ABSTRACT
Introduction
Trials have demonstrated the benefits of methylprednisolone in the treatment of coronavirus disease 2019 (COVID-19). However, data on optimal dose, duration and timing of administration are limited. This study investigates the outcome of various methylprednisolone treatment regimens among hospitalised COVID-19 patients.
Methods
A retrospective cohort study was conducted on hospitalised adult COVID-19 patients admitted between June and August 2021 in general COVID-19 wards, treated with methylprednisolone. Clinical outcomes evaluated include in-hospital mortality, thirty-day mortality, clinical efficacy (C-reactive protein (CRP), total white blood cells (TWBC) and oxygen requirement) as well as the safety of methylprednisolone.
Results
Of 278 patients, 1(0.4%) received weight-based dosing of 1 mg/kg/day, 101(36.3%) received weight-based dosing of 2 mg/kg/day, 130(46.8%) received fixed dosing methylprednisolone 250 mg/day and 46(16.5%) received fixed dosing methylprednisolone 500 mg/day. There was a significant difference in in-hospital mortality rates following different methylprednisolone doses whereby in-hospital mortality occurred in 22.5% (n = 23) of patients with 1 or 2 mg/kg/day methylprednisolone, 32.3% (n = 42) with 250 mg/day and 39.1% (n = 18) with 500 mg/day (p = 0.023). On the other hand, no significant difference in thirty-day mortality, clinical efficacy and safety was observed between different dosing regimens (p > 0.05).
Conclusion
The use of methylprednisolone weight-based dosing in hospitalised COVID-19 patients should be considered due to the positive outcome associated with lower in-hospital mortality.
Introduction
The main cause of severe illness and death in COVID-19 is acute viral pneumonia leading to acute respiratory distress syndrome (ARDS). As the disease progresses, patients often require increasing levels of respiratory support. For early-stage infections in high-risk individuals not needing oxygen supplementation, options include oral antiviral or antiviral monoclonal antibodies (Ministry of Health Malaysia, Citation2023). Hospitalised patients requiring oxygen, however, receive antiviral or immunomodulators with corticosteroids (Ministry of Health Malaysia, Citation2023; National Institute of Health, Citation2024). WHO recommends the use of 6 mg dexamethasone daily or 50 mg hydrocortisone intravenously every 8 h for 7–10 days in severe COVID-19 patients (World Health Organisation Citation2020). Dexamethasone is recommended in most guidelines although methylprednisolone is suggested in COVID-19 patients with multisystem inflammatory syndrome (Ministry of Health Malaysia, Citation2023; National Institute of Health, Citation2024).
The World Health Organisation (WHO) has previously advised against the use of systemic corticosteroids in COVID-19 patients. This is because it was found that in severe acute respiratory syndrome and Middle Eastern respiratory syndrome, viral clearance was delayed with the use of systemic corticosteroids (Corral-Gudino et al., Citation2021; Go et al., Citation2021). However, despite the recommendation by WHO, these drugs have been widely used in China during the COVID-19 outbreak (Tang et al., Citation2021). As such, owing to its anti-inflammatory and anti-fibrotic effects, corticosteroid remains an option among more hypoxic patients (Go et al., Citation2021), with most opting for dexamethasone corticosteroids (Ministry of Health Malaysia, Citation2023; National Institute of Health, Citation2024).
Despite the result of the RECOVERY trial supporting dexamethasone in moderate-to-severe COVID-19 (Horby et al., Citation2021), various studies support the use of methylprednisolone as an alternative corticosteroid (Hasan et al., Citation2021; Wu et al., Citation2020). Initial reports by Wu et al. (Citation2020) showed promising outcomes, whereby it was found that the use of methylprednisolone in COVID-19 patients with ARDS appeared to minimise the risk of death (HR, 0.38; 95% CI, 0.20 −0.72) (Wu et al., Citation2020). A recent meta-analysis concluded that a short course of 3–5 days of methylprednisolone pulse therapy can be a potential alternative to the low-dose dexamethasone therapy in severe COVID-19 infection to avoid death (Hasan et al., Citation2021). In the GLUCOCOVID trial, 64 patients were randomised (35 patients in the methylprednisolone group and 29 patients in the standard-of-care group). In the intention-to-treat analysis, 40% in the methylprednisolone group and 48% in the standard-of-care group reported a composite endpoint of death. In the per-protocol analysis, patients receiving methylprednisolone had a lower chance of developing the composite endpoint of death (age-adjusted risk ratio 0.42; 95% CI 0.20-0.89; p = 0.043). However, both hyperglycaemia and nosocomial infection were higher in the methylprednisolone group (Corral-Gudino et al., Citation2021).
