ABSTRACT
The diagnosis of acute lymphoblastic leukemia (ALL), which is the most common type of cancer in children, has become more accurate with the use of flow cytometry. Here, this technology was used to immunophenotype leukemic cells in peripheral blood samples from Libyan pediatric ALL patients. We recruited 152 newly diagnosed patients at Tripoli Medical Center (Tripoli, Libya) by morphological examination of blood and bone marrow. Twenty-three surface and cytoplasmic antigen markers were used to characterize B and T cells in circulating blood cells by four-color flow cytometry. Six children (3.9%) turned out to have biphenotypic acute leukemia, 88 (57.9%) had B ALL, and 58 (38.1%) had T ALL. There were 68 cases of pro-B ALL CD10-positive (44.7%), 8 cases of pro-B ALL CD10-negative (5.2%), 6 cases of pre-B ALL (3.9%), and 6 of mature-B ALL (3.9%). CD13 was the most commonly expressed myeloid antigen in ALL. We present immunophenotypic data for the first time describing ALL cases in Libya. The reported results indicate that the most common subtype was pro-B ALL, and the frequency of T-ALL subtype was higher compared to previous studies. Six cases were positive for both myeloid and B lymphoid markers. Our findings may provide the basis for future studies to correlate immunophenotypic profile and genetic characteristics with treatment response among ALL patients.
1. Introduction
Leukemia is a heterogeneous group of hematological malignancies. It results from proliferation of uncontrolled immature hematopoietic cells [Citation1]. It represents over one-third of all cancers effecting young children [Citation2]. Depending on the cell type involved, leukemia can be divided into different subtypes that differ in prevalence, treatment strategy, and outcome [Citation3]. The most common leukemia in children is acute lymphoblastic leukemia (ALL), which accounts for nearly 70% of cases [Citation2]. It is characterized by high cure rates and good treatment outcomes [Citation4]. ALL seems to be a heterogeneous group of disorders with subgroups that have distinct clinical and prognostic features, making classification important. Attempts have been made to classify ALL cells in a way that correlates with clinical characteristics such as disease progression and response to therapy. In the French-American-British (FAB) classification system, ALL is classified based on morphological criteria into three subgroups: L1, L2 and L3 [Citation5]. For a long time, morphological and cytochemical evaluation have been considered the principal diagnostic methods, and the FAB classification has been generally accepted. However, this classification does not give information about the lineage or maturation stage of the cells from which the leukemia arises. Alternatively, the WHO classification of ALL is generally based on cell lineage and maturation stages, and recent updates have emphasized the importance of specific cytogenetic and molecular data in the classification of ALL [Citation6,Citation7].
Because normal lymphocytes express a programmed series of specific antigens during development, the antigens expressed by ALL cells can indicate the stage of lymphocyte development at which the malignant transformation occurred. Since the introduction of flow cytometry in clinical diagnosis, markers to classify ALL have been widely used. Both cell surface and cytosolic antigens are detected by flow cytometry, which is currently the preferred method for immunophenotyping [Citation8], while microscopic evaluation of antibody-stained cells is reserved for exceptional situations. Flow cytometry has become a critical tool in both biology and medicine, with a significant impact on hematological diseases. Classification of ALL now depends mainly on flow cytometric results [Citation9–11]. Flow cytometry classifies cells by size, granularity and expression of cell-surface and cytosolic antigens. To that end, leukemic cells stained with monoclonal antibodies are immunophenotyped and identified, and the nature of the cell lineage involved is characterized [Citation12].
Definitive diagnosis of ALL based on cytometric analysis takes into account both the patterns and intensities of antigen expression. ALL is divided into B and T lineages, with more than 80% of ALL originating from B-cell progenitors (B-ALL) [Citation13]. The B lineage is further subdivided into pro-B, pre-B and mature B cell types. Pro-B ALL cells are either CD10+ (known as common B ALL) or CD10–. Markers such as CD79a, CD10, CD19, CD20 and CD22 are commonly used to characterize B-ALL, on the other hand, cyCD3, CD2, sCD3, CD4, CD5, CD7 and CD8 markers are used to characterize T-ALL [Citation12].
