ABSTRACT
Background
The pathogenic Leptospira is maintained in renal tubules of certain animals, mostly rodents, and excreted in the urine which can contaminate the environment. It is necessary to detect pathogenic Leptospira in environmental samples. Knowing the survival of Leptospira in the environment (water and soil) can provide an overview of where and how they can be transmitted to humans.
Objective
Therefore, this study aimed to provide a systematic overview of pathogenic Leptospira presence in water and soil environment, the various species of pathogenic Leptospira that are harmful for human, and the ability to survive using a systematic review method.
Methods
The search process used four databases: PubMed, Science Direct, Scopus, and ProQuest. Furthermore, the articles sought were published from 2000 to July 2021, and 38 were analysed.
Results
The pathogenic Leptospira contamination in water was higher in urban areas, while soil samples were higher in rural areas. Various pathogenic Leptospira detected in the environment were L. alstonii, L. kmetyi, L. noguchii, and L. interrogans. Those pathogenic Leptospira can survive in water at 4–30°C and at pH < 7; in soil, it can survive at a humidity of < 20% and a pH < 6.
Conclusion
Urban and rural areas have the same risk for leptospirosis disease because pathogenic Leptospira (P1).
Introduction
Leptospirosis is an infectious disease caused by Leptospira that is transmitted directly or indirectly from animal to human, known as zoonosis [Citation1]. The pathogenic Leptospira infect renal tubules of reservoir animals, mostly rodents, and shed through urine into the environment. Pathogenic Leptospira can survive in moist soil and surface water for several months [Citation2]. Humans and other animals can be infected through their skin and mucous membranes when they encounter a contaminated environment by pathogenic Leptospira [Citation3].
Leptospirosis outbreak is widely associated with water activities, including flooding, water sports, and work involving contact with water, such as agriculture [Citation4]. There are 1.03 million cases worldwide, with a mortality rate of 58,900 deaths every year. The highest incidence is from Oceania (150.68 cases per 100,000 population), followed by Southeast Asia (55.54), the Caribbean (50.68), and Africa (22.65) [Citation5]. These outbreaks have occurred in several countries such as Guyana, India, Kenya, Lao People’s Democratic Republic, New Caledonia, Nicaragua, Philippines, and Thailand and were reported to have a strong relationship with extreme weather [Citation6]. Furthermore, 3–102 cases in Africa per 100,000 population yearly and 2.3% − 19.8% of hospitalized patients with febrile symptoms were Leptospirosis [Citation7]. Meanwhile, in Fiji, there were 576 cases and 40 deaths from Leptospirosis after a storm that caused flooding in 2012 [Citation8].
Leptospirosis cases in Indonesia increase every year; where in 2017, there were 894 cases, and increase in 2019 of 920 cases, with a death rate of 122 (13.26%); many cases were not reported due to its difficulty to diagnose and the high cost of Leptospirosis laboratory test [Citation9]. The presence of rats, stagnant water around the house, and poor sewage and waste disposal conditions were significantly associated with the incidence of Leptospirosis in Indonesia [Citation10]. A study in Gujarat reported that there was a significant relationship between the incidence of Leptospirosis and working in flooded fields (OR = 4.6, 95% CI = 1.6–17.9), as well as bathing/swimming in canals (OR = 3, 95% CI = 1.8–4.8) [Citation11]. The risk of humans being infected with Leptospira after contact with the environment depends on the ability to survive and infect new hosts [Citation12].
Previously, Leptospira were grouped into two pathogenic and saprophytic. Recently study found that there were 64 genera of Leptospira species and classified into four subclades, namely subclade P1 (pathogen), subclade P2 (intermediate), subclade S1 and S2 (saprophytic) [Citation13]. The name for pathogenic Leptospira now become P1; it is the causative agent of Leptospirosis, which is distributed worldwide [Citation14]. Pathogenic Leptospira or P1 multiply in the kidney tubules of infected mammals (hosts) [Citation15] and excreted through urine into the environment [Citation1]. One of the most threatening and dominant species found in human and animal is L. interrogans. Intermediate Leptospira have recently been discovered in humans and animals, but in animal experiments, they can not reproduce the disease. Meanwhile, the saprophyte is an environmental species non-pathogenic for humans and other animals [Citation16]. The species belonging to subclade P1 (pathogen) are L. interrogans, L. kirschneri, L. noguchii, L. santarosai, L. mayottensis, L. borgpetersenii, L. alexanderi, and L. weilii [Citation13].
