorable properties and well characterized by physicochemical techniques.
In nonaqueous sol-gel process, the formation of metal oxide nanoparticles takes place by providing oxygen from alkali hydroxides or by organic constituent present in the precursor as shown below:
EXPERIMENTAL
Synthesis of Cu2O Nanoparticles via Aqueous Sol-Gel Process
The solution was prepared by dissolving the calculated amount of CuCl (3.7 g, 37.52 mmol) and KOH (5.3 g, 94.64 mmol) in 25 mL of distilled water and stirred for 2 h followed by refluxing for another 6 h. The compound was separated out by filtration and filtrate was washed with distilled water till neutralization is over. The materials were oven dried at 150°C. On calcination (at 450°C, 4 h) in the presence of nitrogen atmosphere, black powder was obtained in a quantitative yield and high purity.
Synthesis of CuO Nanoparticles via Nonaqueous Sol-Gel Process
The calculated amount of CuCl2.H2O (9.7 g, 56.9 mmol) and KOH (8 g, 142.9 mmol) were taken in benzene and hexane mixture and it was stirred for 2 h followed by refluxing for 6 h.
The compound was separated out by filtration and filtrate was washed with methanol till the filtrate reaches pH 7, then it was oven dried (150°C). On calcination (450°C, 4 h) in the presence of nitrogen atmosphere, black powder was obtained according to the calculated yield and with monodispersity.
Characterization
The structural and morphological characterization of as-prepared Cu2O and CuO nanoparticles were carried out with the help of techniques, which are as follow. Fourier transfer infrared (FT-IR) spectra were recorded at room temperature in the range of 4000 to 400 cm−1as KBr pellets using a Perkin-Elmer Spectrometer GX model with a wave number resolution of 4 cm−1. Ultraviolet (UV) absorbance spectra were recorded on a GBC Cintra 10/20/40 spectrometer in dry methanol as dilutor in the range 200–800 nm with a scan speed of 200 nm per minute. The phase purity was confirmed by thermal transformation pattern studied by TGA/DSC (Mettler Toledo star, Columbus, OH) in nitrogen, with a heating rate of 10°C/min from 25°C to 1000°C. X-ray powder diffraction (XRD) patterns were taken in reflection mode CuKα (λ = 1.5406 Å) radiation in the 2θ range from 0° to 80° on a Seimens (Cheshire, UK) D5000 X-ray diffractometer by continuous scanning with a step size of 0.01°. Scanning electron microscope (SEM) images were obtained on a Hitachi S520 scanning electron microscope. Energy dispersive X-ray spectroscopy (EDAX) was taken in an Oxford link ISIS-300 insrument. The morphologies were investigated with a Philips Tecnai G2FEI F12 Transmission Electron Microscope operated at 80–100 KV. The samples for transmission electron microscopy (TEM) were prepared by loading a toluene-suspension of the nanoparticles onto a formvar-coated copper grid. The Brunauer-Emmett-Teller (BET) surface area analysis was done in an AUTOSORB 1 Quantachrome instrument under nitrogen atmosphere after degassing the samples at 200°C for 1 h. All the other solvents and chemicals were obtained from commercial sources and purified using standard methods.[ Citation 12 ]
RESULTS AND DISCUSSION
As part of our research programme to synthesize nanoparticle with improved particle properties, we used a chemie douce approach towards the synthesis of material by controlling the surface energy. The functionalized particle was characterized by the following techniques.