Variations of ALL and its subtypes in Libyan children has not been studied. Therefore, the objective of this study was to use flow cytometry to immunophenotype the leukemic cells in Libyan pediatric patients and to determine the incidence of disease subtypes.
2. Material and methods
2.1. Patients
The study was done on 152 pediatric patients newly diagnosed with ALL by morphologic examination of blood and bone marrow according to standard clinical procedures at the Pediatric Oncology Department, Tripoli Medical Center. No patients were excluded. Age and sex were recorded. Informed consent was obtained from the parents before the children were enrolled in the study. The study was carried out in accordance with the Declaration of Helsinki, national laws and institutional guidelines.
2.2. Monoclonal antibodies
Monoclonal antibodies against cytoplasmic (cy) and surface antigens were used. Cy antigens examined were CD79a and CD22 for B-Cells, CD3 for T-Cells, and Myeloperoxidase (MPO) for myeloid cells, which are used as early identifiable antigens specific for the three cell types. The surface antigens examined were B-cell specific markers (CD19, CD22, CD20, CD10 and IgM), T-cell lineage specific markers (CD3, CD2, CD4, CD5, CD7 and CD8), and myeloid cell markers (CD13, CD14, CD15, CD33, CD64 and CD117). TDT and CD34 were used as markers of immature cells. All antibodies used were obtained from BD Bioscience, USA.
2.3. Sample preparation
Peripheral blood samples were collected from the patients in tubes containing EDTA, stored at 8°C, and analyzed within 24 hours. The samples were processed using the lyse-and-wash technique. In brief, following lysis of erythrocytes using FACS Lysing Solution (BD Biosciences, USA), the cells were washed twice in phosphate buffered saline (PBS) (pH 7.2) and stained with the fluorescence-conjugated monoclonal antibody for 15 min at room temperature. The cells were then washed twice with PBS and resuspended in the same buffer.
2.4. Gates strategy
The gate strategy of CD45 versus side scatter was used at first to identify the lymphocyte population as they appear bright with low side scatter. CD45 is a pan-leukocytic antigen that displays different patterns of expression in different subpopulations of normal leukocytes and malignant cells. CD45 is expressed at lower levels in progenitor cells. Therefore such gating strategy is useful in providing a clear discrimination of the cells of interest. In addition, this strategy minimizes contamination of blast cells with normal hematopoietic cells [Citation14].
2.5. Flow cytometry analysis and immunophenotyping
The BD four-color FACSCalibur system was used (BD Bioscience, USA). Flow cytometry calibration and compensation for the overlap between the different fluorochrome spectra were done automatically using the FacsComp software (BD Biosciences, USA), whereas analysis and calculations were performed using CellQuest (BD Bioscience, USA). Leukemic cells were selected on the basis of CD45 expression versus SSC gating. The expression of an antigen on blast cells was evaluated, and an antigen was considered positive if 30% or more of the cells were stained with the antibody. At least 20,000 events were collected.
3. Results
One hundred and fifty-two pediatric patients newly diagnosed with ALL by morphologic examination of blood and bone marrow were included in the study (83 males and 69 females). Their ages ranged from one month to 14 years, with a median of 4 years. All 152 patient blood samples contained more than 30% of blast cells, and 146 of them were positive for ALL markers and showed reactivity to specific B or T markers. The other six showed both B-lymphoid and myeloid characteristics and were categorized as biphenotypic acute leukemia (BAL).