Many studies have investigated the presence of Leptospira in the environmental samples. In Malaysia, from 40 samples consisting of 20 water and 20 soil samples, 5% Leptospira DNA was found in each after being tested by the qPCR (quantitative-Polymerase Chain Reaction) method [Citation17]. A study in Jakarta took water samples from 20 flood-prone locations, and the result showed that 75% contained Leptospira saprophytes [Citation18]. The saprophytic types live naturally on soil and water surfaces, but these species do not cause disease [Citation3]. Meanwhile, pathogenic types live in the renal tubules of reservoir animals such as rats and then exit into the environment through urine [Citation3].
Knowing the Leptospira’s survival in water and soil provides an overview of where and how it can be transmitted to humans [Citation19]. However, isolation of pathogenic Leptospira (P1) was difficult because saprophytic Leptospira grows rapidly during culture, making it difficult to detect the pathogenic type. This study aimed to provide a systematic overview of pathogenic Leptospira (p1) presence in water and soil environments, the various species of pathogenic Leptospira that are harmful to humans and the ability to survive using a systematic review method.
Methods
Search strategy
This Systematic Review used the PRISMA stage or protocol and has been registered on PROSPERO (Id: CRD42021267260). To determine the research questions, the PICO method was used.
Population (research subject) : Leptospira in environment
Intervention : Detection of Leptospira in environmental samples by Laboratory Test (PCR)
Comparison : Rural and urban research areas (urban & rural)
Outcome : Leptospirosis
The articles were retrieved from four scientific databases, namely PubMed, Science Direct, Scopus, and ProQuest, and some were taken from the Indonesian National Journal, which is not indexed internationally. The search was conducted using the words Leptospira, environmental, water, and soil. The articles in the search database were recorded or cited using the Mendeley Program to facilitate the screening process.
The PubMed search used the keywords ‘Leptospira, environmental, water and soil’, filters applied: Full text, Journal article, English, Indonesian, from 2000/1/1–2021/7/17. ProQuest used ‘leptospiral, environmental, water, and soil’ filters applied: Academic Journal, 1 January 2000 – 17 July 2021, Articles, English. Science Direct ‘leptospira, environmental, water and soil’, 2000–2021, Refined by Article type: Research articles, Subject area: Environmental science, Medicine and Dentistry, Biochemistry, genetics and molecular. Scopus (TITLE-ABS-KEY (leptospira) AND TITLE-ABS-KEY (environmental) AND TITLE-ABS-KEY (water) AND TITLE-ABS-KEY (soil) AND (EXCLUDE (PUBYEAR, 1995) OR (EXCLUDE (PUBYEAR), 1992) OR EXCLUDE (PUBYEAR, 1991), EXCLUDE (PUBYEAR, 1973)) AND (EXCLUDE (SUBJAREA, ‘ENGI’)) AND (LIMIT-TO (DOCTYPE, ‘ar’)) AND (LIMIT-TO (LANGUAGE, ‘English’)) AND (LIMIT-TO (SRCTYPE, ‘j’).
Inclusion and exclusion criteria
The inclusion criteria were articles published in 2000–2021 in English or Indonesian, academic articles or studies with observational designs, and environmental or experimental studies reporting the detection results of pathogenic Leptospira from the environment and/or humans. Furthermore, the criteria include articles on an environment at risk of transmitting the disease to humans and those reporting the bacteria’s survival. Meanwhile, the exclusion criteria were articles that only contained abstracts, systematic reviews, those that reported detection of pathogenic Leptospira in animals, reported detection only in humans without environmental variables, the discovery of pathogenic Leptospira in environments that are rarely touched by humans such as forests and detection of non-pathogenic Leptospira.
Article quality assessment
The quality assessment of the articles reviewed used a list of questions that were assessed according to the information in the selected articles.
Data extraction
The data used in the articles selected based on the inclusion criteria were the main author, year of publication, research period, the country where it was carried out, the design and methods, sampling locations and sample sources. The results related to the presence of pathogenic Leptospira in the environment were the number of samples identified, positive samples, and the species detected. Furthermore, data regarding the ability of these bacteria to survive were also analyzed.
Data synthesis
The data were narratively synthesized, and the study area was categorized into rural, covering places outside the city with low population density, having a lot of lands such as agriculture, rice fields, plantations, livestock, as well as fields, and urban areas located in the city center with high population density.
Overcoming bias
Two reviewers carried out the selection process according their respective fields of expertise; this is to overcome the bias in this review. When there was a conflict between the two reviewers, a third person was used as an intermediary or as a suggestion. Therefore, to overcome the bias of article synthesis, a registered review protocol with Prospero was used to improve review quality, encourage transparency, and avoid duplication.
