ABSTRACT
Background
Data on imported infections in children and young people (CYP) are sparse.
Aims
To describe imported infections in CYP arriving from malaria-endemic areas and presenting to UK emergency departments (ED) who were screened for malaria.
Methods
This is a retrospective, multi-centre, observational study nested in a diagnostic accuracy study for malaria rapid diagnostic tests. Any CYP < 16 years presenting to a participating ED with a history of fever and travel to a malaria-endemic area between 1 January 2016 and 31 December 2017 and who had a malaria screen as a part of standard care were included. Geographical risk was calculated for the most common tropical infections.
Results
Of the 1414 CYP screened for malaria, 44.0% (n = 622) arrived from South Asia and 33.3% (n = 471) from sub-Saharan Africa. Half (50.0%) had infections common in both tropical and non-tropical settings such as viral upper respiratory tract infection (URTI); 21.0% of infections were coded as tropical if gastro-enteritis is included, with a total of 4.2% (60) cases of malaria. CYP diagnosed with malaria were 7.44 times more likely to have arrived from sub-Saharan Africa than from South Asia (OR 7.44, 3.78–16.41).
Conclusion
A fifth of CYP presenting to participating UK EDs with fever and a history of travel to a malaria-endemic area and who were screened for malaria had a tropical infection if diarrhoea is included. A third of CYP had no diagnosis. CYP arriving from sub-Saharan Africa had the greatest risk of malaria.
Abbreviations: CYP: children and young people; ED: emergency department; PERUKI: Paediatric Emergency Research in the UK and Ireland; RDT: rapid diagnostic test; VFR: visiting friends and relatives.
Introduction
Despite a projected 51.6% drop in air passenger departures in 2020 compared with 2019 owing to the COVID-19 pandemic [Citation1], international air travel looks set to continue to rise. Globally, the number of international tourist arrivals in 2018 was 1.4 billion, up 5% on the previous year, with nearly half of these from sub-tropical regions [Citation2].
Global sentinel studies are mainly adult-based and lack detail on imported infections in children and young people (CYP) [Citation2]. However, the number of European imported infections in patients aged 10–20 years is increasing [Citation3]. A Swiss study found that 53.4% of CYP presenting to a Zurich emergency department (ED) with fever and a history of travel to a tropical country had returned from visiting friends and relatives (VFRs), 43.4% were tourists and 2.4% were immigrants [Citation4]. While numbers of cases of malaria imported to Europe have remained steady since 2008 [Citation5], the proportion of cases attributed to immigrant populations has grown from 14% to 84% in the last decade [Citation6]. VFRs are eight times more likely to be diagnosed with malaria than tourists [Citation7], and UK monitoring estimates that around 10% of imported cases of malaria are CYP [Citation8].
Clinicians need an up-to-date understanding of imported tropical infections in CYP to ensure timely diagnosis of potentially fatal infections such as malaria. Data should also inform diagnostic algorithms as travel continues to increase in this age group and patterns of infection change.
In this UK multi-centre study (Wales, Scotland and England), the aim was to describe the number of imported versus common, routine infections diagnosed in CYP presenting to UK EDs with fever and a recent history of travel to a malaria-endemic area, and to calculate the geographical risk of malaria and other common tropical infections.
Subjects and methods
Cases were any CYP <16 years presenting to a participating hospital’s ED with fever and a history of travel to a malarial area (as designated by the http://travelhealthpro.org.uk/ website) between 1 January 2016 and 31 December 2017 and who were screened for malaria as a part of standard clinical care (sites followed their own or the UK national guidelines for imported infections and were managed and treated either by advanced nurse practitioners or doctors specialised in paediatrics or emergency medicine).