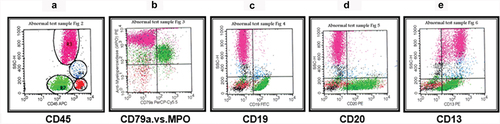
Patients who were positive for ALL markers (146; 96%), were further characterized according to reactivity with the antibodies (). Sixty-eight patients (44.7% of all patients) were pro-B ALL, all of whom were CD10+. The blasts in this group were typically small with minimal forward and side scatter. They showed very low CD45, which left them close to the erythroid cluster on the CD45 versus SSC plot (). Blasts were mainly positive for cyCD79a, CD19, TdT, HLA-DR and CD34 (, ). Eight patients (5.3% of all patients) had pro-B CD10– ALL, and the position of the leukemic cells was the same as for their CD10+ counterparts (). Six patients (3.9% of all patients) were pre-B ALL. The cells were positive for CD19, cyCD79a, cyIgM and CD20 (, ). CD34 was negative in those patients. The defining characteristic of this group was the presence of the cyIgM heavy chain.
Figure 1. Pro-B ALL CD10+ (common B ALL). The blasts were selected and gated as they appear on the CD45 and SSC (green) (a). (b) cyCd79a versus SSC. (c) CD19 versus SSC. (d) CD34 versus SSC. (e) CD10 versus SSC.
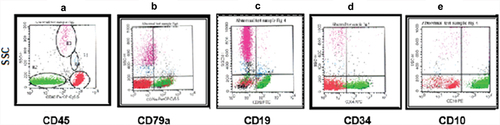
Figure 2. Pro B-ALL CD10–. The blasts were selected and gated as they appear on the CD45 and SSC (green) (a). Shown are plots of cyCd79a versus SSC (b), CD19 versus SSC (c), CD34 versus SSC (d), and CD10 versus SSC (e).
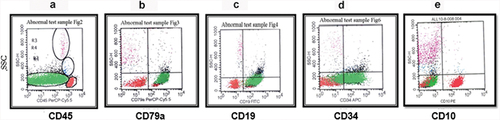
Figure 3. Pre-B ALL. The blasts were selected and gated as they appear on the CD45 and SSC (green) (a). Shown are plots of cyCd79a versus SSC (b), CD19 versus SSC (c), CD34 versus SSC (d), and cyIgM versus SSC (e).
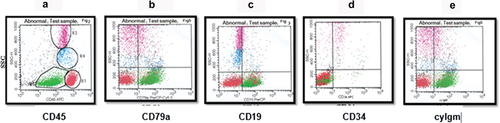
Table 1. Immunophenotyping of acute lymphoblastic leukemia: percentage of patients scoring positive for given markers.
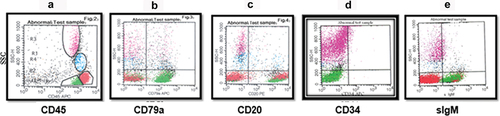
Six cases (3.9%) were mature-B ALL. The blast cells of this subtype of ALL had more forward and side scatter, which merged them close to the lymphocyte and monocyte regions on the CD45 versus SSC plots. The mature-B ALL cells showed positive B-lineage antigen markers (), with a defining characteristic of bright clonal sIgM. The blast cells were CD10+, but the mature antigens and sIgM distinguish them from earlier B-lineage ALL cells.
Figure 4. Mature B-ALL. The blasts were gated and colored green. (a) dot plot of CD45 versus SSC by which leukemic cells are selected. (b) the plot shows the expression of CD79a on blast cells. (c) dot plot of CD20 versus SSC. (d) dot plot shows the absence of CD34 expression. (e) dot plot of IgM versus SSC with the positive expression of IgM.
Fifty-eight specimens (38.1% of all) showed reactivity to T-cell specific antigens: cyCD3, CD2, CD5, CD4, and CD7 (). Many of the cells showed substantial forward and side scatter, which put them in the lymphoblast category (). In contrast to B-ALL, most T-ALL cases were commonly found in males (46 out of 58) ().
Figure 5. Shown are representative dot plots for the expression of surface and intracellular antigens of T-ALL leukemic cells. (a) CD45 versus SSC with a high percentage of blasts (green). Strong expression of cyCD3 (b), CD2 (c), CD7 (d) and CD5 (e) on the blast cells (green).
