ABSTRACT
Information on age-based Taenia solium taeniasis prevalence is crucial for control of cysticercosis. T. solium taeniasis prevalence was determined for a village in Liangshan Prefecture, Sichuan Province, China that was co-endemic for T. solium, Taenia saginata asiatica, and Taenia saginata. Individuals who were Taenia egg-positive by stool microscopy and/or expelled tapeworms or proglottids post-treatment were diagnosed as having taeniasis. Infecting species was identified via multiplex PCR on tapeworm specimens or coproPCR followed by sequencing. In addition, initial stool samples from 10 children with taeniasis suspected of having spontaneous expulsion of tapeworms within the period between diagnosis and treatment were subject to species confirmation via coproPCR and sequencing. Of the 389 study subjects, 194 (49.9%) were diagnosed with taeniasis. Children (< 16 years of age) had a higher T. solium taeniasis prevalence (8.8%) than older individuals (2.5%) (P = 0.0127). Molecular analysis of initial stool samples from 7 of 10 children suspected of spontaneously passing tapeworms indicated 6 infections due to T. solium and 1 infection due to T. saginata. This study found that young children had a higher T. solium taeniasis prevalence than older individuals, providing additional support for the belief that adult T. solium likely has a relatively short lifespan compared to other Taenia species with human definitive hosts.
1. Introduction
Humans act as definitive hosts for the Taenia solium tapeworm after consuming undercooked pork containing the larval form of the parasite (cysticerci) [Citation1]. In addition, humans can act as aberrant intermediate hosts, resulting in cysticercosis and neurocysticercosis. Cysticercosis remains a neglected disease of public health importance in numerous countries in Latin America, sub-Saharan Africa, and Asia [Citation2,Citation3]. Cases of cysticercosis are also found in non-endemic areas due to global travel [Citation4]. In 1992, the International Task Force for Disease Eradication declared that human infection with the adult (taeniasis) and larval (cysticercosis) forms of this tapeworm are potentially eradicable [Citation5].
Understanding how human host age impacts T. solium taeniasis status is crucial for the control of human cysticercosis. Several studies have been undertaken on the epidemiology of taeniasis at the community level [Citation6–16]. However, it remains unclear how host age affects infection status. Our recent study revealed that children 6–15 years of age from an ethnic minority community in Liangshan Prefecture, Sichuan Province, China had a high (6.7%) prevalence of infection with adult T. solium tapeworms [Citation17]. However, additional studies are needed to evaluate how infection frequency changes across the human life span.
In addition to host age, information on parasite life span is important for implementation of effective control practices. In Liangshan Prefecture, we found that some individuals appeared to have spontaneously expelled their tapeworms during the time between case identification via microscopy performed on stool samples and treatment to eliminate the adult worms [Citation17]. For one study site, where treatment was delayed 8 months due to the remote location, 10 of the 23 individuals with Taenia eggs initially seen on microscopy did not expel proglottids or tapeworms upon treatment. The remainder discharged worms belonging to T. solium (11 cases) and Taenia saginata (2 cases). Due to the high efficacy of the purgative agent used [Citation17,Citation18], it is believed that most adult tapeworms present would have been expelled at the time of treatment. In contrast, at another study site where treatment was delivered 3 months after microscopic examination for eggs, all Taenia egg-positive children (8 cases) released tapeworms (all were later confirmed as T. solium). Based on these observations, it was hypothesized that some, if not all, of the worms likely expelled within the 8 months between diagnosis and treatment of the children in Liangshan Prefecture were T. solium. If this was the case, it would provide insight into the adult T. solium lifespan within the human host [Citation9,Citation17,Citation19,Citation20].
Several studies have hypothesized that adult T. solium tapeworms have a shorter life span than T. saginata and Taenia saginata asiatica (an intraspecies variant of T. saginata) tapeworms and are naturally expelled within several years [Citation6,Citation9,Citation21–24]. In the present study, we further assessed the initial stool samples from the 10 children who were suspected to have spontaneously expelled their tapeworms over an 8-month period in order to evaluate the hypothesis that the expelled tapeworms were T. solium. In addition, human age-based taeniasis prevalence of the three locally recognized human Taenia (T. solium, T. saginata, and T. saginata asiatica) was evaluated for samples collected in a village located in Liangshan Prefecture.