In Malaysia, a study compared the clinical outcomes of hospitalised COVID-19 patients receiving a 10-day low-dose corticosteroid (IV methylprednisolone 2 mg/kg/day loading dose then 0.25 mg/kg four times a day q.i.d.) with patients given a 10-day high-dose corticosteroid (IV 20 mg dexamethasone o.d. or a 1.5 mg/kg prednisolone tablet o.d.) (Kori et al., Citation2022). It was demonstrated that there was a significant improvement in clinical outcomes with steroid use but no significant difference between low-dose and high-dose corticosteroid treatments. Consequently, the study recommends low-dose methylprednisolone or corticosteroids for optimising clinical outcomes in hospitalised COVID-19 patients.
To date, it is still unclear whether the mortality benefits of dexamethasone can be extended to other systemic corticosteroids such as methylprednisolone. The evidence on the most appropriate corticosteroid and the recommended dose in the treatment of COVID-19 is scarce. Similarly in our setting, practices vary amongst physicians in terms of methylprednisolone-dosing strategies, with some opting for low-dose methylprednisolone (1-2 mg/kg/day) and some using higher doses of 500 mg per day. With the increasing use of methylprednisolone in our setting, there is a need to investigate the clinical outcomes following the methylprednisolone treatment regimen on COVID-19 patients.
Materials and methods
Study design, population, setting and time period
This was a retrospective cohort study using secondary data obtained from the clinical pharmacist’s Pharmacotherapy Review Form. A Pharmacotherapy Review Form is a standard document developed and validated for use by hospital pharmacists under the Ministry of Health, Malaysia to record patient’s detail, reason for admission, laboratory data, medications, relevant findings, suggestions and interventions performed on pharmaceutical care issues during clerking or reviewing of patients (Pharmaceutical Services Division, Ministry of Health Malaysia, Citation2010). This standard Pharmacotherapy Review Form was used to collect data for the current study. In cases where the Pharmacotherapy Review Form was incomplete data, patients’ medical records will be retrieved from the Hospital Record Office while laboratory data were obtained from the online hospital laboratory information system. The population studied were adults (≥18 years old) and COVID-19 patients who received intravenous methylprednisolone as part of COVID-19 treatment during the 3months The eligibility criteria for the study are listed in .
Table 1. Study eligibility criteria.
Sampling method
A simple random sampling method was applied where subjects were sampled from all cases fulfilling the eligibility criteria in a study period of 3 months. Upon screening for eligibility criteria, the total number of patients that fulfilled the eligibility criteria was numbered consecutively. Then, a random sample generator was generated from Microsoft® Excel® to select study subjects until a minimum sample size was achieved.
Sample size
The minimum sample size achieved was 278, which was based on a study power of 80% and 95% confidence level. The sample size was calculated using the online Raosaoft® software calculator using the formula, n, sample size = Nx/ ((N-1)E2 + X), where x = Z(c/100)2r(100-r), E = Sqrt [(N-n)x/n(N-1)], N is the population size, r is the fraction of responses and Z(c/100) is the critical value for the confidence level c. The margin of error was set at 5% with a confidence interval of 95% while the standard population size of 20,000 was used. The percentage of the study event, r, was set at 24.2%, based on the results published in a recent study which reported a mortality rate of 24.2% among COVID-19 patients receiving methylprednisolone [11].
Data collection and documentation
Pharmacotherapy review forms of patients on methylprednisolone in general COVID-19 wards during the study period were obtained. Upon screening for eligibility and randomisation, the list of study subjects was given a unique identification number in a separate subject identifier form. Data such as patient demographics, medical history, admission history, diagnosis, treatments of COVID-19 received, baseline laboratory investigations, clinical outcomes at day five and day ten and clinical status at day thirty were obtained and recorded. Data collections were performed using a structured data collection form.