The study aimed to estimate in CYP with fever and a recent history of travel to a malaria-endemic area the number and origin of confirmed malarial infections and other febrile illnesses. This was a secondary outcome in a study nested within Travel Fever, a multi-centre, retrospective diagnostic accuracy study for imported malaria which compared the reference test (standard microscopy) with the index test [standard rapid diagnostic text (RDT)], the results of which are presented elsewhere [Citation9]. Hospital sites belonging to Paediatric Emergency Research in the UK and Ireland (PERUKI) were invited to take part in data collection and the study was sponsored by Birmingham Women’s and Children’s NHS Foundation Trust (). As malaria is routinely screened for in any CYP presenting to the ED with a fever and a history of travel to a malaria-endemic region [Citation8], the malaria screen was used as a surrogate marker for CYP who might have a possible imported tropical infection (UK guidelines on imported tropical infections advise a malaria test in all CYP presenting with a fever and a history of tropical travel [Citation8]). The malaria screen for the diagnostic accuracy study comprised at least one blood film (reference test) and one RDT [Citation9], but, for this study, all CYP with a malaria diagnosis based on either test were included.
Table 1. The hospitals which gathered the study data.
Along with the results of malaria screening, data were collected on patient demographics, geographical area of travel, the patient’s haemoglobin and platelets, discharge diagnosis, whether they re-presented to the same ED in the following 30 days, and whether any patient died. Anonymised data were encrypted and sent via a secure network to the chief investigator and to the University of Oxford for statistical analysis. The classification of tropical versus non-tropical infections was based on established epidemiology in which ‘tropical diseases’ are found in tropical regions (but not exclusively) while ‘non-tropical’ infections are routinely diagnosed in temperate regions [Citation2].
Statistical analysis
Patient demographics were summarised using percentages with 95% confidence intervals (CI) or median and interquartile range (IQR), as appropriate. Geographical risk of malaria and other common imported tropical infections was calculated using odds ratios, based on a logistic regression model to compare the characteristics of patients from sub-Saharan Africa with those from South Asia (the two regions with the highest levels of presentation). Data were analysed using R version 4.2.1.
Ethics
The use of anonymised, retrospective data from health records for the study was discussed at a Public Patient Involvement meeting (NIHR Community Healthcare MIC, Oxford, 13 March 2019), and family representatives unanimously accepted this use of the anonymised data. The study was approved by the Health Research Authority (20/HRA/1341) and adhered to RECORD and STROBE guidelines [Citation10,Citation11].
Role of the funding source
The study funders had no role in the study design, data collection, data interpretation or writing the report.
Results
Altogether, there were 1494 cases in 15 PERUKI sites in Wales, Scotland and England, and five of the sites were hospitals in London; 22 cases were excluded because no RDT and/or film had been performed, and a further 58 were excluded because they were over 16 years of age.
Therefore, the total number of cases analysed was 1414, with 47% (n=799) males and a median age of 4 years (IQR 2–9). A history of fever was recorded in 84% (n=1185) of cases, with 5.8% (n=82) recorded as having no fever and the remaining 10% (n=147) unknown (see Discussion for cases with no recorded fever). There were two deaths with the discharge diagnosis recorded as hepatitis and tonsillitis with no further information available. Of the CYP discharged, 85 (6%) re-presented with febrile illness within 30 days. Of the six CYP who re-presented and were diagnosed eventually with malaria, three had a missed diagnosis on initial presentation while three were known to have left the ED before medical assessment (i.e. they did not wait to be seen).
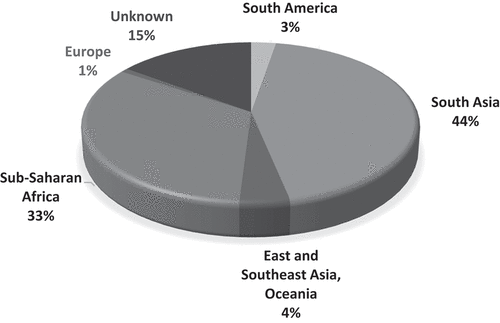
The largest proportion of CYP assessed for imported infections were from South Asia (n=622, 44.0%), followed by sub-Saharan Africa (n=471, 33.3%) (). 50.0% of CYP had infections found in both tropical and non-tropical regions, such as viral upper respiratory tract infection; 21.0% of infections were tropical, if diarrhoeal illness is included ( and Discussion).