2. Materials and methods
2.1. Study site and population
This study was conducted in Shuiluo Township located in Muli County of Liangshan Prefecture, Sichuan Province, China (). The township has a population of approximately 5,000 and a population density of about 4 persons per km2, with inhabitants belonging to the Tibetan, Naxi, and Mongolian ethnic groups. Previous studies have shown that residents of this township are infected with T. solium, T. saginata, and T. saginata asiatica [Citation17,Citation25]. Most township inhabitants own pigs that readily forage in the surrounding environment and have easy access to human feces due to the practice of open defecation. Cattle and yak ownership is more limited, with yaks raised away from the village. Undercooked pork/pork liver and raw yak meat are popular dishes consumed by both adults and children during the slaughtering season (November to January).
Figure 1. A: Map of Sichuan Province indicating the location of Shuiluo Township, Muli County, Liangshan Prefecture, and the provincial capital (Chengdu); B: Map of China with the location of Sichuan Province and the country’s capital (Beijing).
2.1.1 Village prevalence study
The study was carried out from December 2017 to May 2018. In cooperation with the Muli County Center for Disease Control and Prevention (CDC) and the Shuiluo Township medical clinic, residents of the village of Guni (population 695) that were 5–75 years of age were invited to participate after providing informed consent or assent. Ethical approval for this study was granted by the ethics committee of the Sichuan CDC.
2.1.2. School-based study
The 23 children (age 6–15 years) positive for Taenia eggs who attended Shuiluo Primary School in 2016 [Citation17] were also evaluated as part of this study.
2.2. Data and sample collection
2.2.1. Village prevalence study
Study staff administered a questionnaire to each participant or to a participant’s parent (or with the assistance of a teacher) for children under 15 years of age to obtain basic demographic information and any history of expelling tapeworm proglottids within the previous year. A naturally passed fecal sample was requested from all study participants. Approximately 20 g of stool was collected in a 50 ml tube for microscopy to look for the presence of Taenia eggs, and for copro-polymerase chain reaction (coproPCR) if indicated. Direct smears were prepared from stool samples and examined for the presence of Taenia eggs.
Individuals positive for Taenia eggs and/or reporting segment expulsion were treated within several days of fecal sample collection with a combination of pumpkin seeds and areca nut extract, as previously reported, to expel any adult tapeworms [Citation17,Citation18,Citation26]. Expelled tapeworms or proglottids were kept in 75% ethanol for species confirmation by multiplex PCR (see below). For stool samples that were positive for Taenia eggs, but did not contain tapeworm segments, coproPCR followed by sequencing (see below) was employed to identify the infecting species. Individuals who were Taenia egg-positive by stool microscopy and/or expelled tapeworms or proglottids post-treatment were diagnosed as having taeniasis.
2.2.2. School-based study
Age and segment expulsion history, previously obtained from 23 children (age 6–15 years) positive for Taenia eggs who attended Shuiluo Primary School in Shuiluo Township [Citation17], were reviewed. Initial stool samples from 10 of these 23 taeniasis cases who were suspected to have spontaneously expelled tapeworms prior to treatment 8 months later were also subject to species analysis by PCR and sequencing.
2.3. Multiplex PCR, coproPCR, and sequencing
The genomic DNA of parasite isolates was extracted using the DNeasy Blood & Tissue Kit (Qiagen) and subsequently used as a template for PCR. For differentiation of Taenia species, multiplex PCR was conducted as described previously [Citation10,Citation17,Citation18,Citation26,Citation27], with a minor revision. Briefly, one reverse and three forward primers were applied to amplify 984, 827, and 269 bp amplicons, specific for mitochondrial (mt) gene cox1 sequences of T. solium Asian genotype, T. saginata, and T. saginata asiatica, respectively. The PCR cocktail contained mixed primers, 10 µl of GoTaq® Green Master Mix (Promega), and 1 µl of template in 20 µl of reaction mixture. PCR protocols were composed of 1 cycle of initial denaturation (4 min at 95°C), 35 cycles of denaturation (30 sec at 95°C), annealing (30 sec at 55°C), and extension (90 sec at 72°C), plus 1 cycle of final extension (5 min at 72°C).