Results
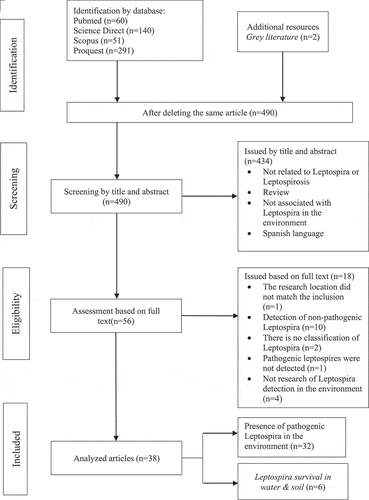
There was no risk of bias in this systematic review. Based on the article search results using four scientific databases, 542 articles were obtained, and two were added from grey literature sources. After deleting the same article, 490 were found, screened by title and abstract. Also, 434 articles were removed after being screened by title and abstract because they unrelated to the bacteria or the disease reviewed, unrelated to Leptospira in the environment, and were in Spanish. Furthermore, the full texts were reviewed to find those that matched the inclusion criteria. From the 56 articles reviewed, 18 were excluded based on the exclusion criteria, namely, the research location did not match the inclusion (n = 1) and detection of non-pathogenic Leptospira (n = 10), no Leptospira classification (n = 2), no pathogenic Leptospira detected (n = 1), and no detection study in the environment (n = 4). Eventually, 38 that met the inclusion criteria were analyzed. A total of 32 articles involved studies on the presence of pathogenic Leptospira in the environment, while six were about the ability to survive in water and soil media (). Quality assessment of 38 selected articles used the scoring table, and they have moderate to good quality with an average score of 75; this information is available in the supplementary appendix.
Article characteristics
Most of the studies came from Southeast Asia (n = 15), followed by South America (n = 8), Western Europe (n = 4), South Asia (n = 3), the United States, and Central America with two articles respectively, while West Africa, West Indies, East Asia, and Southern Europe with one article. Based on the year of publication, the most published articles were in 2018 (n = 6), then 2017 (n = 5), 2014, 2019, and 2020 with four, 2016 (n = 3), 2011, 2013, 2015, as well as 2021 with two articles each, and 2006, 2008, 2010, 2012 with one each. Most articles did not clearly state the type of design, but based on the method used and the time, it can be observed that the design was cross-sectional (n = 27), prospective (n = 2), case study (n = 2), retrospective (n = 1), and six articles used experimental study ().
Table 1. Characteristics of articles.
Presence of Pathogenic Leptospira (P1) in water samples
The presence of pathogenic Leptospira (P1) in water samples was investigated in 30 out of 38 articles. This review compared the presence of pathogenic Leptospira (P1) in water samples based on two research areas, urban and rural. Twelve articles investigate contamination in water samples only from urban area [Citation20–31], 14 articles only from rural areas [Citation19,Citation32–44], and the remaining four articles investigate in both areas [Citation45–48]. In total, water samples from rural and urban were 2.341 samples. The percentage of contamination pathogenic Leptospira (P1) from water samples in urban was higher (18.7%) than in rural area (14.7%). The highest contamination from the urban areas was from puddles around the market in South Amerika (68%) [Citation46]. In rural areas, the highest contamination was from water dams that are used for household activities in the West Indies (62.5%) [Citation34].
In urban area, other Leptospira (P1) contamination from water samples were detected in public toilet 66,7% [Citation23], water canal from slaughterhouse 16,7% [Citation29], rice field 8,3% [Citation26], recreational lake 1,7% [Citation27], open drainage around household between 1,9% − 36% [Citation20,Citation26,Citation28,Citation47,Citation48], human drinking water 8% [Citation45], household water storage between 4% − 17,2% [Citation21,Citation24,Citation25], groundwater and well between 1,6% − 33,3% [Citation21,Citation22,Citation25,Citation46]. Pathogenic Leptospira (P1) were not detected in industrial drainage [Citation48], the puddle at slaughterhouses [Citation29], irrigation and dry canals near the market [Citation30].
In rural area, pathogenic Leptospira (P1) detected in spring that used for drinking 40% [Citation34], waterfall 35,3% [Citation36], river between 2,1%-33,3% [Citation19,Citation35,Citation37,Citation38,Citation44,Citation45], well 25,4% [Citation46], household puddles between 2,2% − 41,1% [Citation37,Citation39,Citation45], household water storage 17%-46,7% [Citation42,Citation45], drinking water 9,3%-15% [Citation37,Citation45], drainage from household 2.1% − 6.7% [Citation35,Citation48] and cowshed drainage 13,3% [Citation48]. There was no contamination detected in the pool, stream, forest land, and river from rural area [Citation34,Citation48]. ()
Table 2. Presence of leptospira in water samples.
