ABSTRACT
Pseudomonas aeruginosa is a multidrug-resistant bacterium capable of forming biofilms. This study aimed to assess resistance of clinical isolates from Libyan hospitals to antipseudomonal antibiotics, the prevalence of selected extended-spectrum β-lactamases and carbapenemase genes among these isolates, and the microorganisms’ capacity for alginate and biofilm production. Forty-five isolates were collected from four hospitals in Benghazi and Derna, Libya. Antimicrobial susceptibility was determined using agar disc diffusion. The presence of resistance genes (blaCTXM, blaTEM, blaSHV-1, blaGES-1, blaKPC, and blaNDM) was screened using PCR. Biofilm formation was quantified via the crystal violet assay, while alginate production was measured spectrophotometrically. Resistance to antipseudomonal antibiotics ranged from 48.9% to 75.6%. The most prevalent resistance gene was blaNDM (26.7%), followed by blaGES-1 (17.8%). Moreover, all isolates demonstrated varying degrees of biofilm-forming ability and alginate production. No statistically significant correlation was found between biofilm formation and alginate production. The dissemination of resistant genes in P. aeruginosa, particularly carbapenemases, is of great concern. This issue is compounded by the bacteria’s biofilm-forming capability. Urgent intervention and continuous surveillance are imperative to prevent further deterioration and the catastrophic spread of resistance among these formidable bacteria.
1. Introduction
Pseudomonas aeruginosa, a Gram-negative common opportunistic pathogen, rarely causes infections in healthy individuals but is considered a potential serious human pathogen due to its intrinsic metabolic diversity, acquired antibiotic resistance, biofilm formation, and production of various virulence factors [Citation1]. The resistance of P. aeruginosa arises from several mechanisms, including the production of diverse classes of β-lactamases and extended-spectrum β-lactamases (ESBLs), overproduction of chromosomal AmpC cephalosporinase, as well as non-enzymatic mechanisms such as efflux pumps and outer membrane impermeability [Citation2].
ESBLs represent one class of beta-lactamase enzymes capable of hydrolyzing cephalosporins and aztreonam and are exclusively found in Gram-negative bacteria such as Enterobacteriaceae, Acinetobacter, and Pseudomonas. The presence of ESBLs poses a growing global health concern, contributing to resistance against essential groups of antibiotics, including penicillins, erythromycin, cephalosporins, and fluoroquinolones [Citation3]. Most ESBL enzymes of P. aeruginosa are derived from the widespread broad-spectrum β-lactamases, TEM-1 and SHV-1 in addition to CTX-M and OXA [Citation4].
Carbapenems serve as last-resort antibiotics to treat severe infections caused by multidrug-resistant microorganisms when other treatments have failed, owing to their limited vulnerability to most beta-lactam resistance determinants [Citation5]. However, in Pseudomonas, carbapenem resistance is emerging due to various resistance mechanisms, such as decreased outer membrane permeability or overexpression of drug efflux pumps, as well as the production of carbapenemase enzymes [Citation6]. Carbapenemases are enzymes capable of hydrolyzing penicillins, cephalosporins, monobactam, and carbapenems, and they are classified into molecular classes A, B, and D β-lactamases. Class A β-lactamase enzymes possess a serine residue that mediates β-lactam hydrolysis and include the KPC, GES, SME, IMI, and NMC families. Class D β-lactamase enzymes also contain a serine residue, mediating β-lactam hydrolysis, and consist of OXA-type β-lactamases [Citation7]. Class B β-lactamase enzymes, on the other hand, are metallo-β-lactamases containing zinc in their active site and encompass VIM, IMP, SPM, GIM, AIM, DIM, and NDM [Citation6]. Although carbapenemase distribution is more common among Enterobacteriaceae than other bacteria, its occurrence in Pseudomonas is garnering increased attention due to its association with severe infections and higher mortality rates [Citation8]. Although some antibiotics are suggested to treat carbapenemase resistant Gram negative bacterial (CRGN) infections such as polymyxin, ceftazidime-avibactam, tigecycline, and sulbactam combination [Citation9]; nevertheless, CRGN are listed by the World Health Organization among the bacteria with a critical priority to which new antibiotics are urgently needed [Citation10].