Measured outcomes
The measured outcomes were divided into primary outcomes and secondary outcomes based on previous work (Jiang et al., Citation2023; Kori et al., Citation2022; Nersesjan et al., Citation2020). Primary outcomes were in-hospital mortality and thirty-day mortality (Jiang et al., Citation2023; Nersesjan et al., Citation2020). Secondary outcomes were clinical efficacy defined as C-reactive protein (CRP), trends of total white blood cells (TWBC) and trends of oxygen requirement measured at day-5 and day-10 and the safety of methylprednisolone (Jiang et al., Citation2023; Kori et al., Citation2022; Nersesjan et al., Citation2020).
Statistical analyses
Data analyses were conducted using IBM® SPSS® Statistics Version 26. For baseline demographics, clinical characteristics and COVID-19 treatment description, categorical data were presented as frequency (n) and percentage (%). Continuous data that were not normally distributed were presented as median and interquartile range (IQR), whilst data that were normally distributed were presented as mean and standard deviation (SD). The normality of data was determined using the Shapiro-Wilks test. Further analysis was then performed, in which categorical data were compared using Chi-square or Fisher’s exact test. Fisher’s exact test was performed when more than 20% of the cells demonstrated an expected count of less than five. ANOVA was used to compare between groups as the variables tested were normally distributed. Statistical significance is defined by a p-value of <0.05.
Ethics approval
The study was conducted in accordance with the ethical standards of the Medical Research and Ethics Committee (MREC), Ministry of Health Malaysia and the University’s Ethics Committee with compliance to the Guidelines on Good Clinical Practice (GCP) and the Declaration of Helsinki. Ethics approval was obtained from MREC (NMRR ID-21-02472-P1Q) and the university’s ethics committee (UKM PPI/111/8/JEP-2022-239) before the commencement of the study.
Results
Patient demographic and baseline characteristics
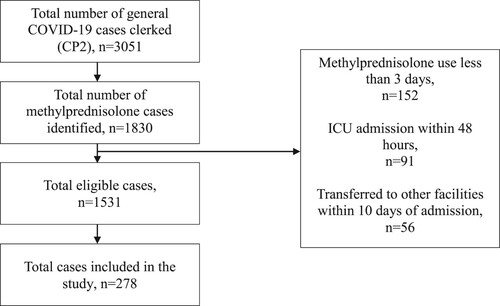
During the study period, a total of 3051 general COVID-19 cases were identified. Upon screening for eligibility, 1531 cases were subjected to sampling. After implementing a simple random sampling method, a total of 278 patients were included in the study, which met the minimum sampling size required. The summary of patient selection is shown in .
Patient demographics and baseline characteristics are summarised in . The mean (SD) age of patients was 54.9 (14.4) years old, with a higher distribution of males (n = 171, 61.5%) than females (n = 107, 38.5%). The majority of patients were of Malay ethnicity (n = 113, 40.6%), followed by Chinese (n = 64, 23%) and Indian (n = 21, 7.6%). The majority of patients were diagnosed as COVID-19 Category 4 (n = 230, 82.7%) followed by COVID-19 Category 3 (n = 34, 12.2%), Category 5 (n = 11, 4%) and Category 2 (n = 3, 1.1%) with most patients requiring the use of nasal prong on admission (n = 117, 42.1%). The mean (SD) day of illness on admission was 6.7 (3.1) days. The median (IQR) CRP and TWBC on admission were 100.9 mg/L (106.7) and 7.9 × 109/L (4.7), respectively. One patient (0.4%) received weight-based dosing of 1 mg/kg/day and 36.3% (n = 101) received weight-based dosing of 2 mg/kg/day. 46.8% (n = 130) of patients received a fixed dose mpf ethylprednisolone of 250 mg/day while 16.5% (n = 46) received a fixed dose of 500 mg/day. 43.2% (n = 120) of patients received a total duration of methylprednisolone of 5 days and below while 56.8% (n = 158) patients received a total duration of more than 5 days.
Table 2. Baseline demographics, clinical characteristics and COVID-19 treatments of all eligible subjects.