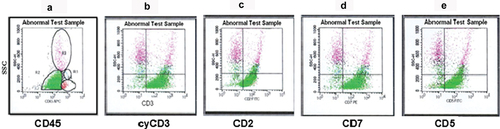
Table 2. Immunophenotyping of acute lymphoblastic leukemia: distribution of ALL subtypes by gender.
Six cases (3.9%) expressed both B-lymphoid (CD19, CD20, CD10, cyCD79a) and myeloid (MPO, CD64, CD13) antigens simultaneously (). These six cases were classified as BAL according to the scoring system of the European Group for Immunological Characterization of Leukemia (EGIL score: 5-point B-lymphoid and 3.5 myeloid points) [Citation15,Citation16].
4. Discussion
Characterization of leukemic cells by flow cytometry in terms of establishing lineage and differentiation stages has become the standard method for ALL classification. Detection of intracellular and surface antigens of leukemic cells has enhanced immunological classification and improved diagnostic accuracy [Citation12,Citation17]. Some markers are important not only for lineage assignment but also as prognosis markers. In this study, blood samples from Libyan children with ALL were used to analyze the expression of surface and cytosolic antigens using multiparameter flow cytometry. To the best of our knowledge, this is the first study to present flow cytometry results following analysis of blood samples taken from Libyan pediatric patients.
In addition to surface antigens, we identified intracellular antigens to establish the correct lineage affiliation according to the 2008 and 2016 WHO recommendations [Citation6,Citation18]. We used MPO in our first panel tube as best myeloid lineage determinant, while CD19 and cytoplasmic CD3 were used to confirm B and T lymphoid lineages, respectively. CD19 has been shown to be expressed in almost every B ALL case [Citation19], and recognized as a key component of both diagnostic and minimal residual disease monitoring. Furthermore, due to the fact that cytoplasmic CD3 expression is much higher in T ALL than the surface CD3 [Citation19], therefore this marker was used to demonstrate the T ALL lineage, followed by the other lineage-specific markers as described in the material and methods section. Intracellular antigens were also helpful in cases lacking surface expression or disclosing ambiguous phenotypic features, and in identifying BAL. These intracellular antigens, the earliest identifiable markers specific for B, T and myeloid cells [Citation20,Citation21], are useful not only in confirming the cell-lineage association of ALL subgroups, but also in identifying immaturity of blasts (). We further used CD34 marker to identify leukemic cells in early stages. Moreover, this antigen can serve as useful follow-up markers because they may remain on blasts at later stages of maturation, providing an abnormal pattern that can be a landmark for follow-up.
Our results have shown that B-ALL is the predominant type of leukemia affecting 57.9% of our cases, which is comparable to other studies [Citation22–27]. On the other hand, the incidence of T-ALL represented 38.1% of all cases, which is approximately similar to that reported in Moroccan children (37%) [Citation28], but is higher than reported in Mexico (6.1%) [Citation26], Saudi Arabia (14.7%) [Citation23], and Holland 14.0% [Citation29]. In our study, T-ALL was more commonly found in males (79.3% of all T-ALL cases). This is in agreement with other studies [Citation30,Citation31]. T-ALL is recognized as a poor prognostic group, however, it is important to correlate this finding with all other prognostic factors before conducting suitable treatment regime.
Differences in the incidence of ALL subtypes have been reported even within the same country. For instance, in eastern India, T-ALL represented 50% of total cases, compared to 15–20% of cases in the western region, in contrast B-ALL was the predominant subtype in the western region, accounting for 60–80% [Citation32]. Risk factors such as inherited genetic susceptibility and environmental exposure have been associated with ALL [Citation33]. Genetic alterations in terms of unique gene mutations and rearrangements have been correlated with ALL variations and subtypes classifications [Citation7]. These genetic abnormalities could improve the diagnosis and prognosis of the disease, which in turn lead to better therapeutic management strategy.