P. aeruginosa harbors various virulence factors that aid its survival in harsh environments and facilitate attacks on host defenses. One of these factors is alginate, an exopolysaccharide that protects the bacteria from adverse conditions [Citation11]. P. aeruginosa strains producing alginate are of particular importance in respiratory infections, contributing to the initial stages of infection, such as bacterial adherence and colonization [Citation12]. Alginate also plays a role in altering P. aeruginosa to the mucoid phenotype in patients with cystic fibrosis, leading to chronic infection [Citation13]. Furthermore, P. aeruginosa’s ability to form biofilms significantly impacts clinical outcomes, as it is implicated in chronic wound infections, ventilator-associated pneumonia, and cystic fibrosis-related respiratory infections [Citation14].
Only a few reports have emerged from Libya, assessing the distribution of ESBL and carbapenemase genes in pseudomonads. Consequently, the primary objective of this study is to investigate antimicrobial resistance and analyze the prevalence of selected ESBL and carbapenemase genes within clinical isolates of P. aeruginosa. These isolates were collected from four hospitals located in the cities of Derna and Benghazi in Libya. Additionally, our investigation aims to assess the capacity of these isolates to produce virulence factors such as alginate and biofilm, while also examining potential correlations between their production. Through this study, we strive to attain valuable insights that can aid in understanding the extent of antibiotic resistance proliferation in order to bolster infection control protocols within clinical settings.
2. Materials and methods
All cultural media used in this study were procured from Oxoid, England. Oxidase strips and antibiotic discs were procured from MAST diagnostics, UK.
3. Isolation and identification of the bacteria
A total of 45 Pseudomonas aeruginosa isolates were obtained from the microbiology laboratories of four hospitals: two located in Derna city and the other two in Benghazi city. The laboratories were instructed to provide any clinical isolate that was confirmed to be Pseudomonas aeruginosa. The isolates were collected between May and October 2019. The isolates were sourced from burn swabs (18), urine samples (15), wound swabs (9), nasal swabs (2), and cerebrospinal fluid (1).
All samples were inoculated on MacConkey agar and Blood agar and incubated aerobically at 37°C for 24 h. Initial identification of P. aeruginosa was based on colony morphology, pigment production, and odor. The isolates were further confirmed by inoculating them on cetrimide media and incubating aerobically at 37°C for 24 h. To identify the bacteria conclusively, urease, Kligler iron, citrate, SIM tests, and the polymerase-chain reaction methods were employed.
The isolates were stored in 15% glycerol in tryptic soybean broth at −20°C for further testing.
4. Antimicrobial susceptibility test
Antimicrobial susceptibility test using disc diffusion method was carried out using Mueller Hinton agar according to Clinical Laboratory and Standards Institute (CLSI, 2015:M100, S25). The following antipseudomonal antibiotics were used: Ceftazidime, Aztreonam, Piperacillin – tazobactam, Ciprofloxacin, Amikacin, and Imipenem. The diameter of zone of inhibition was measured after overnight incubation at 37°C and results interpreted using the breakpoint tables. P. aeruginosa ATCC 27853 was used as a quality control strain.
5. Polymerase chain reaction
PCR was used to confirm the genetic identity of the isolates and to screen for antibiotic resistance genes. From overnight cultures of each isolate and the control strain grown in Mueller–Hinton broth (MHB), 1 ml was centrifuged at 15,000 rpm for 5 min. The supernatant was aspirated, and DNA was extracted using the Wizard® Genomic DNA Purification Kit (Promega, USA), following the manufacturer’s instructions. The rehydrated DNA was stored at −20°C until further analysis. Amplification of the targeted DNA was carried out in a total volume of 20 µL, consisting of primers (0.5 µl of each forward and reverse), DNA (1 µl for 16S rDNA detection, and 8 µl for resistance genes detection), 5× FIREPol® Master Mix (4 µl, Solis BioDyne, Europe), and nuclease-free water up to 20 µl. For each gene, one pair of primers was used ().
Table 1. The primers used in PCR to screen for the relevant genes.