Clinical outcomes
The clinical outcomes following the administration of methylprednisolone are summarised in . Primary outcomes determined were in-hospital and thirty-day mortality. In-hospital mortality involved 29.9% (n = 83) of patients, 55.4% (n = 154) were discharged well and 14.7% (n = 41) of patients (stable patients requiring continuation of care for other conditions) were transferred to other facilities. The percentage of thirty-day mortality was 30.6% (n = 85). The remaining 64.7% (n = 180) were well at day 30 and another 4.7% (n = 13) were unknown.
Table 3. Clinical outcomes following the administration of methylprednisolone (n = 278).
The secondary outcomes investigated were clinical efficacy and safety, as reported in . Clinical efficacy includes trends of CRP, TWBC and oxygen requirement measured at day 5 and day 10 of admission. At day 5, more than 60% (n = 167) of patients had decreasing or normalised CRP and TWBC. However, oxygen requirement at day 5 showed an increasing or elevated trend for 232 (83.5%) patients. The trend seen was similar at day-10 with the CRP trend decreasing or normalised in 152 (54.7%) of patients, the TWBC trend decreasing or normalised in 121 (43.7%) of patients while 107 (38.5%) of patients had an increasing or elevated trend of oxygen requirement. A total of 28.5% (n = 79) of patients had no data taken at day 10. In terms of safety following the administration of methylprednisolone, hyperglycaemia was seen in 171 (61.5%) of patients, nosocomial infection in 113 (40.6%) of patients, and upper gastrointestinal bleeding was seen in 5 (1.8%) patients and 28 (10.1%) patients experienced other adverse events, reported as transaminitis and/or hypernatremia.
The primary clinical outcomes were further analysed based on weight-based or fixed-dose methylprednisolone are summarised in . There was a significant difference in in-hospital mortality rates following different methylprednisolone doses (p = 0.023). In patients that received weight-based doses of either 1 or 2 mg/kg/day methylprednisolone, 23 (22.5%) patients reported in-hospital mortality, 60 (58.8%) were discharged well and 19 (18.6%) were transferred to other facilities. In those receiving a fixed dose of 250 mg/day methylprednisolone, 42 (32.3%) patients reported in-hospital mortality, 76 (58.5%) were discharged well and 12 (9.2%) were transferred to other facilities. In-hospital death and discharge were the same in patients receiving fixed-dose methylprednisolone of 500 mg/day (n = 18, 39.1%, respectively) whilst 10 (21.7%) patients were transferred to other facilities. On the other hand, there was no significant difference in thirty-day mortality between the three groups (p > 0.05). Thirty-day mortality for weight-based dose was 23 (22.5%), fixed-dose 250 mg/day was 43 (33.1%) and fixed-dose 500 mg/day was 19 (41.3%).
Table 4. Clinical outcomes following weight-based (n = 102), fixed-dose (250 mg/day), (n = 130) or fixed-dose 500 mg/day (n = 46) of methylprednisolone.
The secondary outcomes following different methylprednisolone doses are presented in . The overall outcome of the trend of CRP, TWBC and oxygen requirement did not differ significantly between groups. The weight-based dose group had the highest rate of achieving decreasing or normalised CRP trend at day 5 while the fixed-dose 250 mg/day group had the highest rate of increasing or elevated CRP trend at day 5. For TWBC at day 5, the rate of decreasing or normalised TWBC trend was similar between groups while the weight-based dose had a higher rate of increasing or elevated TWBC trend (n = 36, 35.3%) as compared to the fixed dose of 250 mg/day (n = 40, 30.8%) and fixed dose of 500 mg/day (n = 15, 32.6%) respectively. Decreasing or normalised oxygen requirements were the lowest in the fixed-dose 500 mg/day group at day 5 (n = 5. 10.9%). At day 10, the weight-based dose group had the highest rate of decreasing or normalised CRP trend (n = 65, 63.7%) while the fixed dose 500 mg/day group had the highest rate of increasing or elevated CRP trend (n = 11, 23.9%). As for TWBC and oxygen requirement trends at day 10, the weight-based dose group showed the highest rate of achieving decreasing or normalised trends compared to the other two groups (n = 55, 53.9%) and (n = 40. 39.2%), respectively. The incidences of adverse events between the groups did not differ significantly. The rate of developing subsequent nosocomial infection was similar between the three groups (weight-based dose = 41.2%; a fixed dose of 250 mg/day = 40% and a fixed dose of 500 mg/day = 41.3%).