The most common subtype of B-ALL we observed was related to the common B ALL (pro-B with positivity of CD10) (44.7%). Predominance of this subtype has been reported in different studies [Citation23,Citation24,Citation29,Citation34]. Future research on more samples from different parts of the country are needed to provide insights into the observed prevalence differences in ALL subtypes. Six cases in our study expressed cell markers of more than one lineage. EGIL defines those biphenotypic subtypes as a group in which blasts simultaneously express myeloid and lymphoid antigens. Our immunophenotyping data show that the acute leukemia cases with this type of aberrant antigen expression represented 3.9% of all cases. This rate compares well with others [Citation35–39]. In our study, the biphenotypic cases were B/myeloid leukemia expressing the myeloid markers MPO, CD64, CD13 and the B-cell antigens CD19, CD20, CD10 and cyCD79a. B/myeloid is the most common immunophenotype among ambiguous lineage [Citation40,Citation41]. The biphenotypic subtype normally has a worse prognosis than other ALL subtypes, and it is diagnostically and therapeutically challenging. Although these subtypes have a low incidence, accounting for 2–5% of ALL cases [Citation36,Citation40], it is recommended to include lineage-specific antigens as advised by the WHO into routine examinations, so that biophenotypic subtypes are not excluded during diagnosis of leukemia samples [Citation6]. This would provide accurate descriptive information about the blast cells of those subtypes that is useful for disease monitoring, provides clues to disease genetics, and most importantly can help to select effective treatment.
5. Conclusion
Flow cytometry analysis of ALL subtypes can help to evaluate and predict the outcome of patients with ALL, and in order to design treatment effectively, it is important to rely on reasonably accurate prognostic markers. More research on large numbers of patients are needed, using the data presented in this study as a reference, to establish an optimal number of markers and to standardize immunophenotyping techniques. Furthermore, the identification of genetic abnormalities in Libyan pediatric patients with ALL is importantly needed to incorporate the immunophenotypic results and genetic characteristics with clinical outcome.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7(6):e577. doi: 10.1038/bcj.2017.53
- Ssenyonga N, Stiller C, Nakata K, et al. Worldwide trends in population-based survival for children, adolescents, and young adults diagnosed with leukaemia, by subtype, during 2000–14 (CONCORD-3): analysis of individual data from 258 cancer registries in 61 countries. Lancet Child Adolesc Health. 2022;6(6):409–7. doi: 10.1016/S2352-4642(22)00095-5
- Inaba H, Pui CH. Advances in the diagnosis and treatment of pediatric acute lymphoblastic leukemia. J Clin Med. 2021;10(9):1926. doi: 10.3390/jcm10091926
- Abboud M, Ghanem K, Muwakit S. Acute lymphoblastic leukemia low and middle-income countries: disease characteristics and treatments. Curr Opin Oncol. 2014;26(6):650–655. doi: 10.1097/CCO.0000000000000125
- Bennett J, Catovsky D, Daniel MT, et al. The morphological classification of acute lymphoblastic leukaemia: concordance among observers and clinical correlations. Br J Haematol. 1981;47(4):553–561. doi: 10.1111/j.1365-2141.1981.tb02684.x
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544
- Duffield AS, Mullighan CG, Borowitz MJ. International consensus classification of acute lymphoblastic leukemia/lymphoma. Virchows Arch. 2023;482(1):11–26. doi: 10.1007/s00428-022-03448-8
- Del Principe M, De Bellis E, Gurnari C, et al. Applications and efficiency of flow cytometry for leukemia diagnostics. Expert Rev Mol Diagn. 2019;19(12):1089–1097. doi: 10.1080/14737159.2019.1691918
- Arber DA, Borowitz MJ, Cessna M, et al. Initial diagnostic workup of acute leukemia: guideline from the college of American Pathologists and the American society of hematology. Arch Pathol Lab Med. 2017;141(10):1342–1393. doi: 10.5858/arpa.2016-0504-CP