The reaction components were mixed in a PCR tube and placed in a PCR thermocycler (Bioer XP cycler, China). Amplification conditions were as follows: For 16S rDNA: an initial denaturation at 95°C for 5 min, followed by 25 cycles each consisting of 20 s at 94°C for denaturation, then annealing at 58°C for 20 sec, 40 sec extension at 72°C, and a final extension at 72°C for 5 min [Citation15]. For the resistance genes: an initial denaturation at 94°C for (2 min for blaTEM and blaGES-1; 3 min for CTX-M; 5 min for blaSHV-1, and 10 min for blaKPC and blaNDM), followed by 30 cycles (for blaTEM, blaSHV-1, blaGES-1) or 36 cycles (for blaCTXM, blaKPC and blaNDM) each of denaturation at 94°C for (30 sec: blaTEM, blaKPC and blaNDM; 1 min for blaCTXM, blaSHV-1 and blaGES-1); annealing at (58°C for 30 sec for blaCTXM; 52°C for 1 min for blaTEM\; 54°C for 1 min for blaSHV-1; 50°C for 1 min for blaGES-1; and 52°C for 40 sec (for blaKPC and blaNDM)); extension at 72°C for (1 min for blaCTXM, blaTEM, blaSHV-1 and blaGES-1; 45 sec for blaKPC and blaNDM) and final extension at 72°C for (5 min for blaSHV-1, blaKPC and blaNDM; 7 min for blaGES-1; 10 min for blaTEM and blaCTXM) [Citation21–24]. Nuclease-free water was used as a negative control, and P. aeruginosa ATCC 27853 was used as a positive control for P. aeruginosa identification. Klebsiella pneumoniae clinical isolates (88, 89, 96, 97 and 100) carrying the relevant genes were obtained from Dr Asem Alshehabi laboratory and used as positive controls for blaTEM, blaCTXM, blaSHV-1, blaKPC and blaNDM respectively.
Each PCR product, including positive and negative controls was electrophoresed on a 2% agarose gel using Bio-Rad equipment (USA). The gel was run for 60 min at 120 V and then visualized under ultraviolet light (UVP, USA) at 260 nm using Redsafe (Intron Biotechnology, Seongnam, Korea).
6. Quantification of alginate production
Quantification of alginate production was carried out according to Wang et al., (2005). The isolates were cultured in MHB and incubated with shaking overnight at 37°C. Bacterial suspensions were vortexed, and 1 ml was centrifuged at 15,000 rpm for 5 min to pellet the cells. The supernatant was aspirated for use in the assay [Citation25]. From the same bacterial suspension, 100 µl from each isolate suspension was transferred to the wells of a 96-well microtiter plate to measure the optical density at 600 nm in triplicate. A calibration standard was prepared from alginic acid solution with concentrations ranging from 0.1 to 1 mg/ml. Glucose (1 mg/ml) in water served as a negative control. To 100 µl of each sample, negative control, and standards, 1000 µl of tetraborate solution (75 mM sodium tetraborate in concentrated sulfuric acid) was added, followed by 50 µl of 0.25% carbazole in ethanol. Samples were vortexed and then boiled for 15 min. After heating, samples were placed on ice for 5 min and then transferred to a 96-well plate for reading in a spectrophotometer at a wavelength of 530 nm. The absorption value of each sample was normalized by dividing it by the optical density of that sample. Alginate concentration (mg/ml) was calculated by dividing the normalized absorption by the slope of the calibration curve.
7. Evaluation of biofilm production
The crystal violet assay, adapted from Stepanovic et al. (2000), was applied to all the isolates [Citation26]. The optical density (OD) of the prepared samples was measured at 550 nm using a plate reader (BioTek, USA). Broth medium was used as a negative control, and each isolate and the control were measured in triplicate. A cut-off value (ODc) was calculated from the negative control, defined as three standard deviations (SD) above the mean OD of the negative control: ODc = average OD of the negative control + (3 × SD of the negative control). The isolates were then classified into the following categories based on the OD [Citation26]
Non-biofilm producer (OD < ODc).
Weak-biofilm producer (ODc < OD < 2 × ODc).