The QIAamp Fast DNA Stool Mini Kit was used to extract DNA from fecal samples. Prior to using the kit, glass beads were added to the fecal samples. The samples were then agitated using a tissue breaker to release as much DNA as possible from the parasite eggs. Extracted DNA was used as a template for multiplex PCR to amplify DNA specific for the three Taenia, as described previously [Citation10,Citation18,Citation26,Citation27], with a minor amendment. Briefly, double amplifications were performed for coproPCR, using the first PCR product as a template for the second PCR. The same protocols as described for multiplex PCR were used, except that for coproPCR, two µl of template were added to 50 µl of reaction mixture, and annealing was performed at 52°C. Subsequently, the second PCR products were subject to sequencing for reconfirmation of species. All diagnostic testing was conducted at the Sichuan CDC (China).
2.4. Statistical analysis
Age was evaluated for normality and presented using means or medians depending on outcome. Infection frequencies by age group were expressed as proportions. Chi-square tests were used to evaluate differences in T. solium taeniasis, T. saginata taeniasis, T. saginata asiatica taeniasis, and overall taeniasis prevalence between children less than 16 years of age and individuals 16 years of age and older (considered adults for this study) from the village of Guni. Fifteen years of age was evaluated as the cutoff for children since this is the oldest age typically attending primary school. The Student’s t-test was employed to assess mean age of children attending Shuiluo Primary School who were positive for T. solium by coproPCR, with supposed natural elimination of their tapeworms within 8 months, versus the mean age of Taenia egg-positive children with expulsion of T. solium tapeworms following treatment 8 months after diagnosis via microscopy. Significance was set at P < 0.05.
3. Results
3.1. Village prevalence study
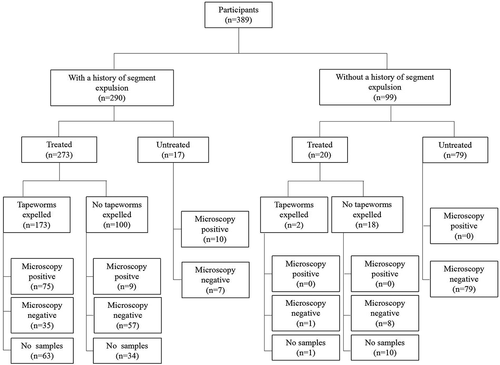
A total of 389 subjects were enrolled from the village of Guni, of which 202 (51.9%) were male and 187 (48.1%) were female. Participant age ranged from 5 to 73 years (median of 31 years), with 276 adults (71.0%) and 113 children (29.0%). Of the 389 subjects investigated, 290 (74.6%) reported a history of passing tapeworm segments within the previous year ().
Figure 2. Diagram showing study process and the number of participants from the village of Guni, Liangshan Prefecture, Sichuan Province, China.
3.1.1 Results of stool microscopy
Fecal samples were available from 281 of the 389 enrolled individuals from the village of Guni. Overall, 67.0% (n = 185) of adults and 85.0% (n = 96) of children provided samples. Of the 281 samples, 94 (33.5%) were Taenia egg positive by microscopy, with 47.6% of samples from adults positive and 6.3% of samples from children positive.
3.1.2. Tapeworms expelled post-treatment
A total of 293 residents of the village of Guni received treatment, of which 185 had originally provided a fecal sample. Of these 185 individuals, 84 were positive for Taenia eggs and had a history of segment expulsion, 92 were negative for eggs but reported a history of segment expulsion, and 9 were negative for both but had requested treatment. There were no instances where an individual who was positive for Taenia eggs did not have a history of segment expulsion. For the remaining 108 treated subjects who did not provide an initial fecal sample, 97 reported expelling proglottids in the past year and 11 did not have a history of segment expulsion but requested treatment (). Tapeworms or proglottids were produced in 175 (59.7%) of 293 treated individuals (), but parasite material was disposed of by one subject prior to collection.