Discussion
Overall, the administration of methylprednisolone in the current study resulted in 30% in-hospital mortality and thirty-day mortality, respectively. These findings are consistent with the literature, where previous investigations found that methylprednisolone use in the treatment of COVID-19 had comparable clinical outcomes in terms of mortality (Corral-Gudino et al., Citation2021; Papamanoli et al., Citation2021). Although the use of low-dose dexamethasone has been reported (Kori et al., Citation2022), the use of methylprednisolone, particularly among the local population is limited. To the best of our knowledge, this is the first study that compares the use of different doses of methylprednisolone among COVID-19 patients.
Interestingly, weight-based doses of either 1 mg/kg/day or 2 mg/kg/day methylprednisolone were more favourable compared to fixed doses of 250 and 500 mg/day. Several studies investigated short courses of high methylprednisolone dose (Edalatifard et al., Citation2020; Pinzón et al., Citation2021) and long courses of low-dose weight-based dosing (Corral-Gudino et al., Citation2021; Jeronimo et al., Citation2021; Papamanoli et al., Citation2021; Tang et al., Citation2021). Short-course high-dose methylprednisolone of up to 1 g per day significantly reduced recovery time, prevented admission into ICU and decreased severity markers such as CRP (Pinzón et al., Citation2021). Similar to the current work, low doses of 1 or 2 mg/kg/day methylprednisolone resulted in lower in-hospital mortality, demonstrating the basis for further work on the use of low-dose methylprednisolone in this group of patients. Indeed, methylprednisolone is associated with decreased reliance on mechanical ventilation and lower utilisation of intensive care resources, all without undue increase in complications (Papamanoli et al., Citation2021). Despite this, the high in-hospital mortality among those treated with 500 mg/day methylprednisolone could be attributed to the use of rescue methylprednisolone, when patients with more severe illness received higher doses. Therefore, death could be due to advanced disease rather than the use of high-dose methylprednisolone, which required further investigation.
Overall, clinical efficacy, defined by decreasing or normalising CRP and TWBC levels, and achievement of normal oxygen saturation under room air at day 5 and day 10, were not statistically significant between the groups, similar to previous findings (Corral-Gudino et al., Citation2021 and Lu & Wang, Citation2020). On the other hand, more than 80% of the patients in this study required a higher level of oxygen support on day 5. This finding is possibly because most patients’ condition worsened on day 5 of admission, requiring a higher level of oxygen support, hence requiring a higher administration of methylprednisolone dose. On day 10, only the weight-based dose group showed a higher proportion of patients that achieved favourable clinical outcomes in terms of oxygen support. This may be because higher doses of methylprednisolone (250 mg per day or 500 mg per day) were given to the patients who were more hypoxic and hence were at a more severe infection stage.
In terms of safety, results from this study showed that higher doses of methylprednisolone were not associated with increased rates of adverse effects. This finding is supported by other studies which found no significant increase in the risk of adverse effects with the use of high-dose methylprednisolone (Edalatifard et al., Citation2020; Papamanoli et al., Citation2021). Edalatifard et al. (Citation2020) investigated the use of intravenous methylprednisolone 250 mg per day for three days over standard of care. Only two patients from each group (5.8% and 7.1%, respectively) experienced significant adverse events (Edalatifard et al., Citation2020). The median daily dose of methylprednisolone used by Papamanoli et al. (Citation2021) was 160 mg (120-180 mg). The rates of bacteraemia, nosocomial pneumonia and gastrointestinal bleeding among patients, who received methylprednisolone, were non-inferior to those who did not receive methylprednisolone (Papamanoli et al., Citation2021).