Moderate-biofilm producer (2 × ODc < OD < 4 × ODc)
Strong-biofilm producer (4 × ODc < OD)
8. Results
8.1. Antimicrobial susceptibility test
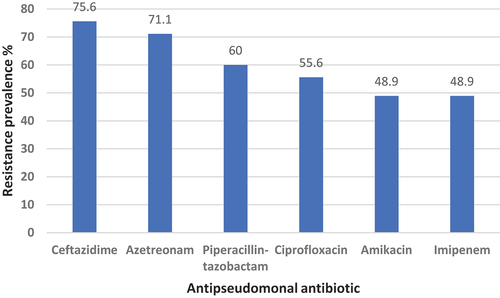
A substantial proportion of the isolates showed a remarkable resistance to the antipseudomonal antibiotics ( and Supplementary Table S1). The highest resistance was found against ceftazidime (75.6%) followed by aztreonam (71.1%). Even the drugs with the lowest resistance rate (amikacin and imipenem), the isolates have reported a notable resistance against them (48.9% each).
8.2. Polymerase chain reaction analyses
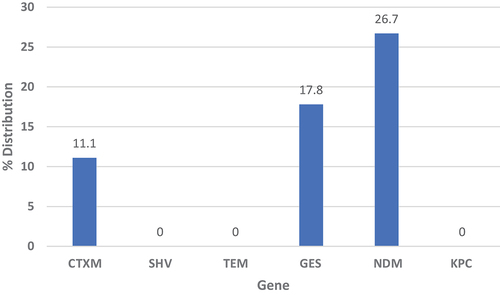
and Supplementary Table S1 show the distribution of blaCTXM, blaTEM, blaSHV-1, blaGES-1, blaKPC and blaNDM genes in the isolates. The distribution of carbapenemase resistance genes blaGES-1 and blaNDM was remarkable (17.8% and 26.7% respectively), although blaKPC was 0%.
8.3. Alginate production
All the isolates produced alginate to variable degrees.
8.4. Biofilm
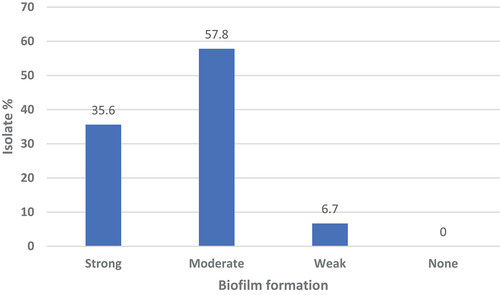
All the isolates were able to form biofilm. The majority were moderate biofilm formers (57.8%) followed by strong formers (35.6%), .
A potential relationship between alginate concentration and biofilm formation was tested by applying one way ANOVA test on the isolates. No significant difference in alginate production was found among different biofilm formation strengths (p = 0.179)
9. Discussion
In this study, antimicrobial resistance, and the presence of selected ESBL genes and carbapenemase genes were evaluated in P. aeruginosa clinical isolates collected from four hospitals in the cities of Derna and Benghazi, Libya. The production of the virulence factors alginate, and biofilm, was investigated in these isolates, and the potential correlation between alginate production and biofilm strength was examined.
In Libya, over the last decade, only a few published studies have investigated the antimicrobial resistance of clinical P. aeruginosa isolates. Due to its inherent resistance, only a few antibiotics are effective against it, including fluoroquinolones, gentamicin and carbapenems [Citation27]. In our study, high resistance rates were found in P. aeruginosa isolates against different antipseudomonal antibiotic classes (ceftazidime, piperacillin-tazobactam, ciprofloxacin and amikacin), with resistance rates ranging from 48.9% to 75.6%. This elevation in resistance rates was observed in a previous study in Libya, which was performed on 45 clinical P. aeruginosa isolates collected between October 2013 and May 2014 [Citation28]. In their study, the resistance rates for the same antibiotics ranged from 76% to 95%. Similarly, in our study, high resistance to imipenem was found in almost half of the isolates (48.9%), denoting an alarming situation where one of the last-line drugs has been rendered ineffective. In the last two decades, there has been an obvious increase in P. aeruginosa’s resistance to carbapenems. A six-month study in four hospitals in Benghazi, Libya, between September 2004 and February 2005 found that the resistance rate among the isolated P. aeruginosa strains to imipenem was 19.8% [Citation27]. However, in a study on clinical isolates collected between 2013 and 2014 the rate increased to 85.8% [Citation29]. These results collectively show that over time, there is an increase in P. aeruginosa’s resistance against antipseudomonal antibiotics, including last-line drugs.