Presence of Pathogenic Leptospira (P1) in soil sample
The presence of Pathogenic Leptospira (P1) in soil samples was investigated in 15 out of 38 articles. Six articles investigate contamination in soil samples only from urban areas [Citation23,Citation25,Citation28,Citation30,Citation31,Citation50], eight articles investigate only from rural areas [Citation16,Citation19,Citation33,Citation41,Citation43,Citation44,Citation51,Citation52], the remain 1 article investigate in both area [Citation48]. In total, soil samples from rural and urban were 989 samples. The percentage of contamination pathogenic Leptospira (P1) from water samples in rural were higher (28%) than in urban area (14%). The highest contamination from the urban areas was from soil from a pond in the lethal case of Leptospirosis area in Southern Europe (100%) [Citation23]. While in rural, the highest contamination was from coastal areas in Southeast Asia (47.8%) [Citation51]. In urban areas, pathogenic Leptospira (P1) not only found around slums and poor communities [Citation25,Citation28,Citation30,Citation50], but it also found in industrial areas [Citation48] and around campus [Citation31]. In rural areas, contamination of pathogenic Leptospira was detected in soil samples from markets [Citation41,Citation48], stables [Citation48], rice fields [Citation48], rivers [Citation19], beach, and coastal area [Citation19,Citation51], even from recreational area [Citation16,Citation33,Citation41]. ()
Table 3. Presence of pathogenic leptospira in soil samples.
Identification results of Pathogenic Leptospira (P1) species in water and soil samples
Various Leptospira species that are harmful and able to infect animals (agent) and humans were detected in water and soil samples. They are L. alstonii, L. kmetyi, L. noguchii, and L. interrogans. Those species were detected in a rural and urban settings.
Pathogenic Leptospira (P1) survival ability in environmental samples
Pathogenic Leptospira (P1) can survive in soil with humidity < 20% [Citation31] and can survive for four days in the soil after a storm [Citation51] but cannot reproduce in the environment [Citation49]. It is still able to infect even in poor nutritional conditions [Citation6] and can survive in low pH water for at least 20 months [Citation53] as well as in nutrient-free water for more than a year [Citation6]. In addition, they can outlive and move faster or slower than the average speed of flowing water [Citation54] ().
Table 4. Leptospira survival in the environmental samples.
Discussion
Pathogenic Leptospira (P1) live and reproduce in the kidney tubules of animal, mostly rodents, and excreted into the environment through the urine of infected animals. Survival of pathogenic Leptospira (P1) in the environment after being shed via animal urine is a key factor in estimating the risk of infection. This review showed that once pathogenic Leptospira (P1)is excreted into the environment through the urine of the infected animal, Leptospira immediately needs to be in a humid environment or body of water to survive and remain capable of infecting pathogenic Leptospira (P1) could not survive on hard surface [Citation54].
Pathogenic Leptospira (P1) can survive in wet soil during dry days and emerge to the surface during rainy days. Furthermore, it can survive in soil with a humidity of < 20% and pH levels have no effect on distribution and survival in the soil [Citation31]. Pathogenic Leptospira (P1) only survived 12 hours in sodium chloride, while it lasted for three days in natural seawater [Citation51]. Meanwhile, freshwater pathogenic Leptospira (P1) survived for more than one year or at least 20 months, and even under conditions of nutritional deficiency, it still can infect susceptible animals [Citation6].
Pathogenic Leptospira can not multiply in the environment [Citation49]. Based on a study using water with different temperatures and pH levels, it is known that pathogenic Leptospira (P1) is able to survive at 4°C for 130 days, at 20°C for 236 days, and at 30°C for 316 days. Based on pH levels, pathogenic Leptospira (P1) survived for 344 days at pH 7 while at pH < 7 for 129 days. Pathogenic Leptospira (P1) is also capable of infecting experimental animals even though the pH of the water was reduced to < 6. Therefore, it was concluded that even at cold temperatures and low levels of nutrients in the water, pathogenic Leptospira was able to survive and become infected for at least 20 months [Citation53].
Pathogenic Leptospira (P1) was rarely detected in soil samples because it prefers water bodies or very humid environments. In this review, the proportion of soil samples was less than that of water samples. However, the presence of pathogenic Leptospira (P1) in soil samples has been proven; it is important to be known to increase awareness and prevention of Leptospirosis transmission. To detect Leptospira in soil samples, it is necessary to pay attention to sampling location, which should include wet, mud, or humus soil. The samples were obtained in wet and shady places [Citation16,Citation25]. Pathogenic Leptospira (P1) contamination in soil samples from urban areas is mostly found in industrial and recreational areas, while in rural areas, they are more commonly found around livestock, rice fields, and the patient’s home [Citation16,Citation52].