3.1.3. Results of multiplex PCR and coproPCR
Multiplex PCR-determined infecting species for 174 individuals who expelled tapeworms or proglottids post-treatment are shown in . CoproPCR was performed on samples from 18 of 19 Taenia egg-positive cases without tapeworm specimens. Species-specific DNA was successfully amplified for T. saginata asiatica in 16 cases and for T. saginata in 2 cases. Subsequent sequencing of coproPCR products succeeded in all 18 cases, exhibiting identical results to the multiplex PCR. That is, 15 were 99% genetically similar and 1 was 97% similar to T. saginata asiatica [NCBI Accession number KJ187963.1], and the other 2 were 99% similar to a published genetic sequence for T. saginata [NCBI Accession number AB984351.1] (). Species differentiation was not performed for 2 cases, 1 due to lack of an available stool sample and the other due to inadvertent disposal of the parasitic material by the infected individual.
Table 1. Species-specific distribution of tapeworms in children (n = 113) and adults (n = 276) in the village of Guni, Sichuan Province, China (2017)
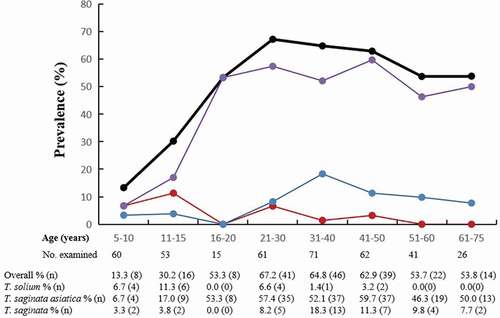
Combining the results of stool microscopy and tapeworm expulsion post-treatment, the overall taeniasis prevalence was 49.9% (194/389) in the village of Guni. Subjects aged 11–15 years were found to have the highest prevalence of T. solium taeniasis (6/53; 11.3%), while those in the 41–50 years age group had the highest prevalence of T. saginata asiatica (37/62; 59.7%) and those in the 31–40 years age group had the highest prevalence of T. saginata (13/71; 18.3%) (). The overall prevalence of taeniasis increased with age, reaching a peak in the 21–30-year age group and then remaining stable (). This finding was largely driven by T. saginata asiatica prevalence in the older age groups. Children were more likely to have T. solium taeniasis than adults (8.8% vs 2.5%) (xCitation2= 6.210, P = 0.0127). In contrast, adults were more likely than children to have T. saginata asiatica taeniasis (54.0% vs 11.5%) (xCitation2= 57.802, P < 0.001) or T. saginata taeniasis (11.2% vs 3.5%) (xCitation2= 4.892, P = 0.0270). Similarly, the overall prevalence of taeniasis due to any Taenia species was significantly different between adults (61.6%) and children (21.2%) (xCitation2= 50.626, P < 0.001).
3.2. School-based study
Of the 23 children positive for Taenia eggs who attended Shuiluo Primary School, 14 (60.9%) were male and 9 (39.1%) were female. Twenty-one (91.3%) reported passing tapeworm segments within the past year (). Initial stool samples were available from 7 of the 10 children who were suspected of having passed tapeworms spontaneously. CoproPCR performed on these 7 samples indicated 6 infections due to T. solium and 1 infection due to T. saginata (). Six of the PCR products were successfully sequenced. Five of the sequences were over 99% identical to a published genetic sequence for T. solium [NCBI Accession number AB984354.1], while the sixth sequence was 99% identical to a published sequence for T. saginata [NCBI Accession number AB984351.1] (). Sequencing findings were consistent with coproPCR findings for all 6 cases. The mean age (13.0 years) of the six cases identified as having possible natural expulsion of T. solium tapeworms was significantly older than the mean age (10.3 years) of the 11 individuals who expelled T. solium tapeworms following treatment (P = 0.017).