In this study, the occurrence of selected ESBL and carbapenemase genes was investigated. These genes were chosen since they are among the commonly prevailing resistance genes in Gram negative bacteria isolated from Libya and neighboring countries [Citation29–35]. The distribution of blaCTXM was found to be 9.8%, while blaTEM and blaSHV-1 were not detected. Unfortunately, we could not find any study investigating ESBL genes dissemination (other than carbapenemase) among P. aeruginosa in Libya to compare our results with. However, compared to other bacteria, a study on Escherichia coli and K. pneumoniae isolated from two hospitals in Benghazi, Libya by Mathlouthi et al. (2016), has shown that blaCTXM was the most prevalent ESBL gene (65.4% and 41.7% respectively), followed by blaTEM (61.5 and 16.7% respectively) and blaSHV (11.5% and 16.7% respectively) [Citation29]. In another study, the distribution of blaCTXM among E. coli urine isolates from five hospitals in Libya was 17.6% [Citation31]. Due to the limited studies on clinical pseudomonas isolates in Libya, we can’t assert that the dissemination of blaTEM, blaSHV-1 or blaCTXM is lower in them compared to Enterobacteriaceae. However, a study in Sudan, a neighboring country, had shown low distribution rates of ESBL genes among P. aeruginosa clinical isolates [Citation32]. In their study, the distribution rate of selected ESBL genes among 70 P. aeruginosa isolates was 6.6% for blaCTXM, 10% for blaSHV-1 and 3.3% for blaTEM, which are in line with our findings. On the contrary, in Algeria, another neighboring country, a study on pseudomonas clinical isolates collected between 2016 and 2019 from burn patients showed higher dissemination rates of blaCTXM (48.9%), blaTEM (34%), and none of blaSHV [Citation33]. These reports indicate that even though the dissemination of ESBL genes may vary between countries or locations within a country, their spread among pseudomonads is alarming.
Carbapenem resistance is a major and persistent public health problem worldwide, occurring mainly among Gram-negative pathogens and may be intrinsic or mediated by transferable carbapenemase-encoding genes. These resistance genes are already widespread in several parts of the world, especially in Europe, Asia, and South America [Citation36]. In the current study, blaNDM genes and blaGES-1 were found in 26.7% and 17.8% of P. aeruginosa isolates, respectively, whereas blaKPC gene was not detected. Published reports on the prevalence of carbapenemase resistance among P. aeruginosa isolates from Libya are scarce. Only a few were found. A recent study has shown that the most prevalent carbapenemase genes detected in Pseudomonas spp. clinical isolates collected from Libyan hospitals were blaNDM, followed by blaVIM [Citation30]. In another study, eight Gram-negative bacilli isolates, which included one P. aeruginosa and one P. putida, were screened for carbapenemase genes. Among the two Pseudomonads, blaGES was detected in P. aeruginosa and blaVIM was detected in the P. putida isolate [Citation34].
A study to phenotypically screen for carbapenemase-resistant metalo-beta-lactamase in P. aeruginosa isolates collected from patients in Burn and Plastic Surgery Center, Tripoli, had detected these enzymes in 32.6% of the isolates [Citation28]. However, compared to a nearby country, Egypt, Azab et al., (2015) studied 45 clinical strains of P. aeruginosa isolated from cases of surgical site infections [Citation35]. In their study, blaGES-1 gene was detected in 26.7% of the isolates. Collectively, our results and the previous reports imply that regardless of the prevailing carbapenemase genes, their presence in pseudomonas isolates collected from Libyan hospitals is persistent through the years. Therefore, to avoid further dissemination of these multiply resistant bacteria, screening and surveillance systems should be applied in all Libyan hospitals, and the guidelines set by the WHO regarding the control and combat of carbapenemase-resistant bacteria should be implemented (Implementation manual to prevent and control the spread of carbapenem-resistant organisms at the national and health care facility level) [Citation37]. In addition, it is essential to establish and empower infection control committees in hospitals to rigorously implement all hygienic practices and proper infection control protocols among healthcare workers and within the facilities. Furthermore, antimicrobial stewardship programs should be implemented. However, we believe that international organizations such as the WHO should intervene to help the country control this dissemination of resistant bacteria among healthcare facilities.