FT-IR
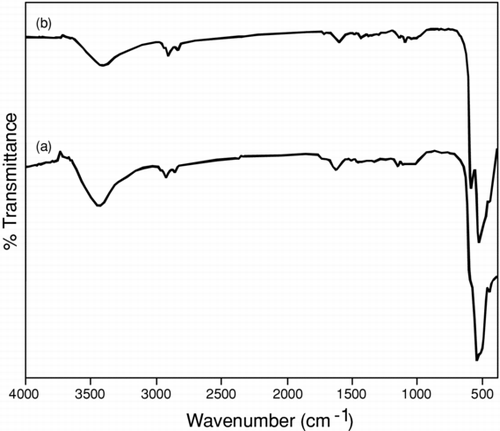
Metal oxide generally gives absorption bands below 1000 cm−1 that arise from interatomic vibrations; by observing significant frequencies helps in the confirmation of particle formation. In Cu2O nanoparticle, the symmetric and asymmetric frequencies were observed at 1162 and 532 cm−1, respectively (), which corresponds to M-O overtones frequencies.[ Citation 13 , Citation 14 ]CuO nanoparticle has similar characteristic frequencies at 1115 cm−1; however, the appearance of two frequency peaks at 593 and 527 cm−1 relate to M-O bond vibrational frequencies support the presence of monoclinic phases.[ Citation 15 ] Therefore it was assumed that the formation of phase takes place depending on the synthetic methodology. It was further supported by thermal analysis and XRD. In general, the close similarity was observed between Cu2O and CuO nanoparticles probably due to small particle size by observing peaks below1000 cm−1.
UV-Vis absorption
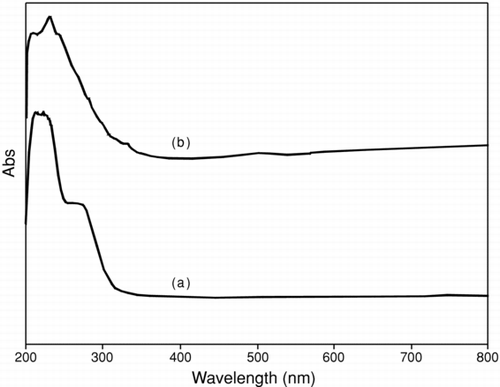
A prominent absorption band was observed at around 240 nm for both particles (). The absorption peak is probably related to the electronic transition taking place from valence band to the conduction banddue to quantum size of particle.[ Citation 16 ] Peaks at 289 and 275 nm may be attributed to the formation of Cu2O and CuO, respectively. These bands suggest higher structural organization in the particle that takes place due to the donor-acceptor nature of metal ion, which leads to size-quantization effect due to band transition. A weak, broad peak centered at ∼ 500 nm is attributed to the band gap transition of CuO present at the surface of the nanocrystal.[ Citation 17 ] The sharp absorbance leads to an increase in particle size due to high surface energy of smaller particles with controlled shape, supporting the formation of uniform-particle in solution chemistry. The difference in absorption bands in both cases illustrates the degree of crystallinity increases with surface purity, as supported by thermal analysis. It is concluded that broadness of the absorption edge shift to lower wavelength due to the small particle size with wide distribution, with the help of tuning the synthetic methodology.
TGA and DSC
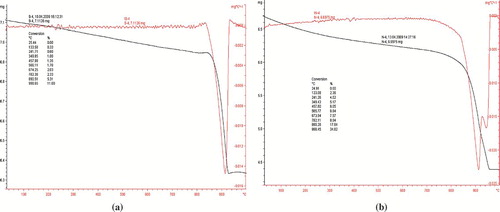
The thermal analysis was carried out to find the crystallinity temperature (). In both nanopowders, the endothermic peaks were attributed to first weight loss was related to the removal of water from the surface at 150°C. The second weight loss occurs at 850°C can be attributed to the removal of organic moieties. At higher temperatures, no further significant weight loss was observed, thereby supporting the formation of crystallinity with high purity. The DSC of CuO and Cu2O shows the presence of a common sharp peak at 916°C; however, an additional peak at 960°C was observed in CuO, thereby supporting the existence of monoclinic phase with its self- assembled structure depending on the oxidation state of copper ion.
XRD
X-ray diffraction analysis reveals that amorphous phases was obtained in as-synthesized condition, and subsequently on heat treatment at 450°C for 4 h in the presence of inert atmosphere, the amorphous phase transforms into crystalline phase with enhanced particle properties (). On removal of impurities, the sharp diffraction peaks was observed with high intensity. By applying the Debye-Scherer equation, the size of the particle was calculated to be in the range of 32–31 nm.[ Citation 18 ] All the diffraction peaks can be indexed with lattice constant and compared to the Joint Committe on Powder Diffraction Standards (JCPDS).