Table 2. Demographic information and infecting species for Taenia egg-positive children attending Shuiluo Primary School, Sichuan Province in 2016 without tapeworm expulsion (n = 10) and with tapeworm expulsion (n = 13) following treatment 8 months after diagnosis
4. Discussion
This is the first study to evaluate the relationship between host age and infection with adult T. solium tapeworms, with infecting species confirmed in all but two taeniasis carriers. Prior data about age-related tapeworm infections are scarce, primarily due to the low number of tapeworm carriers identified in individual studies [Citation13–16]. The largest previous study, which was conducted in Guatemala, showed infections with Taenia spp. tapeworms increasing with age, and peaking in the 30–39 years age group in areas co-endemic for T. solium and T. saginata [Citation6]. This pattern was similar to the current study’s findings for overall taeniasis prevalence. Unfortunately, in the Guatemala study, infecting species was not available for more than one-third of the individuals with taeniasis. However, it was shown that T. solium was the infecting species for more than half of the evaluated tapeworm carriers and that over half of the detected taeniasis cases in these Guatemalan communities were less than 20 years of age [Citation6]. Another study conducted in Ecuador indicated that infection with Taenia spp. also increased with age [Citation8]. However, the study was based on individuals who reported passing tapeworms after treatment rather than those shown to be infected parasitologically.
In two previous studies conducted in Peru and the Democratic Republic of the Congo, young children were found to be at greatest risk for tapeworm infections through detection of coproantigen [Citation9,Citation11]. In these two studies, information on infecting species was also not available. However, T. solium was known to be locally endemic through reported cases of human and porcine cysticercosis. Studies in other regions have shown that young children, such as a 4-year-old girl from Bali, can harbor T. solium tapeworms [Citation28]. In the study village of Guni, adults and children likely had similar access to infected pork within the household, while the pork provided to children by the school was confirmed to have been purchased from areas in China considered non-endemic for T. solium. Although pork consumed at school was likely safe, it was very common for children to grill pork for their own consumption when a pig was slaughtered at home. Lack of sufficient oversight creates the opportunity for children to consume improperly cooked pork. The method by which children incompletely chew food has also been proposed as a risk for acquiring taeniasis [Citation19,Citation29].
Detection of concurrent infection with two or three tapeworm species is not uncommon in co-endemic areas [Citation17,Citation18], and co-infections were also found in the village of Guni. Therefore, there is no reason to think that the low number of T. solium infections in adults, in the current study, was due to existing T. saginata asiatica or T. saginata infections. The larger numbers of T. saginata asiatica and T. saginata infections in adults compared to children are likely due to adults having accumulated the parasites over a longer duration in combination with the long life span (up to decades) of these parasites. The relatively low prevalence of T. saginata in all age groups most likely reflects a diet that more commonly includes pork rather than yak meat or beef.
It has previously been speculated that T. solium has a shorter life span than T. saginata and T. saginata asiatica. Lightowlers estimated the average longevity of T. solium adults to be less than 3 years by evaluating the rate of taeniasis reoccurrence in populations after mass treatment with cestocidal drugs [Citation20]. [Citation9, Citation11], both inferred that intestinal infection with T. solium is short lived since the highest infection prevalence was found in the 5–10 years age group in both Peru and Congo and estimated that the lifespan of T. solium tapeworms was most likely less than 5 years. Very early studies with experimental infection of adult Japanese volunteers with T. solium cysticerci in the 1930s found that tapeworms produced the greatest number of gravid proglottids during the first month of patency, suggesting a relatively short reproductive life span [Citation19,Citation29].
Other case reports have reinforced the belief that the T. solium life span is no longer than several years. In two instances, travelers from Japan and Mongolia both visited India where they reported consuming local pork dishes and spontaneous expelled their tapeworms within 3 years of returning home [Citation30,Citation31]. The current study suggests that a large number of T. solium parasites were expelled from children within an 8-month time period. However, the authors acknowledge that stool-based evidence was absent after treatment to ensure worm removal. Overall, the spontaneous passing of T. solium tapeworms in children was estimated to occur within 5 years of infection based on children with naturally expelled worms reporting segment expulsion for no longer than three years.
The possibility of reinfection is high in highly endemic areas, such as the village of Guni. However, it is not clear if repeated infections impact immunogenicity and worm expulsion. In the current study, all T. solium carriers reported segment expulsion for no longer than five years. Preliminary work done by our research group has shown similar findings in low endemic areas (prevalence less than 1%) of China (unpublished), suggesting that repeated infection likely has no or only a limited effect on parasite longevity. Moreover, new infections are likely deterred through concomitant immunity caused by infection with the same species of tapeworm. This hypothesis is supported by the observation of cases infected with multiple tapeworms of the same species that likely have the same source of infection based on worm morphology and size [Citation32].