All the isolates tested were able to produce biofilm. The biofilm formation ability ranged from weak to strong, with the vast majority of isolates being strong (35.3%) to moderate (58.8%) biofilm formers, and only (5.9%) were weak biofilm formers. It is well known that biofilm formation has an immense impact on the microbial resistance to antimicrobials. Therefore, the formation of biofilms by pseudomonas will augment the inherent resistance of these bacteria to antibiotics and will create a significant challenge for patient therapeutics.
The stability and structure of P. aeruginosa biofilm are determined by at least three different polysaccharides: alginate, Pel, and Psl [Citation38]. Several studies have attempted to investigate the role of alginate in biofilm. One study has shown that alginate is able to stabilize biofilm structure and to participate in water retention and nutrient accumulation inside its matrix [Citation39]. In another study, the isolation of alginate-producing P. aeruginosa from cystic fibrosis patients was suggested to be associated with hyper-immune response and poor clinical condition [Citation40]. Since in our study, all isolates produced alginate with varying concentrations, we analyzed the effect of alginate production on the strength of biofilm formation. The results indicated no significant difference in alginate production among the isolates with strong, moderate, or weak biofilm formation ability (ANOVA, p = 0.179), implying no correlation between alginate production and biofilm formation capability. This result may support other studies which showed that alginate is not required for the initiation or maturation of biofilms [Citation40] and that it does not act as a primary adhesion for the P. aeruginosa cells on the surfaces [Citation12]. However, alginate was suggested to play a major role in the biofilm architecture and the formation of a thick three-dimensional structure [Citation12].
In conclusion, this study sheds light on the escalating challenges posed by antimicrobial resistance and the dissemination of resistance genes among clinical isolates of P. aeruginosa in Libyan hospitals. The findings underscore the pressing need for robust screening and surveillance systems within healthcare facilities along with rigorous implementation of infection control measures and antimicrobial stewardship to curb the persistent and alarming prevalence of resistance, especially with the emergence of carbapenemase-encoding genes. The study’s outcomes emphasize the critical importance of international collaboration and support, to effectively address the dissemination of resistant bacteria and safeguard patient care.
Furthermore, the investigation into biofilm formation by P. aeruginosa isolates highlights the potential impact of this phenomenon on antimicrobial resistance. While alginate production was observed in all isolates, the absence of a significant correlation between alginate production and biofilm strength suggests complex mechanisms at play in biofilm formation.
Supp_table_R1.docx
Download MS Word (26.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19932820.2024.2344320.
Additional information
Funding
References
- Balasubramanian D, Schneper L, Kumari H, et al. A dynamic and intricate regulatory network determines pseudomonas aeruginosa virulence. Nucleic Acids Res. 2013;41(1):1. doi: 10.1093/NAR/GKS1039
- Bokaeian M, Shahraki Zahedani, Shahram M, et al. Frequency of PER, VEB, SHV, TEM and CTX-M Genes in resistant strains of pseudomonas aeruginosa producing extended spectrum β-lactamases. Jundishapur J Microbiol. 2015;8(1):13783.