The presence of pathogenic Leptospira (P1) in soil samples was not related to environmental characteristic such as vegetation, rat density, distance from open sewers to houses, or clay content. However, samples with high water content showed a high prevalence [Citation50]. The survival of pathogenic Leptospira (P1) in the soil influenced by the nutrients contained, such as iron, manganese, and copper. The major content is iron, which helps to maintain the virulence and growth of pathogenic Leptospira (P1) [Citation40]. The study in New Caledonia reported that pathogenic Leptospira (P1) was detected in soil samples from the site of suspected exposure at nine weeks from the estimated time, but not after 12 weeks [Citation16].
People who work in agriculture, farmers, veterinarians, forest surveyors, mining, and laboratory workers are the high risk of Leptospirosis because pathogenic Leptospira (P1) is able to survive in wet soil (aquatic ecosystem) [Citation47,Citation56,Citation57]. To avoid transmission of leptospirosis, the worker is recommended to wear special clothing, such as boots, masks, and gloves, to avoid contact with the skin or soil or materials that have been contaminated. After carrying out the work, laboratory workers and abattoirs are advised to wash work tools with sodium hypochlorite in a dilution of 1:4000 or use detergents [Citation58].
Pathogenic Leptospira (P1) species detected in water and soil samples both in urban and rural areas, there were L. alstonii [Citation27,Citation31,Citation36,Citation41], L. kmetyi [Citation30,Citation33,Citation36,Citation43,Citation44,Citation51], L. interrogans [Citation16,Citation22,Citation28], L. weilii [Citation45,Citation59], and L. noguchii [Citation28]. L. interrogans and L. kirschneri are the most infectious and harmful for humans [Citation59,Citation60]. Even L. wolffii from the intermediate group (P2) has also been found in human samples. The P2 was dominant in environmental samples. This condition emphasizes that the environment is a high-risk factor for transmission [Citation22]. A study in Thailand and Colombia detected L. interrogans in humans, pigs, dogs, and water samples. The water samples containing L. interrogans came from livestock drinking water sources and sewage. Many cases in rural areas of Leptospira were unreported because some infected humans did not show any symptoms [Citation22,Citation32]. Furthermore, studies in Italy found L. interrogans in public toilet. This investigation was carried out after a 65-year-old toilet worker was found dead, and L. interrogans was found in his body [Citation23].
Leptospirosis is commonly known as a disease that originates from water; the presence of pathogenic Leptospira (P1) in the soil cannot be ignored. Therefore, knowledge of environmental risk factors, especially in the soil, can prevent transmission in risky environments such as markets, agriculture, livestock, and natural recreational areas.
Limitations
The limitation of this study was that it could not perform a meta-analysis due to the limited quantitative data available in the articles obtained. The information was obtained from primary reports sourced from reliable databases. For further study, when the bacteria in water samples in urban areas are to be investigated, the samples should be taken from open household sewers, rainwater, or floodwaters. In rural areas, it should be obtained from wells, rain-fed water, other open reservoirs, and surface water around the house, such as rivers or rainwater puddles. When Leptospira in soil samples is being investigated, it should be carried out on the soil around the case’s house, and the time for environmental sampling should be 1–2 months after infection. In addition, the soil sample should be wet and taken in a shady location.
Conclusion
Our review suggests that pathogenic Leptospira (P1) spreads in the environment and is able to survive in the water body for months and in moist soil for a few days. Urban and rural areas have the same risk for leptospirosis disease because pathogenic Leptospira (P1) was detected in both areas. The contamination of pathogenic Leptospira (P1) was not only detected in slums or poor household areas, but it was also detected in industrial and recreational areas. This information could be used to increase awareness and prevent the transmission of leptospirosis.
Supplemental Material
Download MS Word (14.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20008686.2024.2324820
Additional information
Funding
References
- World Health Organization. Human leptospirosis: guidance for diagnosis, surveillance and control. WHO; 2003.