This study is the first that compared different dosing regimens of methylprednisolone in COVID-19 patients in Malaysia. The findings may aid physicians in determining optimal doses of methylprednisolone in the treatment of COVID-19, requiring methylprednisolone. However, this study has several limitations. First, the study's design was retrospective and hence may be limited by the information available in the patient’s medical records. Therefore, CRP and TWBC levels were not available for all patients, which may affect the outcome of the results. There was also no standardisation of methylprednisolone dosing schedule (duration and tapering of dose) within the group, which could give rise to variations in outcomes. The study cohort only involved patients in the general COVID-19 ward and therefore, the results may not be extrapolated to patients in COVID-19 ICU. Finally, other medications were not taken considered, which could have also affected patients’ outcomes. A larger multicentred randomised-controlled trial involving standardised dosing of methylprednisolone among COVID-19 patients is required.
Conclusion
There is still uncertainty with regard to the ideal dose and duration of methylprednisolone therapy in various clinical settings, despite accumulating evidence of its benefit in severe COVID-19 patients. This study was able to investigate the clinical impact of various methylprednisolone doses. In hospitalised COVID-19 patients receiving methylprednisolone, the use of weight-based methylprednisolone of 1 or 2 mg/kg/day is associated with lower in-hospital mortality without significant adverse effects.
Acknowledgement
We express sincere appreciation to the Faculty of Pharmacy, Universiti Kebangsaan Malaysia, and Medical Department, Hospital Kuala Lumpur for the continuous support and resources. We would also like to thank the Director General of Health Malaysia for the permission to publish this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Corral-Gudino, L., Bahamonde, A., Arnaiz-Revillas, F., Gómez-Barquero, J., Abadía-Otero, J., García-Ibarbia, C., Mora, V., Cerezo-Hernández, A., Hernández, J. L., López-Muñíz, G., Hernández-Blanco, F., Cifrián, J. M., Olmos, J. M., Carrascosa, M., Nieto, L., Fariñas, M. C., & Riancho, J. A. (2021). Methylprednisolone in adults hospitalized with COVID-19 pneumonia : An open-label randomized trial (GLUCOCOVID). Wiener Klinische Wochenschrift, 133(7–8), 303–311. https://doi.org/10.1007/s00508-020-01805-8
- Edalatifard, M., Akhtari, M., Salehi, M., Naderi, Z., Jamshidi, A., Mostafaei, S., … Rostamian, A. (2020). Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: Results from a randomised controlled clinical trial. European Respiratory Journal, 56(6), 1–13. https://doi.org/10.1183/13993003.02808-2020.
- Go, R. C., Shah, R., Nyirenda, T., Oe, Y., Sarfraz, K., Panthappattu, J. J., Philip, L., Bheeman, C., Shah, N., Shah, S., Dar, S., Hung, S., Rahman, W., Im, H., Marafelias, M., Omidvari, K., Pradhan, A., Sadikot, S., Rose, K. M., … Josephs, J. (2021). Methylprednisolone and 60 days in hospital survival in coronavirus disease 2019 pneumonia. Critical Care Explorations, 3, e0493. https://doi.org/10.1097/cce.0000000000000493
- Hasan, S. S., Kow, C. S., Mustafa, Z. U., & Merchant, H. A. (2021). Does methylprednisolone reduce the mortality risk in hospitalized COVID-19 patients? A meta-analysis of randomized control trials. Expert Review of Respiratory Medicine, 15(8), 1049–1055. https://doi.org/10.1080/17476348.2021.1925546
- Horby, P., Lim, W. S., Emberson, J. R., Mafham, M., Bell, J. L., Linsell, L., Staplin, N., Brightling, C., Ustianowski, A., Elmahi, E., Prudon, B., Green, C., Felton, T., Chadwick, D., Rege, K., Fegan, C., Chappell, L. C., Faust, S. N., Jaki, T., … Landray, M. J. (2021). Dexamethasone in hospitalized patients with Covid-19. New England Journal of Medicine, 384(8), 693–704. https://doi.org/10.1056/NEJMoa2021436
- Jeronimo, C. M. P., Farias, M. E. L., Val, F. F. A., Sampaio, V. S., Alexandre, M. A. A., Melo, G. C., Safe, I. P., Borba, M. G. S., Netto, R. L. A., Maciel, A. B. S., Neto, J. R. S., Oliveira, L. B., Figueiredo, E. F. G., Oliveira Dinelly, K. M., de Almeida Rodrigues, M. G., Brito, M., Mourão, M. P. G., Pivoto João, G. A., Hajjar, L. A., … Lacerda, M. V. G. (2021). Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID-19; Metcovid): A randomized, double-blind, phase IIb, placebo-controlled trial. Clinical Infectious Diseases : An Official Publication of the Infectious Diseases Society of America, 72(9), e373–e381. https://doi.org/10.1093/cid/ciaa1177
- Jiang, X., Zhang, C., Pan, Y., Cheng, X., & Zhang, W. (2023). Effects of C-reactive protein trajectories of critically ill patients with sepsis on in-hospital mortality rate. Scientific Reports, 13(1), 15223. https://doi.org/10.1038/s41598-023-42352-2
- Kori, N., Islahudin, F., Abd Rahim, M. Y., Periyasamy, P., Shah, N. M., Hatah, E. M., & Lan, L. C. (2022). Corticosteroid effectiveness among hospitalised COVID-19 patients in Malaysia. The Journal of Infection in Developing Countries, 16(09), 1390–1397. https://doi.org/10.3855/jidc.16039
- Lu, G., & Wang, J. (2020). Dynamic changes in routine blood parameters of a severe COVID-19 case. January.