Multiple programs have been carried out in an attempt to control T. solium taeniasis and cysticercosis at a regional scale [Citation8,Citation33–36]. However, success has been limited. While the current study does have the limitation of using a convenience sample, 56.0% (389/695) of villagers were evaluated in Guni. Overall, children less than 16 years of age were shown to have a higher T. solium taeniasis prevalence than older individuals. Therefore, children need to be included in T. solium control programs carried out in endemic settings. This study also provided additional support for the hypothesis that T. solium likely has a relatively short life span based on the large number of T. solium parasites that were apparently expelled within an 8-month time period. Information obtained in this study allows us to further understand local T. solium transmission dynamics and develop effective intervention measures.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author(s).
Acknowledgments
We thank the staff of Shuiluo Township medical clinic and the Muli CDC for their contributions to field work.
Additional information
Funding
References
- Grove DI. A history of human helminthology. Wallingford: CAB International; 1990.
- Garcia HH, Gonzalez AE, Evans CA, et al., Cysticercosis Working Group in Peru. Taenia solium cysticercosis. Lancet. 2003b;362:547–556.
- Ito A, Nakao M, Wandra T. Human taeniasis and cysticercosis in Asia. Lancet. 2003;362:1918–1920.
- Ito A, Budke CM. Culinary delights and travel? A review of zoonotic cestodiases and metacestodiases. Travel Med Inf Dis. 2014;12:582–591.
- Schantz PM, Cruz M, Sarti E, et al. Potential eradicability of taeniasis and cysticercosis. Bull Pan Am Health Organ. 1993;27:397–403.
- Allan JC, Velasquez-Tohom M, Garcia-Noval J, et al. Epidemiology of intestinal taeniasis in four rural Guatemalan communities. Ann Trop Med Parasitol. 1996;90(2):157–165.
- Conlan JV, Vongxay K, Khamlome B, et al. A cross-sectional study of Taenia solium in a multiple taeniid-endemic region reveals competition may be protective. Am J Trop Med Hyg. 2012;87:281–291.
- Cruz M, Davis A, Dixon H, et al. Operational studies on the control of Taenia solium taeniasis/cysticercosis in Ecuador. Bull World Health Organ. 1989;67(4):401–407.
- Garcia HH, Gilman RH, Gonzalez AE, et al., Cysticercosis Working Group in Peru. HYPERENDEMIC HUMAN AND PORCINE Taenia solium INFECTION IN PERÚ. Am J Trop Med Hyg. 2003a;68(3):268–275.
- Li T, Craig PS, Ito A, et al. Taeniasis/cysticercosis in a Tibetan population in Sichuan Province, China. Acta Trop. 2006;100:223–231.
- Madinga J, Kanobana K, Lukanu P, et al. Geospatial and age-related patterns of Taenia solium taeniasis in the rural health zone of Kimpese, Democratic Republic of Congo. Acta Trop. 2017;165:100–109.
- Okello A, Ash A, Keokhamphet C, et al. Investigating a hyper-endemic focus of Taenia solium in northern Lao PDR. Parasit Vectors. 2014;7:134.
- Rodriguez-Canul R, Fraser A, Allan JC, et al. Epidemiological study of Taenia solium taeniasis/cysticercosis in a rural village in Yucatan State, Mexico. Ann Trop Med Parasitol. 1999;93:57–67.
- Rodriguez-Hidalgo R, Benitez-Ortiz W, Dorny P, et al. Taeniosis-cysticercosis in man and animals in the Sierra of northern Ecuador. Vet Parasitol. 2003;118:51–60.
- Sanchez AL, Gomez O, Allebeck P, et al. Epidemiological study of Taenia solium infections in a rural village in Honduras. Ann Trop Med Parasitol. 1997;91:163–171.
- Somers R, Dorny P, Nguyen VK, et al. Taenia solium taeniasis and cysticercosis in three communities in North Vietnam. Trop Med Int Health. 2006;11:65–72.