- Smith DJW, Self HRM. Observations on the survival of leptospira Australis a in soil and water. J Hyg. 1955;53(4):436–12. doi: 10.1017/S0022172400000942
- Adler B, de la Peña Moctezuma A. Leptospira and leptospirosis. Vet Microbiol. 2010;140(3–4):287–296. doi: 10.1016/j.vetmic.2009.03.012
- Wynwood SJ, Graham GC, Weier SL, et al. Leptospirosis from water sources. Pathog Glob Health. 2014;108(7):334–338. doi: 10.1179/2047773214Y.0000000156
- Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PloS Negl Trop Dis. 2015;9(9):e0003898. doi: 10.1371/journal.pntd.0003898
- Bierque E, Thibeaux R, Girault D, et al. A systematic review of Leptospira in water and soil environments. PloS One. 2020;15(1):1–22. doi:10.1371/journal.pone.0227055
- Allan KJ, Halliday JEB, Moseley M, et al. Assessment of animal hosts of pathogenic leptospira in northern Tanzania. PloS Negl Trop Dis. 2018;12(6):1–19. doi: 10.1371/journal.pntd.0006444
- Guernier V, Goarant C, Benschop J, et al. A systematic review of human and animal leptospirosis in the Pacific Islands reveals pathogen and reservoir diversity. PloS Negl Trop Dis. 2018;12(5):1–32. doi: 10.1371/journal.pntd.0006503
- Kementerian Kesehatan Republik Indonesia. Profil Kesehatan Indonesia. Kementerian Kesehatan Republik Indonesia. 2019; p. 206.
- Haq A, Anggraini S, Masnarivan Y. Environmental factors related to leptospirosis in Indonesia: a systematic review. AIPHC. 2020. doi: 10.4108/eai.9-10-2019.2297166
- Desai NPRBK, Patel F, Patel PB, et al. A case–control study of epidemiological factors associated with leptospirosis in South Gujarat region. J Postgrad Med. 2016;62(4):223–227. doi: 10.4103/0022-3859.188551
- Barragan V, Olivas S, Keim P, et al. Critical knowledge gaps in our understanding of environmental cycling and transmission of leptospira spp. Appl Environ Microbiol. 2017;83(19):1–10. doi:10.1128/AEM.01190-17
- Vincent AT, Schiettekatte O, Goarant C, et al. Revisiting the taxonomy and evolution of pathogenicity of the genus leptospira through the prism of genomics. PloS Negl Trop Dis. 2019;13(5):e0007270. doi: 10.1371/journal.pntd.0007270
- Sun A, Liu X, Yan J. Leptospirosis is an invasive infectious and systemic inflammatory disease. Biomed J. 2020;43(1):24–31. doi:10.1016/j.bj.2019.12.002
- Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296–326. doi:10.1128/CMR.14.2.296-326.2001
- Thibeaux R, Geroult S, Benezech C, et al. Seeking the environmental source of Leptospirosis reveals durable bacterial viability in river soils. PloS Negl Trop Dis. 2017;11(2):1–14. doi: 10.1371/journal.pntd.0005414
- Muhammad N, Nik H, Halim A, et al. Leptospirosis Occurrence in Agricultural Communities in Setiu, Terengganu. IJRTE. 2019;8(2S3):259–263.
- Widiyanti D, Astuti IIP. Study of leptospira sp in several flood-vulnerable areas in Jakarta. J Kedokt Yars. 2016;24(1):80–088.
- Miller E, Barragan V, Chiriboga J, et al. Leptospira in river and soil in a highly endemic area of Ecuador. BMC Microbiol. 2021 Jan;21(1):17.
- Arnau Cassanovas-Massana AIK, Costa F, Riediger IN, et al. Spatial and temporal dynamics of pathogenic Leptospira in surface waters from the urban slum environment. PMC. 2017;1(203):176–184. doi: 10.1016/j.watres.2017.11.068
- Houéménou H, Gauthier P, Houéménou G, et al. Pathogenic leptospira and water quality in African cities: a case study of Cotonou, Benin. Sci Total Environ. 2021;774:145541. doi: 10.1016/j.scitotenv.2021.145541
- Kurilung A, Chanchaithong P, Lugsomya K, et al. Molecular detection and isolation of pathogenic Leptospira from asymptomatic humans, domestic animals and water sources in Nan province, a rural area of Thailand. Res Vet Sci. 2017;115(October 2016):146–154. doi: 10.1016/j.rvsc.2017.03.017
- Luchini D, Meacci F, Oggioni MR, et al. Molecular detection of Leptospira interrogans in human tissues and environmental samples in a lethal case of leptospirosis. Int J Legal Med. 2008 May;122(3):229–233.
