 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background: The COVID-19 pandemic has led to millions of fatalities globally. Kidney transplant (KT) patients, given their comorbidities and under immunosuppressant drugs, are identified as a high-risk group. Though vaccination remains pivotal for pandemic control, some studies indicate that KT exhibits diminished immune reactions to SARS-CoV-2 vaccines. Therefore, evaluating the vaccine responses in KT, especially the humoral responses against emergent variants is crucial.
Methods: We developed a multiplexed SARS-CoV-2 variant protein microarray, incorporating the extracellular domain (ECD) and the receptor binding domain (RBD) of the spike proteins from the variants. This was employed to investigate the collective humoral responses after administering two doses of mRNA-1273 and AZD1222 vaccines in KT under immunosuppressive drugs and in healthy controls.
Results: After two doses of either mRNA-1273 or AZD1222, the KT generally showed lower surrogate neutralizing and total antibodies against spike ECD in multiple variants compared to healthy controls. Although two doses of mRNA-1273 induced 1.5–2 fold more surrogate neutralizing and total antibodies than AZD1222 in healthy controls, the KT subjects with two doses of mRNA-1273 generally exhibited higher surrogate neutralizing but similar total antibodies against spike ECD in multiple variants. There were moderate to high correlations between the surrogate neutralizing and total antibodies against spike ECDs.
Conclusion: This study offers pivotal insights into the relative vulnerability of KT concerning humoral immunity and the evolving mutations of SARS-CoV-2. Such findings are useful for evaluating vaccine responses and recommending vaccine episodes for KT.
Introduction
Since the emergence of SARS-CoV-2 in Wuhan in late 2019, the virus has continually mutated, leading to multiple variants including the alpha, beta, gamma, delta, omicron, and subsequent omicron subtypes. The RNA virus has caused multiple transmission waves due to its high mutation rate, culminating in over 752 million infections and 6.8 million deaths worldwide. Kidney transplant (KT) patients have been endowed with a kidney from either a living or deceased donor due to kidney failure [Citation1]. Immunosuppressant drugs to counteract the rejection of the transplanted kidney often compromise the immune system, leading to a higher fatality rate among KT [Citation2–4].
Vaccination stands as a paramount tool in mitigating severe COVID-19. Given the elevated risk faced by KT due to their inherent comorbidities and continuous immunosuppression, an optimized vaccination approach is indispensable [Citation5]. While mRNA and viral vector vaccines are among the commonly administered SARS-CoV-2 vaccines, their efficacy has been challenged by the evolving virus variants, compounded further by the immunosuppressed status in KT [Citation6]. Thus, consistent monitoring of humoral responses in KT against a spectrum of SARS-CoV-2 variants is pivotal to averting detrimental outcomes [Citation7–9].
To evaluate humoral antibody responses against a range of SARS-CoV-2 variants, we previously designed a multiplexed SARS-CoV-2 Variant (CoVariant) protein array [Citation10–12] This array allows for the simultaneous measurement of both binding and surrogate-neutralizing antibodies against multiple SARS-CoV-2 variants [Citation12]. In investigating vaccine-induced responses among KT, we compared healthy subjects and KT who received either two doses of mRNA-1273 (M2) or AZD1222 (AZ2). Our findings using the CoVariant protein microarray revealed that KT administered with M2 exhibited elevated neutralizing antibodies against various variants compared to KT vaccinated with AZ2.
Materials and methods
Preparation of CoVariant protein microarray
Multiplexed SARS-CoV-2 Variant (CoVariant) protein microarrays were created by printing various SARS-CoV-2 variant spike proteins, nucleocapsid proteins, and controls, all dissolved in a solution of 30% glycerol in PBS (Table S1). All the samples were printed on precoated slides as previously described [Citation13]. The array printed triplicate for each protein in 9 × 7 format with 14 identical blocks by using a contact printer (CapitalBio, #SmartArrayer 136), immobilized for 12 hours, and then stored at −80 °C.
CoVariant protein microarray quality control
CoVariant protein microarrays were examined for protein immobilizations and protein functions via ACE2 staining and anti-His staining. Briefly, the arrays were washed in TBST for 10 minutes, blocked with SuperBlock Stock solution (Thermo) for 15 minutes, and incubated with biotinylated ACE2 (Sino Biological) using a serial dilution starting at 0.125 ng/mL for 1 hour. After a TBST rinse, Cy5-conjugated anti-His antibody (Jackson Lab) and Cy5-conjugated streptavidin (Jackson Lab) were added and incubated for 1 hour. After five washes, the arrays were dried and scanned with SpinScan from Caduceus Biotechnology using PMT settings at Cy5 30% and Cy3 25%.
Subjects and ethical statement
The study was carried out at the outpatient Kidney Transplantation Department, National Cheng Kung University Hospital from 22 July 2021, to 18 February 2022. Serums were harvested and stored at − 80 °C until needed. This study followed the principles of the Declaration of Helsinki, and the protocols were approved by the Institutional Review Boards of National Cheng Kung University Hospital (Approval No. A-ER-110-263, A-ER-110-203).