Table 2. Discharge diagnoses in 1414 children who underwent screening for malaria in the ED.
Of CYP tested for malaria, 4.2% (60 reported cases) were diagnosed with Plasmodium infection (). Of the CYP with malaria infection who had a complete screen (both film and RDT, n=47), 36 (77%) were diagnosed with P. falciparum, seven (15%) P. vivax, two (4.3%) P. malariae, one P. ovale, and one with mixed infection (P. vivax and P. ovale). No case of P. knowlesi was recorded. P. falciparum infections totalled 2.7% in the total cohort screened (95% CI 1.9–3.7).
The geographical risk of malaria was greatest for travellers returning from sub-Saharan Africa. CYP diagnosed with malaria were 7.44 times more likely to have arrived from sub-Saharan Africa than from South Asia (OR 7.44, 3.78–16.41). CYP diagnosed with malaria were also more likely to be >5 years (OR 3.26, 1.76–6.45) ().
Table 3. Odds ratios of geographical region associated with a diagnosis of malaria and gastro-enteritis based on a logistic regression model (age- and sex-adjusted) with 95% confidence intervals.
A total of 29% (n=408) had either no or an incomplete discharge diagnosis (from ED or if admitted to hospital): no diagnosis was coded for 19.9% of patients (n=282), 6.8% (n=96) were coded as having fever and 2.1% (n=30) were recorded as having pyrexia of unknown origin. A further 14.8% (n=209) were coded as having a ‘viral illness’.
Of CYP arriving from sub-Saharan Africa, 10.2% had malaria (n=48, 95% CI 7.7–13.4), 10.2% gastro-enteritis (n=48, 95% CI 7.7–13.4) and 0.6% typhoid (n=3, 95% CI 0.2–2.0%). Of CYP arriving from South Asia, 15.8% had gastro-enteritis (n=98, 95% CI 13.0–18.9), 2.6% typhoid (n=16, 95% CI 1.5–4.2), 1.4% malaria (n=9, 95% CI 0.7–2.8) and 0.6% dengue (n=4, 95% CI 0.2–1.8). The available clinical coding did not differentiate between viral gastro-enteritis and imported traveller’s diarrhoea (12 CYP, 0.8%, were diagnosed with dysentery).
Discussion
This multi-centre study of over 1400 acute presentations to UK EDs showed that around three quarters of CYP screened for malaria presented from either South Asia or sub-Saharan Africa. About a fifth of CYP were diagnosed with tropical infections, half were diagnosed with infections found in both tropical and non-tropical regions and over a quarter had no discharge diagnosis.
General findings
Obtaining a true picture of imported infections in CYP is problematic as data often come from small, single-centre studies rather than the better data from specialist travel health clinics, largely the preserve of adult medicine [Citation12,Citation13].
A GeoSentinel survey based on 19 travel clinics across the globe assessed the diagnoses of 1591 CYP presenting with travel-related illness between 1997 and 2007 and found that the most common diagnosis was diarrhoeal illness (28%), followed by skin disorders (25%) and febrile illness (23%), the latter including malaria (8%) [Citation14]. Our data identified half that number of malaria diagnoses, which might reflect a global decrease in its incidence, especially in South Asia from where most of our cohort had travelled [Citation15].
A German study found a high relative risk for all febrile tropical diseases in CYP arriving from West and Central Africa compared with other regions [Citation16], but did not specify the malaria risk. A study from a French ED cited only length of stay in an endemic country of >30 days as being associated with an increased risk of malaria infection in CYP (OR 3.13, 1.02–9.59) [Citation17].