- Ministry of Health Malaysia. (2023). COVID-19 management guidelines in Malaysia No.5 / 2020. Annex 2e: clinical management of confirmed COVID-19 case in adult and paediatric - updated 28/12/2023. Retrieved March 2, 2024.
- National Institute of Health. (2024). COVID-19 treatment guidelines panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/
- Nersesjan, V., Amiri, M., Christensen, H. K., Benros, M. E., & Kondziella, D. (2020). Thirty-day mortality and morbidity in COVID-19 positive vs. COVID-19 negative individuals and vs. individuals tested for influenza A/B: a population-based study. Frontiers in Medicine, 7, 598272. https://doi.org/10.3389/fmed.2020.598272
- Papamanoli, A., Yoo, J., Grewal, P., Predun, W., Hotelling, J., Jacob, R., Mojahedi, A., Skopicki, H. A., Mansour, M., Marcos, L. A., & Kalogeropoulos, A. P. (2021). High-dose methylprednisolone in nonintubated patients with severe COVID-19 pneumonia. European Journal of Clinical Investigation, 51(2), 1–10. https://doi.org/10.1111/eci.13458
- Pharmaceutical Services Division Ministry of Health Malaysia. (2010). Guidelines for Inpatient Pharmacy Practice.
- Pinzón, M. A., Ortiz, S., Holguín, H., Betancur, J. F., Arango, D. C., Laniado, H., Arias, C. A., Munõz, B., Quiceno, J., Jaramillo, D., & Ramirez, Z. (2021). Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia. PLoS One, 16(5 May), 1–13. https://doi.org/10.1371/journal.pone.0252057
- Tang, X., Feng, Y. M., Ni, J. X., Zhang, J. Y., Liu, L. M., Hu, K., Wu, X. Z., Zhang, J. X., Chen, J. W., Zhang, J. C., Su, J., Li, Y. L., Zhao, Y., Xie, J., Ding, Z., He, X. L., Wang, W., Jin, R. H., Shi, H. Z., & Sun, B. (2021). Early use of corticosteroid may prolong SARS-CoV-2 shedding in non-intensive care unit patients with COVID-19 pneumonia: A multicenter, single-blind, randomized control trial. Respiration; International Review of Thoracic Diseases, 100(2), 116–126. https://doi.org/10.1159/000512063
- World Health Organization. (2020). Corticosteroids for COVID-19. Living guidance 2 September 2020. World Health Organization. Available at https://iris.who.int/rest/bitstreams/1299344/retrieve.
- Wu, C., Chen, X., Cai, Y., Xia, J., Zhou, X., Xu, S., Huang, H., Zhang, L., Zhou, X., Du, C., Zhang, Y., Song, J., Wang, S., Chao, Y., Yang, Z., Xu, J., Zhou, X., Chen, D., Xiong, W., … Song, Y. (2020). Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Medicine, 180(7), 934–943. https://doi.org/10.1001/jamainternmed.2020.0994