- Solly F, Angelot-Delettre F, Ticchioni M, et al. Standardization of flow cytometric immunophenotyping for hematological malignancies: the France flow group experience. Cytometry Pt A. 2019;95(9):1008–1018. doi: 10.1002/cyto.a.23844
- Lhermitte L, Bottcher S. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26(9):1908–1975. doi: 10.1038/leu.2012.120
- DiGiuseppe J, Wood BL. Applications of flow cytometricImmunophenotyping in the diagnosis and posttreatment monitoring of B and T lymphoblastic leukemia/lymphoma. Cytometry B Clin Cytom. 2019;96(4):256–265. doi: 10.1002/cyto.b.21833
- Liu YF, Wang BY, Zhang WN, et al. Genomic profiling of adult and pediatric B-cell acute lymphoblastic leukemia. EBioMedicine. 2016;8:173–183. doi: 10.1016/j.ebiom.2016.04.038
- Lacombe F, Durrieu F, Briais A, et al. Flow cytometry CD45 gating for immunophenotyping of acute myeloid leukemia. Leukemia. 1997;11(11):1878–1886. doi: 10.1038/sj.leu.2400847
- Bene MC, Castold G, Knapp W, et al. Proposals for the immunological classification of acute leukemias. European group for the immunological characterization of leukemias (EGIL). Leukemia. 1995;9:1783–1786.
- Lee HG, Baek HJ, Kim HS, et al. Biphenotypic acute leukemia or acute leukemia of ambiguous lineage in childhood: clinical characteristics and outcome. Blood Res. 2019;54(1):63–73. doi: 10.5045/br.2019.54.1.63
- Paredes-Aguilera R, Romero-Guzman L, Lopez-Santaigo N, et al. Flow cytometric analysis of cell-surface and intracellular antigens in the diagnosis of acute leukemia. Am J Hematol. 2001;68(2):69–74. doi: 10.1002/ajh.1155
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. doi: 10.1182/blood-2009-03-209262
- Rezaei MS, Esfandiari N, Refoua S. Characterization of immunophenotypic aberrancies in adult and childhood acute lymphoblastic leukemia: lessons from regional variation. Iran J Pathol. 2020;15(1):1–7. doi: 10.30699/ijp.2019.93974.1926
- Dworzak MN, Fritsch G, Fröschl G, et al. Four-color flow cytometric investigation of terminal deoxynucleotidyl transferase–positive lymphoid precursors in pediatric bone marrow: CD79a expression precedes CD19 in early B-cell ontogeny. Blood. 1998;92(9):3203–3209. doi: 10.1182/blood.V92.9.3203
- Basso G, Buldini B, De Zen L, et al. New methodologic approaches for immunophenotyping acute leukemias. Haematologica. 2001;86:675–692.
- Addakiri S, Bencharef H, Jaddaoui S, et al. Immunophenotype of acute lymphoblastic leukemia: the experience of University Hospital Centre Casablanca - Morocco. Clin Lab. 2023;69(9):10.7754. doi: 10.7754/Clin.Lab.2023.230307
- Bawazir A, Al-Zamel N, Amen A, et al. The burden of leukemia in the Kingdom of Saudi Arabia: 15 years period (1999–2013). BMC Cancer. 2019;19(1):703. doi: 10.1186/s12885-019-5897-5
- Ibagy A, Silva DB, Seiben J, et al. Acute lymphoblastic leukemia in infants: 20 years of experience. J Pediatr. 2013;89(1):64–69. doi: 10.1016/j.jped.2013.02.010
- Santos MMD, Santos ASD, Santos HHM, et al. Immunophenotypic characterization of acute leukemias in Bahia, Brazil. Einstein (Sao Paulo). Einstein (São Paulo). 2023;21:eAO0117. doi: 10.31744/einstein_journal/2023AO0117
- Vázquez-Cornejo E, Morales-Ríos O, Hernández-Pliego G, et al. Incidence, severity, and preventability of adverse events during the induction of patients with acute lymphoblastic leukemia in a tertiary care pediatric hospital in Mexico. PLoS One. 2022;17(3):e0265450. doi: 10.1371/journal.pone.0265450
- Wimalachandra M, Prabashika M, Dissanayake M, et al. Immunophenotypic characterization of acute lymphoblastic leukemia in a flowcytometry reference centre in Sri Lanka. Ceylon Med J. 2020;65(1&2):23–27. doi: 10.4038/cmj.v65i1-2.9133
- Dakka N, Bellaoui H, Khattab M, et al. Immunologic profile and outcome of childhood acute lymphoblastic leukemia (ALL) in Morocco. J Pediatr Hematol Oncol. 2007;29(8):574–580. doi: 10.1097/MPH.0b013e3181256b8f
- Kaspers GJ, Veerman AJ, Van Wering ER, et al. Prognostic significance of peanut agglutinin binding in childhood acute lymphoblastic leukemia. Leukemia. 1996;10:675–681.