Malaria
The present study provides data showing that the risk of malaria in travellers is significantly greater in CYP returning from sub-Saharan Africa than from other malaria-endemic regions. It is unclear why there was an increased risk in CYP >5 years (malaria morbidity and mortality is higher in the under-5s) but it could be owing to families refraining from tropical travel with younger, more vulnerable CYP, or that older CYP are less compliant with preventive measures. Information on bed-net use and other malaria prophylaxis was not collected, and, ideally, such data would be captured in any future prospective study to measure the effect on prevention and severity of malaria in children [Citation18]: a UK study [Citation19] and a more recent Belgian study found that the use of malaria prophylaxis was decreasing in CYP [Citation20].
The French study reported twice as many cases of imported malaria (9.5%) [Citation16] than in this study (4.2%), but this might be explained by established high traffic routes for imported malaria. Tatem et al. reported that West Africa accounts for 56% of imported cases, with France diagnosing the largest volume of both adult and CYP cases (2169 cases on average annually during 2005–2015), closely followed by the UK (1898 cases in 2005–2015) [Citation7], and both countries have a similar paediatric-to-adult proportion of malaria cases (France, 12% [Citation18]; UK, 10% [Citation8]).
Enteric infections
The higher proportion of typhoid infections in CYP arriving from South Asia than from sub-Saharan Africa is similar to that in other studies [Citation2,Citation14]. A recent Australian study in adults and CYP found that 72% of typhoid notifications were patients who had returned from South Asia [Citation21] where high transmission is maintained by rapid, unplanned urbanisation, poor sanitation and open defaecation [Citation22].
This study diagnosed about half the number (13.4% if dysentery included) of diarrhoeal illnesses than in other studies (25% in a Munich travel clinic [Citation16] and 27.1% in a French children’s ED [Citation17]), with the geographical risk of diarrhoeal disease higher in patients returning from South Asia than from sub-Saharan Africa. Diarrhoeal episodes might have been managed at home, via the UK’s national telephone health advice service, in primary care, or they might have been coded incorrectly − 14.8% of CYP were coded as a ‘viral illness’ only – or the absence of fever during the diarrhoeal episode could have precluded a malaria screen. The gap in time between arrival in the UK and a test for malaria might have made diarrhoeal illness less likely, while current limited clinical coding in many UK EDs also means that cases of diarrhoeal illnesses were not coded as traveller’s diarrhoea.
Dengue
Only four (0.3%) CYP were diagnosed with dengue compared with 18% in a single-centre Israeli study [Citation23], 2% in the Munich travel clinic [Citation16], 2% in the GeoSentinel survey [Citation14], 5% in a systematic review [Citation2] and 12% in a recent multi-centre, prospective European study in adults [Citation24]. Currently, a test to confirm imported dengue infection in the UK must be sent to the country’s central Imported Fevers Service so this infection is probably under-diagnosed. With growing urbanisation and increased travel, the annual incidence of dengue has doubled in the last decade [Citation25], with autochthonous outbreaks reported recently in France, Italy, Spain, Portugal, Croatia [Citation26] and North America [Citation27].
Schistosomiasis
No cases of schistosomiasis were found, compared with 2% in the Munich study [Citation16] and 6.4% in the Israeli study [Citation23], but many CYP with schistosomiasis do not present with fever.
Diagnostics and data capture
UK clinical guidelines advise screening for malaria (to rule out potentially fatal P. falciparum) in any unwell CYP with fever who has recently returned from a malaria-endemic area [Citation8], and, if the travel history and clinical findings warrant, blood cultures for typhoid [Citation24]. However, besides these routine blood tests, UK EDs lack the diagnostics that could improve screening for tropical infections, e.g. RDTs for arboviral infections such as dengue. In addition, the RDTs to screen for malaria in the UK are used in hospital laboratories rather than in primary care or children’s EDs, adding unnecessary delay to the diagnosis of this potentially fatal infection [Citation28].