FIGURE 4 X-ray diffraction patterns of copper oxide nanoparticles after calcination at 450°C in nitrogen for 4 h support the presence of phase in Cu2O and CuO, respectively, whereas the diffraction peaks for Cu2O matches with CuO peaks, as illustrated in the figure: (a) Cu2O and (b) CuO nanoparticles.
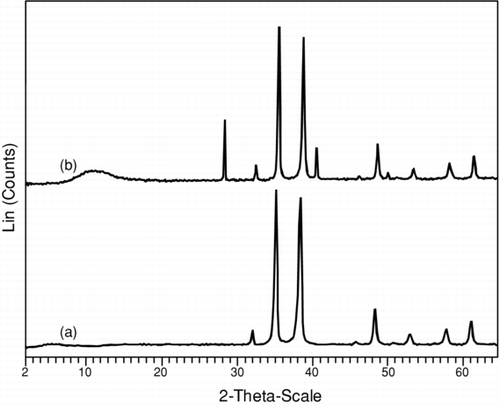
λ θ D = Kλ/β cos θ The d-spacing values of two samples matches well with JCPDS files for Cu2O and CuO (No. 05-0661, 45-0937), corresponding to cubic and monoclinic crystal system, respectively.[ Citation 19 ]The particles have approximately the same density due to average shape with same surface area. In Cu2O particle, the diffraction peaks can be indexed to cubic structure of distorted octahedral shape.[ Citation 14 ] The value of peak with 2θ corresponds to the crystal planes of crystalline CuO, supporting all reflections can be indexed to correlate the monoclinic phase.[ Citation 17 ] In general, the diffraction peak pattern are same for both the particles; however, the additional peaks in CuO can be corelated to the oxidation state of Cu2 + ion.
SEM-EDX
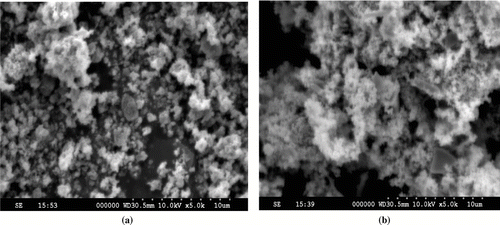
The micrographs as displayed in support that the particle size distribution depends on the effect of time, synthetic methodology, morphology, and the nature of molecular precursor used. It also support the formation of well-dispersed spherical and regular polyhedron shape for the Cu2O and CuO nanoparticles, respectively, with clear porosity. Due to high surface charge, the process of agglomeration takes place due to Ostwald ripening process. The primary particles appeared in the form of agglomerates, ranging from submicron to few microns in size. An ultrafine, uniform, and aggregated spherical crystallite particle, with modal diameters around 10 nm, was observed, depending on experimental conditions. From the SEM images, it is clearly observed that the powders synthesized at pH 3 have regular shape. At pH 7–8, the rate of nucleation process increases due to presence of charges at the particle surface, which leads to crystallinity. At the nanosized scale, the individual nanoparticle has limited aggregation. In case of Cu2O, the individual particle has nearly round shape with aggregation.[ Citation 14 ] In case of cuprous oxide, limited crystallinity was observed with octahedral shape, thereby supporting the presence of limited aggregation.[ Citation 15 , Citation 17 ] It is assumed that the polarity of solvent with synthetic methodology affects the properties of nanoparticles.
FIGURE 5 SEM images of (a) Cu2O and (b) CuO nanoparticles after calcination at 450°C in nitrogen for 4 h, showing agglomerated particles due to weak inter-intra particle forces.
The elemental quantification and stoichiometry ratio of copper oxide nanoparticles were confirmed by X-ray dispersive analysis shows the presence of uniform distribution of copper to oxygen with atomic ratio 2:1 and 1:1 in Cu2O and CuO, respectively, which agrees well with XRD studies.