- Dores GM, Devesa SS, Curtis RE, et al. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood. 2012;119(1):34–43. doi: 10.1182/blood-2011-04-347872
- Kakaje A, Alhalabi MM, Ghareeb A, et al. Rates and trends of childhood acute lymphoblastic leukemia: an epidemiology study. Sci Rep. 2020;10(1):6756. doi: 10.1038/s41598-020-63528-0
- Mukhopadhyay A, Gangopadhyay S, Dasgupta S, et al. Surveillance and expected outcome of acute lymphoblastic leukemia in children and adolescents: an experience from Eastern India. Indian J Med Paediatr Oncol. 2013;34(4):280–282. doi: 10.4103/0971-5851.125245
- Jin MW, Xu SM, An Q, et al. A review of risk factors for childhood leukemia. Eur Rev Med Pharmacol Sci. 2016;20:3760–3764.
- Hjalgrim LL, Rostgaard K, Schmiegelow K, et al. Age- and sex-specific incidence of childhood leukemia by immunophenotyping in the Nordic countries. J Natl Cancer Inst. 2003;95(20):1539–1544. doi: 10.1093/jnci/djg064
- Maruffi M, Sposto R, Oberley MJ, et al. Therapy for children and adults with mixed phenotype acute leukemia: a systematic review and meta-analysis. Leukemia. 2018;32(7):1515–1528. doi: 10.1038/s41375-018-0058-4
- Matutes E, Pickl WF, Van’t Veer M, et al. Mixed-phenotype acute leukemia: clinical and laboratory features and outcome in 100 patients defined according to the WHO 2008 classification. Blood. 2011;117(11):3163–3171. doi: 10.1182/blood-2010-10-314682
- Rubnitz JE, Onciu M, Pounds S, et al. Acute mixed lineage leukemia in children: the experience of St Jude children’s research hospital. Blood. 2009;113(21):5083–5089. doi: 10.1182/blood-2008-10-187351
- Weinberg OK, Seetharam M, Ren L, et al. Mixed phenotype acute leukemia: a study of 61 cases using World Health Organization and European group for the immunological classification of leukaemias criteria. Am J Clin Pathol. 2014;142(6):803–808. doi: 10.1309/AJCPPVUPOTUVOIB5
- Weinberg OK, Arber DA. Mixed-phenotype acute leukemia: historical overview and a new definition. Leukemia. 2010;24(11):1844–1851. doi: 10.1038/leu.2010.202
- Yan L, Ping N, Zhu M, et al. Clinical, immunophenotypic, cytogenetic, and molecular genetic features in 117 adult patients with mixed-phenotype acute leukemia defined by WHO-2008 classification. Haematologica. 2012;97(11):1708–1712. doi: 10.3324/haematol.2012.064485
- Tian H, Xu Y, Liu L, et al. Comparison of outcomes in mixed phenotype acute leukemia patients treated with chemotherapy and stem cell transplantation versus chemotherapy alone. Leuk Res. 2016;45:40–46. doi: 10.1016/j.leukres.2016.04.002