Meanwhile, inaccurate coding in UK EDs only widens the diagnostic gap, as shown by this study’s large proportion of children with no diagnosis. Ideally, a multi-centre prospective study with a wider array of diagnostics, including point-of-care tests, as in previous adult studies, would help clarify the true prevalence of imported tropical infections and rationalise management.
Climate change
Ecological alteration owing to climate change will probably be conducive to vector-, water- and food-borne diseases in regions not currently considered to be sub-tropical, blurring traditional boundaries between tropical and non-tropical illness [Citation29]. There have been autochthonous outbreaks of chikungunya, a mosquito-borne viral disease [Citation27] in France and Italy, along with localised outbreaks of autochthonous P. vivax malaria in southern Europe [Citation30] alongside the dengue outbreaks mentioned above.
Limitations
Limited resources meant that seasonality and the reason for travel, e.g. tourism, migration and, most importantly, VFRs were not captured. In addition, the length of stay in the country of origin was not recorded; several studies have observed an increased length of stay in a malaria-endemic setting as a risk factor for malaria infection in children, especially VFRs [Citation13,Citation15,Citation16]. Also not recorded was the interval between travel and testing for malaria, which could have affected the number of tropical infections diagnosed, especially traveller’s diarrhoea. Also, the data on disposition (a marker for severity of infection) were not analysed, and, as with all retrospective studies using electronic health records, there is the possibility of unreliable or incomplete coding of diagnoses.
The undertaking of a malaria screen, the most critical infection to diagnose promptly in the ED [Citation31], was used as a marker for a possible imported tropical infection in CYP who arrived from malaria-endemic areas with fever. CYP might have presented elsewhere (to primary care, a private clinic), not presented to a healthcare provider at all, or clinicians might have forgotten to take a travel history or they had little concern for malaria and so did not screen. However, using a malaria screen as a key inclusion criterion was identified by the PERUKI research committee (consisting of senior clinicians) to be the most feasible method of patient selection as it was likely to be the most robustly documented and least resource-intensive data point.
Inclusion for malaria screening required a history of fever or a documented fever but in this retrospective study, 5.8% were recorded as having no fever, with a further 10% unknown, but all of the cohort had been screened for malaria. It was concluded that there could have been confusion during data collection between a ‘history of fever’ and a ‘documented fever’ at presentation. As there were two cases of malaria in the cohort who were recorded as ‘fever –unknown’, it was decided to include these patients but this could have affected the results.
Conclusion
This study describes infections in UK CYP imported from malaria-endemic areas and underlines the continued importance of screening for malaria in all patients with a fever and a history of recent travel to a tropical country, especially CYP arriving from sub-Saharan Africa. It shows a diagnostic gap in diagnosing imported infections in children, with UK EDs probably missing dengue infections.
Contributions
CB, TF, PT and GH conceived and designed the study. TF made the study power calculations and undertook the statistical analyses. CB, TF, PT, ML and GH analysed and interpreted the data. DW, VM, ML and NM ensured PERUKI network involvement, generated data and provided clinical perspectives. CB drafted the manuscript, which was edited by TF, ML and GH. CB and TF had full access to the data in the study and had final responsibility for the decision to submit for publication.