- Allwood P, Muñoz-Zanzi C, Chang M, et al. Knowledge, perceptions, and environmental risk factors among Jamaican households with a history of leptospirosis. J Infect Public Health. 2014;7(4):314–322. doi: 10.1016/j.jiph.2014.03.004
- Flores B, Escobar K, Muzquiz JL, et al. Detection of pathogenic leptospires in water and soil in areas endemic to leptospirosis in Nicaragua. Trop Med Infect Dis. 2020;5(3):149. doi: 10.3390/TROPICALMED5030149
- Nugroho A, Joharina AS, Susanti L. Karakteristik Lingkungan Abiotik dan Potensi Keberadaan Leptospira Patogenik di Air dalam Kejadian Luar Biasa Leptospirosis di Kota Semarang. Vektora J Vektor dan Reserv Penyakit. 2017;9(1):37–42. doi: 10.22435/vk.v9i1.5317.37-42
- Benacer D, Woh PY, Mohd Zain SN, et al. Pathogenic and saprophytic leptospira species in water and soils from selected urban sites in peninsular Malaysia. Microbes Environ. 2013;28(1):135–140. doi: 10.1264/jsme2.me12154
- Pui CF, Bilung LM, Apun K, et al. Diversity of leptospira spp. In rats and environment from urban areas of Sarawak, Malaysia. J Trop Med. 2017;2017:1–8. doi: 10.1155/2017/3760674
- Tabo NA, Villanueva SYAM, Gloriani NG. Molecular detection of leptospira from environmental samples around abattoirs of Cavite province, Philippines. Southeast Asian J Trop Med Public Health. 2019;50(2):290–299.
- Tantengco OAG, Gloriani NG. Isolation and genetic detection of pathogenic leptospira spp. From environmental soils and water in Central Luzon, Philippines. Asian Pacific J Trop Dis. 2017;7(12):748–752. doi:10.12980/apjtd.7.2017D7-220
- Saito M, Villanueva SYAM, Chakraborty A, et al. Comparative analysis of leptospira strains isolated from environmental soil and water in the Philippines and Japan. Appl Environ Microbiol. 2013;79(2):601–609. doi: 10.1128/AEM.02728-12
- Calderón A, Rodríguez V, Máttar S, et al. Leptospirosis in pigs, dogs, rodents, humans, and water in an area of the Colombian tropics. Trop Anim Health Prod. 2014;46(2):427–432. doi:10.1007/s11250-013-0508-y
- Zaki AM, Hod R, Shamsusah NA, et al. Detection of leptospira kmetyi at recreational areas in Peninsular Malaysia. Environ Monit Assess. 2020 Oct;192(11):703. doi: 10.1007/s10661-020-08639-x
- Rawlins J, Portanova A, Zuckerman I, et al. Molecular detection of leptospiral DNA in environmental water on St. Kitts. IJERPH. 2014;11(8):7953–7960. doi: 10.3390/ijerph110807953
- Ridzlan FR, Bahaman AR, Khairani-Bejo S, et al. Detection of pathogenic leptospira from selected environment in Kelantan and Terengganu, Malaysia. Trop Biomed. 2010;27(3):632–638.
- Chaiwattanarungruengpaisan S, Suwanpakdee S, Sangkachai N, et al. Potentially pathogenic Leptospira species isolated from a waterfall in Thailand. Jpn J Infect Dis. 2018;71(1):65–67. doi:10.7883/yoken.JJID.2017.363
- Muñoz-Zanzi C, Mason MR, Encina C, et al. Leptospira contamination in household and environmental water in rural communities in Southern Chile. IJERPH. 2014;11(7):6666–6680. doi: 10.3390/ijerph110706666
- Viau EJ, Boehm AB. Quantitative PCR-based detection of pathogenic Leptospira in Hawaiʻian coastal streams. J Water Health. 2011 Dec;9(4):637–646. doi: 10.2166/wh.2011.064
- Verma A, Beigel B, Smola CC, et al. Evidence of leptospiral presence in the Cumberland Gap Region. PloS Negl Trop Dis. 2019;13(12):e0007990. doi: 10.1371/journal.pntd.0007990
- Lall C, Kumar KV, Raj RV, et al. Correlation between physicochemical properties of soil and presence of leptospira. Ecohealth. 2018;15(3):670–675. doi: 10.1007/s10393-018-1346-1
- Azali MA, Yean Yean C, Harun A, et al. Molecular Characterization of Leptospira spp. in environmental samples from North-Eastern Malaysia revealed a pathogenic strain, Leptospira alstonii. J Trop Med. 2016;2016:2060241. doi:10.1155/2016/2060241
- Widiastuti D, Anggun R, Djati P. Kontaminasi Leptospira Patogenik pada Air Konsumsi di Pemukiman Kabupaten Demak. Balaba J Litbang Pengendali: Penyakit Bersumber Binatang Banjarnegara. 2015;11(16):89–96. doi:10.22435/blb.v11i2.4446.89-96
- Mohd Ali MR, Mohamad Safiee AW, Yusof NY, et al. Isolation of leptospira kmetyi from residential areas of patients with leptospirosis in Kelantan, Malaysia. J Infect Public Health. 2018;11(4):578–580. doi: 10.1016/j.jiph.2017.12.008
- Neela VK, Azhari NN, Joseph N, et al. An outbreak of leptospirosis among reserve military recruits, Hulu Perdik, Malaysia. Eur J Clin Microbiol Infect Dis. 2019 Mar;38(3):523–528.