- Li T, Chen X, Wang H, et al. High Prevalence of taeniasis and Taenia solium cysticercosis in children in western Sichuan, China. Acta Trop. 2019;199:105133.
- Li T, Ito A, Chen X, et al. Usefulness of pumpkin seeds combined with areca nut extract in community-based treatment of human taeniasis in northwest Sichuan Province, China. Acta Trop. 2012;124:152–157.
- Ito A, Saito M, Donadeu M, et al. Kozen Yoshino’s experimental infections with Taenia solium tapeworms: an experiment never to be repeated. Acta Trop. 2020;205:105378.
- Lightowlers MW. Eradication of Taenia solium cysticercosis: a role for vaccination of pigs. Int J Parasitol. 2010;4:1183–1192.
- Dixon HBF, Hargreaves WH. Cysticercosis (Taenia solium): a further ten years’ clinical study, covering 284 cases. Q J Med. 1944;13:107–121.
- Ito A, Budke CM. Perspectives: genetic diversity of Taenia solium and its relation to clinical presentation of cysticercosis. Yale J Biol Med. 2021a; 94: 1-7.
- Ito A, Budke CM. Perspectives on intestinal tapeworm infections: evaluation of direct life cycles, with a special emphasis on Hymenolepis species. Cur Res Parasitol Vector-Bone Dis. 2021b; 1:100023.
- Lescano AG, Garcia HH, Gilman RH, et al., Cysticercosis Working Group in Peru. Taenia solium cysticercosis hotspots surrounding tapeworm carriers: clustering on human seroprevalence but not seizures. PLoS Negl Trop Dis. 2009;3:e371.
- Openshaw JJ, Medina A, Felt SA, et al. Prevalence and risk factors for Taenia solium cysticercosis in school-aged children: a school based study in western Sichuan, People’s Republic of China. PLoS Negl Trop Dis. 2018;12:e0006465.
- Li T, Chen X, Yanagida T, et al. Detection of human taeniasis in Tibetan endemic areas, China. Parasitology. 2013;140:1602–1607.
- Yamasaki H, Allan JC, Sato MO, et al. DNA differential diagnosis of taeniasis and cysticercosis by Multiplex PCR. J Clin Microbiol. 2004;42:548–553.
- Sutisna P, Kapti IN, Wandra T, et al. Towards a cysticercosis-free tropical resort island: a historical overview of taeniasis/cysticercosis in Bali. Acta Trop. 2019;190:273–283.
- Yoshino K. On the subjective symptoms caused by the parasitism of Taenia solium and its development in man. Taiwan Igakkai. Zasshi. 1934;33: 183–194. in Japanese.
- Davaasuren A, Davaajav A, Ukhnaa B, et al. Neurocysticercosis: a case study of a Mongolian traveler who visited China and India with an updated review in Asia. Travel Med Infect Dis. 2017;20:31–36.
- Kobayashi K, Nakamura-Uchiyama F, Nishiguchi T, et al. Rare case of disseminated cysticercosis and taeniasis in a Japanese traveler after returning from India. Am J Trop Med Hyg. 2013;89:58–62.
- Ito A, Li T, Chen X, et al. Minireview on chemotherapy of taeniasis and cysticercosis due to Taenia solium in Asia, and a case report with 20 tapeworms. Trop Biomed. 2013;30:164–173.
- Diaz Camacho SP, Candil Ruiz A, Suate Peraza V, et al. Epidemiologic study and control of Taenia solium infections with praziquantel in a rural village of Mexico. Am J Trop Med Hyg. 1991;45(4):522–531.
- Garcia HH, Gonzalez AE, Tsang VC, et al., Cysticercosis Working Group in Peru. Elimination of Taenia solium transmission in northern Peru. N Engl J Med. 2016;374:2335–2344.
- Keilbach NM, De Aluja AS, Sarti-Gutierrez E. A programme to control taeniasis-cysticercosis (Taenia solium): experiences in a Mexican village. Acta Leiden. 1989;57:181–189.
- Sarti E, Schantz PM, Avila G, et al. Mass treatment against human taeniasis for the control of cysticercosis: a population-based intervention study. Trans R Soc Trop Med Hyg. 2000;94:85–89.