Acknowledgments
This multi-centre study would not have been possible without the work of the following teams: Sheffield Children’s Hospital: Glenda Amenos Barraza, Shammi Ramlakhan, Fiona Shackley, Mark Simmerson, Emma Wynne; John Radcliffe Hospital, Oxford: Emily Tough, Sally Beer, Charlotte Brown, Jiske Steensma; Bristol Royal Hospital for Children: Sarah Blakey; Royal Hospital for Children, Glasgow: Eleanor Shone, Steve Foster; Watford General Hospital: Michelle Jacobs, Mohamed Rineesh; Royal Berkshire Hospital: Katie Palmer, Manish Thakker; University Hospital of Wales, Cardiff: Jennifer Muller, Jeff Morgan; Chelsea and Westminster Hospital, London: Sophie McEvoy; Royal Hospital for Children and Young People, Edinburgh: Jen Browning; Addenbrookes Hospital, Cambridge: Kashif Malik, Jude Okoye; Royal Free Hospital, London: Shye-Wei Wong, Cynthia Diaba, Sudeepta Hemraj; North Middlesex Hospital, London: Poonam Patel, George Lawson, Katie Knight, Deborah McCartney; Northwick Park Hospital, London: Paul Tanto, Lauren Fraser, Sarah Al-Rawi, Kazim Ghafoor, Behrouz Nezavat, Anna Silva Ferreira; Whittington Hospital, London: Erum Jamall; Birmingham Children’s Hospital: Sarah Hadfield, Karen Davies, Stuart Hartshorn; St George’s Hospital, London: Heather Jarman.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bielecki M, Patel D, Hinkelbein J, et al. Air travel and COVID-19 prevention in the pandemic and peri-pandemic period: a narrative review. Travel Med Infect Dis. 2021;39:101882. doi: 10.1016/j.tmaid.2020.101915
- Buss I, Genton B, D’Acremont V. Aetiology of fever in returning travellers and migrants: a systematic review and meta-analysis. J Travel Med. 2020;27:taaa207. doi: 10.1093/jtm/taaa207
- Grobusch MP, Weld L, Goorhuis A, et al. Travel-related infections presenting in Europe: a 20-year analysis of EuroTravNet surveillance data. Lancet Reg Health Eur. 2020;12:100001. doi: 10.1016/j.lanepe.2020.100001
- Leuthard D, Berger C, Staubli G, et al. Management of children with travel-related illness evaluated in a pediatric emergency room. Pediatr Infect Dis J. 2015;34:1279–1282. doi: 10.1097/INF.0000000000000890
- European Centre for Disease Prevention and Control. Malaria: Annual Epidemiological Report for 2018. Stockholm; 2020.
- Mischlinger J, Rönnberg C, Álvarez-Martínez MJ, et al. Imported malaria in countries where malaria is not endemic: a comparison of semi-immune and nonimmune travelers. Clin Microbiol Rev. 2020;33:e00104–119. doi: 10.1128/CMR
- Tatem AJ, Jia P, Ordanovich D, et al. The geography of imported malaria to non-endemic countries: a meta-analysis of nationally reported statistics. Lancet Infect Dis. 2017;17:98–107. doi: 10.1016/S1473-3099(16)30326-7
- Lalloo DG, Shingadia D, Bell DJ, et al. UK malaria treatment guidelines 2016. J Infect. 2016;72:635–649. doi: 10.1016/j.jinf.2016.02.001
- Bird C, Hayward GN, Turner PJ, et al. A diagnostic accuracy study to evaluate standard rapid diagnostic test (RDT) alone to safely rule out imported malaria in children presenting to UK emergency departments. J Pediatr Infect Dis Soc. 2023;12:290–297. doi: 10.1093/jpids/piad024
- Benchimol EI, Smeeth L, Guttmann A, et al. The reporting of studies conducted using observational routinely collected health data (RECORD) statement. PLOS Med. 2015;12:e1001885. doi: 10.1371/journal.pmed.1001885
- von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLOS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296
- Klein JL, Millman GC. Prospective, hospital based study of fever in children in the United Kingdom who had recently spent time in the tropics. BMJ. 1998;316:1425–1426. doi: 10.1136/bmj.316.7142.1425