- Mason MR, Encina C, Sreevatsan S, et al. Distribution and diversity of pathogenic leptospira species in peri-domestic surface waters from South Central Chile. PloS Negl Trop Dis. 2016 Aug;10(8):e0004895.
- Ganoza CA, Matthias MA, Collins-Richards D, et al. Determining risk for severe leptospirosis by molecular analysis of environmental surface waters for pathogenic Leptospira:. PLOS Med. 2006 Aug;3(8):e308.
- Vinod Kumar K, Lall C, Vimal Raj R, et al. Molecular detection of pathogenic leptospiral protein encoding gene (lipL32) in environmental aquatic biofilms. Lett Appl Microbiol. 2016;62(4):311–315. doi: 10.1111/lam.12533
- Zala DB, Khan V, Sanghai AA, et al. Leptospira in the different ecological niches of the tribal union territory of India. J Infect Dev Ctries. 2018;12(10):849–854. doi: 10.3855/jidc.10541
- Arnau Casanovas-Massana AIK, Ghizzi Pedra G, Wunder EA Jr., et al. Quantification of Leptospira interrogans survival in soil and water microcosms. Am Soc Microbiol. 2018;84(13):1–11. doi:10.1128/AEM.00507-18
- Schneider AG, Casanovas-Massana A, Hacker KP, et al. Quantification of pathogenic Leptospira in the soils of a Brazilian urban slum. PloS Negl Trop Dis. 2018 Apr;12(4):e0006415.
- Saito M, Miyahara S, Villanueva SYAM, et al. PCR and culture identification of pathogenic leptospira spp. From coastal soil in Leyte, Philippines, after a storm surge during super typhoon Haiyan (Yolanda). Appl Environ Microbiol. 2014;80(22):6926–6932. doi: 10.1128/AEM.02568-14
- Fuh Y-B, Shia W-Y, Lee W-M, et al. The use of commercial soil nucleic acid extraction kit and nested PCR for detection of leptospira in farm environment after flooding in Taiwan. Thai J Vet Med. 2011 Dec;41(4):493–498. doi: 10.56808/2985-1130.2342
- André-Fontaine G, Andre-Fontaine G, Thorin C. Waterborne leptospirosis: survival and preservation of the virulence of pathogenic leptospira spp. In fresh water. Curr Microbiol. 2015;71(1):136–142. doi: 10.1007/s00284-015-0836-4
- Nau LH, Obiegala A, Król N, et al. Survival time of Leptospira kirschneri serovar Grippotyphosa under different environmental conditions. PloS One. 2020;1–12. doi: 10.1371/journal.pone.0236007
- Bierque E, Soupé-Gilbert M-E, Thibeaux R, et al. Leptospira interrogans retains direct virulence after long starvation in water. Curr Microbiol. 2020 Oct;77(10):3035–3043. doi: 10.1007/s00284-020-02128-7
- Adler B, Lo M, Seemann T, et al. Pathogenesis of leptospirosis: the influence of genomics. Vet Microbiol. 2011;153(1–2):73–81. doi: 10.1016/j.vetmic.2011.02.055
- Centers for Disease Control and Prevention (U.S.). Leptospirosis fact sheet for clinicians. 2018 Jan 30. https://stacks.cdc.gov/view/cdc/52537.
- Kementerian Kesehatan Republik Indonesia. Petunjuk Teknis Pengendalian Leptospirosis. Kementerian Kesehatan Republik Indonesia; 2017.
- Philip N, Bahtiar Affendy N, Ramli SNA, et al. Leptospira Interrogans and Leptospira Kirschneri are the dominant Leptospira species causing human leptospirosis in Central Malaysia. PloS Negl Trop Dis. 2020;14(3):1–14.
- Meny P, Menéndez C, Ashfield N, et al. Seroprevalence of leptospirosis in human groups at risk due to environmental, labor or social conditions. Revista Argentina de Microbiología. 2019;51(4):324–333. doi: 10.1016/j.ram.2019.01.005