- Colodner R. Extended-spectrum β-lactamases: a challenge for clinical microbiologists and infection control specialists. Am J Infect Control. 2005;33(2):104–8. doi: 10.1016/j.ajic.2004.07.010
- Bradford PA. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001;14(4):933. doi: 10.1128/CMR.14.4.933-951.2001
- Meletis G. Carbapenem resistance: overview of the problem and future perspectives. Ther Adv Infect Dis. 2016;3(1):15–21. doi: 10.1177/2049936115621709
- Dortet L, Poirel L, Nordmann P, et al. Worldwide dissemination of the NDM-Type carbapenemases in gram-negative bacteria. Bio Med Res Inter. 2014;2014:1–12. doi: 10.1155/2014/249856
- Queenan AM, Bush K. Carbapenemases: the versatile β-lactamases. Clin Microbiol Rev. 2007;20(3):440. doi: 10.1128/CMR.00001-07
- Reyes J, Komarow L, Chen L, et al. Global epidemiology and clinical outcomes of carbapenem-resistant Pseudomonas aeruginosa and associated carbapenemases (POP): a prospective cohort study. Lancet Microbe. 2023;4(3):e159–e170. doi: 10.1016/S2666-5247(22)00329-9
- Zeng M, Xia J, Zong Z, et al. Guidelines for the diagnosis, treatment, prevention and control of infections caused by carbapenem-resistant gram-negative bacilli. J Microbiol Immunol Infect. 2023;56:653–671. doi: 10.1016/j.jmii.2023.01.017
- WHO. WHO publishes list of bacteria for which new antibiotics are urgently needed. 2017. [cited 2020 Aug 21]. Available from: https://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed
- Boyd A, Chakrabarty AM. Pseudomonas aeruginosa biofilms: role of the alginate exopolysaccharide. J Ind Microbiol. 1995;15(3):162–168. doi: 10.1007/BF01569821
- Nivens DE, Ohman DE, Williams J, et al. Role of alginate and its O acetylation in formation of Pseudomonas aeruginosa microcolonies and biofilms. J Bacteriol. 2001;183(3):1047–1057. doi: 10.1128/JB.183.3.1047-1057.2001
- Wood SR, Firoved AM, Ornatowski W, et al. Nitrosative stress inhibits production of the virulence factor alginate in mucoid Pseudomonas aeruginosa. Free Radic Res. 2009;41(2):208–215.
- Mulcahy LR, Isabella VM, Lewis K. Pseudomonas aeruginosa biofilms in disease. Microb Ecol. 2014;68(1):1. doi: 10.1007/S00248-013-0297-X
- Spilker T, Coenye T, Vandamme P, et al. PCR-Based assay for differentiation of Pseudomonas aeruginosa from other pseudomonas species recovered from cystic fibrosis patients. J Clin Microbiol. 2004;42(5):2074–2079. doi: 10.1128/JCM.42.5.2074-2079.2004
- Pitout JDD, Gregson DB, Poirel L, et al. Detection of Pseudomonas aeruginosa producing metallo-β-lactamases in a large centralized laboratory. J Clin Microbiol. 2005;43(7):3129. doi: 10.1128/JCM.43.7.3129-3135.2005
- Nanvazadeh F, Khosravi AD, Zolfaghari MR, et al. Genotyping of Pseudomonas aeruginosa strains isolated from burn patients by RAPD-PCR. Burns. 2013;39(7):1409–1413. doi: 10.1016/J.BURNS.2013.03.008
- Fatima A, Naqvi SB, Khaliq SA, et al. Antimicrobial susceptibility pattern of clinical isolates of Pseudomonas aeruginosa isolated from patients of lower respiratory tract infections. Springerplus. 2012;1(1):1–4. doi: 10.1186/2193-1801-1-70
- Akpaka PE, Swanston WH, Ihemere HN, et al. Emergence of KPC-producing Pseudomonas aeruginosa in Trinidad and tobago. J Clin Microbiol. 2009;47(8):2670. doi: 10.1128/JCM.00362-09
- Nordmann P, Poirel L, Carrër A, et al. How to detect NDM-1 producers. J Clin Microbiol. 2011;49(2):718–721. doi: 10.1128/JCM.01773-10
- Seyedjavadi SS, Goudarzi M, Sabzehali F. Relation between blaTEM, blaSHV and blaCTX-M genes and acute urinary tract infections. J Acute Dis. 2016;5(1):71–76. doi: 10.1016/J.JOAD.2015.07.007
- Cao V, Lambert T, Nhu DQ, et al. Distribution of extended-spectrum beta-lactamases in clinical isolates of Enterobacteriaceae in vietnam. Antimicrob Agents Chemother. 2002;46(12):3739–3743. doi: 10.1128/AAC.46.12.3739-3743.2002