- West NS. Fever in returned travellers: a prospective review of hospital admissions for a 2½ year period. Arch Dis Child. 2003;88:432–434. doi: 10.1136/adc.88.5.432
- Hagmann S, Neugebauer R, Schwartz E, et al. Illness in children after international travel: analysis from the Geosentinel surveillance network. Pediatrics. 2010;125:e1072–e1080. doi: 10.1542/peds.2009-1951
- Marks M, Armstrong M, Whitty CJM, et al. Geographical and temporal trends in imported infections from the tropics requiring inpatient care at the hospital for tropical diseases, London – a 15 year study. Trans R Soc Trop Med Hyg. 2016;110:456–463. doi: 10.1093/trstmh/trw053
- Herbinger KH, Drerup L, Alberer M, et al. Spectrum of imported infectious diseases among children and adolescents returning from the tropics and subtropics. J Travel Med. 2012;19:150–157. doi: 10.1111/J.1708-8305.2011.00589.X
- Naudin J, Blondé R, Alberti C, et al. Aetiology and epidemiology of fever in children presenting to the emergency department of a French paediatric tertiary care centre after international travel. Arch Dis Child. 2012;97:107–111. doi: 10.1136/archdischild-2011-300175
- Leblanc C, Vasse C, Minodier P, et al. Management and prevention of imported malaria in children. Update of the French guidelines. Med Mal Infect. 2020;50:127–140. doi: 10.1016/j.medmal.2019.02.005
- Ladhani S, Aibara RJ, Blaze M, et al. Trends in imported childhood malaria in the UK: 1999–2003. Arch Dis Child. 2006;91:911–914. doi: 10.1136/adc.2005.089433
- Selimaj Kontoni V, Goetghebuer T, Hainaut M, et al. Imported malaria in children: a study over an 11-year period in Brussels. Pediatr Infect Dis J. 2023;42:733–738. doi: 10.1097/INF.0000000000003986
- Forster DP, Leder K. Typhoid fever in travellers: estimating the risk of acquisition by country. J Travel Med. 2021;28:taab150. doi: 10.1093/jtm/taab150
- Basnyat B, Karkey A. Tackling typhoid fever in South Asia: lessons from Vietnam. Lancet Glob Health. 2019;7:e1317–e1318. doi: 10.1016/S2214-109X(19)30320-1
- Rabinowicz S, Schwartz E. Morbidity among Israeli paediatric travellers. J Travel Med. 2017;24:1–7. doi: 10.1093/jtm/tax062
- Camprubí-Ferrer D, Cobuccio L, Van Den Broucke S, et al. Causes of fever in returning travelers: a European multicenter prospective cohort study. J Travel Med. 2022;29:taac002. doi: 10.1093/jtm/taac002
- Halstead S, Wilder-Smith A. Severe dengue in travellers: pathogenesis, risk and clinical management. J Travel Med. 2019;26:1–15. doi: 10.1093/jtm/taz062
- Ahmed AM, Mohammed AT, Vu TT, et al. Prevalence and burden of dengue infection in Europe: a systematic review and meta‐analysis. Rev Med Virol. 2020;30:e2093. doi: 10.1002/rmv.2093
- Fredericks AC, Fernandez-Sesma A. The burden of dengue and chikungunya worldwide: implications for the southern United States and California. Ann Glob Health. 2015;80:466. doi: 10.1016/j.aogh.2015.02.006
- Enane LA, Sullivan KV, Spyridakis E, et al. Clinical impact of malaria rapid diagnostic testing at a US children’s hospital. J Ped Infect Dis Soc. 2020;9:298–304. doi: 10.1093/jpids/piz022
- Semenza JC, Paz S. Climate change and infectious disease in Europe: impact, projection and adaptation. Lancet Reg Health Eur. 2021;9:100230. doi: 10.1016/j.lanepe.2021.100230
- Emms H, Lee R, Thomas A, et al. Return of vivax malaria in Cyprus. Arch Dis Child. 2020;105:102.1–103. doi: 10.1136/archdischild-2018-316459
- Wilson ME, Freedman DO. Etiology of travel-related fever. Curr Opin Infect Dis. 2007;20:449–453. doi: 10.1097/QCO.0b013e3282a95e27