TEM
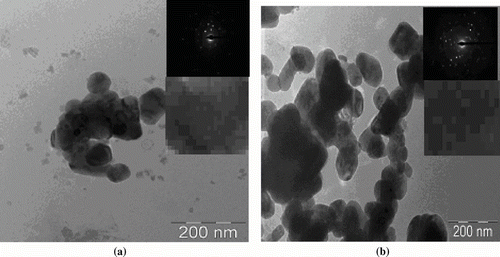
The micrographs in show the presence of irregularly shaped and moderate size distribution network of larger particles due to agglomeration, with average well-dispersed particle size of 31 and 32 nm for Cu2O and CuO, respectively, which agree well with XRD analysis.[ Citation 20 ]These particles are joined closely to form nanosphere. The existence of uniform size, shape, and phase purity with clear morphology and crystallinity was confirmed by electron diffraction analysis. The presence of diffraction rings supports the existence of well-dispersed spherical and regular polyhedron shape for the Cu2O and CuO nanoparticles, respectively. The octahedral Cu2O particles has symmetrical morphology and cubic symmetry, supported by selected area electron diffraction (SAED) pattern of the particle with single crystalline nature with uniformity.[ Citation 16 ]The presence of dark and light spots in CuO suggests the presence of many small tiny nanoparticles. SAED supports the defect-free single-crystalline structure.[ Citation 15 , Citation 17 ] The particle mapping shows the well-defined crystallinity with uniform spacing, it can be assume that the nanoparticles are embedded into gel intermediate and agglomerates itself after washing. It can be concluded that self-assembled structure has potential application based on high surface to volume ratio of particle.
BET
The specific surface area for the nanocrystals synthesized via sol-gel techniques was determined experimentally. The BET surface area and pore volume was found to be (3.3 and 1.3 m2/g) (0.761 and 0.299 cm3· g−1) for the Cu2O and CuO nanoparticle samples, respectively.[ Citation 8 ]Synthetic methodology has an effect on particle properties depending on the oxidation state of copper ion, as evident with steady decrease in specific surface area with increase of temperature. The gradual increase in particle size is related to grain growth at higher temperatures, manifesting itself to the reduction of surface area with required geometry at a particular temperature. The analyses support the high porosity with characteristic applications for catalytic and sensitive devices.
In summary, Cu2O and CuO nanoparticles have been successively synthesized via soft-chemistry approach with controlled particle properties in a quantitative yield. As a result, the particle size of the Cu2O and CuO nanoparticles was found to be 31 and 32 nm, respectively. This synthetic methodology is useful for fabricating efficient and cost-effective gas sensors and other allied potential applications. The calcination temperature has more effect on the nanocrystallite growth and is in potential use for an advanced functional materials. The methodology has promising applications such as sensors that requires a high reactivity and good sensitivity. These results are extremely promising and further research is currently underway to develop and optimize this methodology for other multimetal (more than two) oxide nanopowders, as well as metal-oxide composite nanopowders.
Acknowledgments
Organic Chemistry Division III, Indian Institute of Chemical Technology, Tarnaka, Hyderabad (A.P.), India
REFERENCES
- Jolivet , J. P. 2000 . Metal Oxide Chemistry and Synthesis , Chichester : Wiley .
- Jolivet , J. P. , Cassaignon , S. , Chaneac , C. , Chiche , D. and Tronc , E. 2008 . Design of oxide nanoparticles by aqueous chemistry . Sol-Gel Sci Technol. , 46 : 299 – 305 .
- Frietsch , M. , Zudock , F. , Goschnick , J. and Bruns , M. 2000 . CuO catalytic membrane as selectivity trimmer for metal oxide gas sensors . Sens. Actuators B , 65 : 379 – 381 .
- Maruyama , T. 1998 . Copper oxide thin films prepared by chemical vapor deposition from copper dipivaloylmethanate . Sol. Energy. Mater. Sol. Cells. , 56 : 85 – 92 .