- Warjri I, Dutta TK, Lalzampuia H, et al. Detection and characterization of extended-spectrum β-lactamases (blaCTX-M-1 and blaSHV) producing Escherichia coli, Salmonella spp. And Klebsiella pneumoniae isolated from humans in Mizoram. Vet World. 2015;8(5):599–604. doi: 10.14202/VETWORLD.2015.599-604
- Remya P, Shanthi M, Sekar U. Prevalence of blaKPC and its occurrence with other beta-lactamases in Klebsiella pneumoniae. J Lab Physicians. 2018;10(4):387. doi: 10.4103/JLP.JLP_29_18
- Wang EW, Jung JY, Pashia ME, et al. Otopathogenic Pseudomonas aeruginosa strains as competent biofilm formers. Arch Otolaryngol Head Neck Surg. 2005;131(11):983–989. doi: 10.1001/ARCHOTOL.131.11.983
- Stepanović S, Vuković D, Dakić I, et al. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40(2):175–179. doi: 10.1016/S0167-7012(00)00122-6
- ALshaiki JM, Toweir A. Prevalence Pseudomonas aeruginosa among Libyan patients and its association with hospital’s environment in Benghazi. J Med Microb Diagn. 2017;6(2):1–8. doi: 10.4172/2161-0703.1000257
- Kraiem AG, Zorgani A, Elahmer O, et al. Carbapenem-resistant gram-negative bacilli in Tripoli, Libya. Am J Infect Control. 2016;44(10):1192–1194. doi: 10.1016/j.ajic.2016.04.245
- Mathlouthi N, Areig Z, Al Bayssari C, et al. Emergence of carbapenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii clinical isolates collected from some Libyan hospitals. Microb Drug Resist. 2015;21(3):335–341. doi: 10.1089/MDR.2014.0235
- Slimene K, El SA, Dziri O, et al. Epidemiology, phenotypic and genotypic characterization of carbapenem-resistant gram-negative bacteria from a Libyan hospital. Microb Drug Resist. 2023;29(8):333–343. doi: 10.1089/MDR.2022.0060
- Zorgani A, Almagatef A, Sufya N, et al. Detection of CTX-M-15 among uropathogenic Escherichia coli isolated from five major hospitals in Tripoli, libya. Oman Med J. 2017;32(4):322. doi: 10.5001/OMJ.2017.61
- Babour IA, Mohamed MB, Shehabi AA. Molecular characterization of Pseudomonas aeruginosa isolates from various clinical specimens in Khartoum/Sudan: Antimicrobial resistance and virulence genes. Int Arab J Antimicrob Agents. 2020;10(1). doi: 10.3823/840
- Tchakal-Mesbahi A, Metref M, Singh VK, et al. Characterization of antibiotic resistance profiles in Pseudomonas aeruginosa isolates from burn patients. Burns. 2021;47(8):1833–1843. doi: 10.1016/J.BURNS.2021.03.005
- Kraiem AG, Zorgani A, Elahmer O, et al. New Delhi metallo-β-lactamase and OXA-48 carbapenemases in Gram-negative bacilli isolates in Libya. Libyan Journal Of Medicine. 2015;10(1):29206. doi: 10.3402/ljm.v10.29206
- Azab MM, Shehata A, Mohamed M-O. 10 and GES-1 Extended-spectrum β-lactamases play a major role in causing antibiotic resistance of pseudomonas aeruginosa isolated from nosocomial infections in Ismailia, egypt. Egypt J Med Microbiol. 2015;24(4):81–88. doi: 10.12816/0030399
- Codjoe FS, Donkor ES. Carbapenem Resistance: a review. Med Sci. 2018;6(1):1. doi: 10.3390/MEDSCI6010001
- World Health Organization (WHO). Implementation manual to prevent and control the spread of carbapenem-resistant organisms at the national and health care facility level. 2019. [cited 2023 Aug 9]. Available from: https://apps.who.int/iris/bitstream/handle/10665/312226/WHO-UHC-SDS-2019.6-eng.pdf
- Franklin MJ, Nivens DE, Weadge JT, et al. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front Microbiol. 2011 2. doi: 10.3389/fmicb.2011.00167
- Pericolini E, Colombari B, Ferretti G, et al. Real-time monitoring of Pseudomonas aeruginosa biofilm formation on endotracheal tubes in vitro. BMC Microbiol. 2018;18(1):1–10. doi: 10.1186/s12866-018-1224-6
- Wozniak DJ, Wyckoff TJO, Starkey M, et al. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa biofilms. Proc Natl Acad Sci U S A. 2003;100(13):7907–7912. doi: 10.1073/pnas.1231792100