- Dai , P. C. , Mook , H. A. , Aeppli , G. , Hayden , S. M. and Dogan , F. 2000 . Resonance as a measure of pairing correlations in the high-Tc superconductor YBa2Cu3O6.6 . Nature , 406 : 965 – 305 .
- Deng , J. F. , Sun , Q. , Zhang , Y. L. , Chen , S. Y. and Wu , D. 1996 . A novel process for preparation of a Cu/ZnO/A12O3 ultrafine catalyst for methanol synthesis from CO2+ H2: comparison of various preparation methods . Appl. Catal. A , 139 : 75 – 85 .
- Kumar , R. V. , Elgamiel , R. E. , Diamant , Y. and Gedanken , A. 2001 . Sonochemical preparation and characterization of nanocrystalline copper oxide embedded in poly (vinyl alcohol) and its effect on crystal growth of copper oxide . Langmuir , 17 : 1406 – 1410 .
- Carnes , C. L. , Stipp , J. and Klabunde , K. J. 2002 . Synthesis, characterization and adsorption studies of nanocrystalline copper oxide and nickel oxide . Langmuir , 18 : 1352 – 1359 .
- Wang , H. W. , Xu , J. Z. , Zhu , J. J. and Chen , H. Y. 2002 . Preparation of CuO nanoparticles by microwave method . J. Cryst. Growth. , 244 : 88 – 94 .
- Fan , H. , Yang , L. , Hua , W. , Wu , X. , Wu , Z. , Xie , S. and Zou , B. 2004 . Controlled synthesis of monodispersed CuO nanocrystals . Nanotechnology , 15 : 37 – 42 .
- Yang , Y. , Chen , H. , Zhao , B. and Bao , X. 2004 . Size control of ZnO nanoparticles via thermal decomposition of zinc acetate coated on organic additives . J. Cryst. Growth , 263 : 447 – 453 .
- Vogel , A. I. 1989 . Textbook of Quantitative Chemical Analysis, , 5th ed. , London : Longman .
- Guedes , M. , Ferreira , J. M. F. and Ferro , A. C. 2009 . Dispersion of Cu2O particles in aqueous suspensions containing 4,5-dihydroxy-1,3-benzenedisulfonic acid disodium salt . J. Ceram. Int. , 35 : 1939 – 1945 .
- Du , F. , Liu , J. and Guo , Z. 2009 . Shape controlled synthesis of Cu2O and its catalytic application to synthesize amorphous carbon nanofibers . J. Mater. Res. Bull. , 44 : 25 – 29 .
- Liu , J. , Huang , X. , Li , Y. , Sulieman , K. M. , He , X. and Sun , F. 2006 . Hierarchical nanostructures of cupric oxide on a copper substrate: controllable morphology and wettability . J. Mater. Chem. , 16 : 4427 – 4434 .
- Yin , M. , Wu , C. K. , Lou , Y. , Burda , C. , Koberstein , J. T. , Zhu , Y. and O'Brien , S. 2005 . Copper oxide nanocrystals . J. Am. Chem. Soc. , 127 : 9506 – 9511 .
- Wang , J. , He , S. , Li , Z. , Jing , X. , Zhang , M. and Jiang , Z. 2009 . Self-assembled CuO nanoarchitectures and their catalytic activity in the thermal decomposition of ammonium perchlorate . Colloid. Polym. Sci. , 287 : 853 – 858 .
- Brinker , C. J. and Scherer , G. 1990 . Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing , San Diego : Academic Press .
- Jia , W. , Reitz , E. , Shimpi , P. , Rodriguez , E. G. , Gao , P. X. and Yu , L. 2004 . Spherical CuO synthesized by a simple hydrothermal reaction: concentration-dependent size and its electrocatalytic application . Mat. Mat. Res. Bull. , 44 : 1681 – 1686 .
- Wang , Z. L. 2000 . Transmission electron microscopy of shape-controlled nanocrystal and their assemblies . J. Phys. Chem. B , 104 : 1153 – 1175 .