Serum profiling
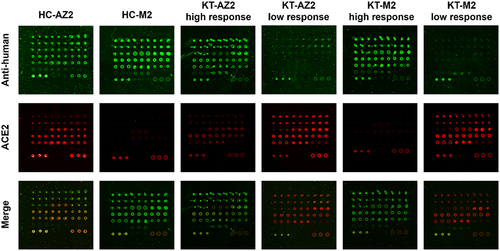
After the blocking step, the CoVariant microarrays underwent incubation with 50-fold diluted serum for an hour. Subsequently, the arrays were washed and subjected to another incubation step with biotinylated human ACE2, Cy5-Streptavidin, and Cy3-anti-human IgG+IgA+IgM (Jackson Lab, #109-165-064) for one hour. After a few washes, the arrays were dried and scanned with SpinScan from Caduceus Biotechnology using PMT settings at Cy5 30% and Cy3 25% as previously instructed [Citation7,Citation8]. We use two fluorescent channels to quantify surrogate neutralization activity and antibody levels. We show the antibody responses of normal humans with the immunosuppressive effect of KTR under two different vaccines. Both vaccines have antibody responses (green fluorescence) in the healthy control, but Moderna has a better inhibitory effect (red fluorescence).
Figure 1. The images of total antibodies and ACE2 bindings in HC and KT. The assay procedures involve the simultaneous quantification of total antibodies and surrogate neutralizing. The total antibodies in the serum were quantified using a Cy3-labelled anti-human IgG+IgA+IgM antibody. Concurrently, the surrogate neutralizing was quantified using Cy5-labelled ACE2.
Data analysis
GenePix Pro software was used to analyse signals, employing foreground minus background calculations. Surrogate neutralizing activity was determined by comparing data from the sample and blank. The inhibition of ACE2 binding, denoting surrogate neutralizing, was defined as the inhibition percentage of ACE2 binding and calculated as 1-
For total antibody (IgG+IgA+IgM) quantification, fluorescence signals were normalized by dividing the protein amounts based on the anti-His signals. One-way ANOVA with Tukey’s post-hoc test (p < 0.05) was used to compare surrogate neutralizing and total antibodies in different groups. Baseline characteristics and negative counts of surrogate neutralizing were calculated by Mann-Whitney tests for continuous variables and Chi-square tests for categorical variables. GraphPad Prism software was used for statistical analysis and figure generation. Data were presented as mean ± SD, with n indicating the number of subjects.
Results
To evaluate the humoral responses against emergent SARS-CoV-2 variants in KT, the reactivity of the neutralizing and total antibodies against multiple variants was needed. Here, we employed a previously developed CoVaraint protein microarray including spike proteins from wild-type, Alpha, Beta, Gamma, Delta, and Omicron variants [Citation10,Citation12,Citation14]. Also, we previously developed a multiplexed surrogate method as an alternative way to perform multiple plaque reduction assays [Citation12]. By updating the CoVariant arrays to include Omicron, we can now access the surrogate neutralizing and total antibodies at the same time against emergent SARS-CoV-2 variants.
To assess the humoral responses in KT after vaccination, we collected serum samples from both HC and KT who received the two-dose vaccines. Due to the time of sampling, only AZD-1222 and mRNA-1273 were imported into Taiwan. Therefore, in this study, we only focused on two-dose mRNA-1273 (M2) or AZD1222 (AZ2) vaccination. Blood samples were obtained from KT on regular immunosuppressive medications and healthy controls (HC) using aseptic phlebotomy techniques, 7 to 120 days after two doses of either mRNA-1273 (M2) or AZD1222 (AZ2). The number of HC-AZ2, KT-AZ2, HC-M2, and KT-M2 subjects were 15, 17, 15, and 23, respectively. The mean and standard deviation of the days after vaccination were 31.3 ± 24.9, 93.1 ± 32.7, 56.1 ± 27.8, and 83.4 ± 35.5 for HC-AZ2, KT-AZ2, HC-M2, and KT-M2, respectively. The baseline characteristics of KT-AZ2 and KT-M2 were summarized in Table S2. There were no differences in gender, glomerular filtration rate, underlying diseases, and medications. However, there were significant differences in age and creatinine in KT-M2 (Table S2).
We selected the red colour to represent surrogate neutralization activity and the green colour to monitor the antibody levels via anti-human IgG+IgA+IgM. The representative images from the KT and HC who underwent two doses of either mRNA-1273 (M2) or AZD1222 (AZ2) were listed in . Both HC-AZ2 and HC-M2 had total antibody signals (green colour), but HC-M2 showed higher surrogate neutralizing (inhibiting red colour) than HC-AZ2. In the KT-AZ2 or KT-M2 groups, some of the subjects exhibited high antibody signal and surrogate neutralizing while others demonstrated low antibody signal and surrogate neutralizing (). The dramatic differences in the images demonstrated the different immunosuppressive status and the need for monitoring humoral responses in KT.
The ECD spike represented a more comprehensive structure than the RBD spike. Therefore, this study mainly focused on the data from ECD spikes. Compared to HC-AZ2, KT-AZ2 suppressed surrogate neutralizing in wild-type, Alpha, and Gamma ECDs but had no effects on Beta, Delta, and Omicron ECDs (). Compared to HC-M2, KT-M2 exhibited lowered surrogate neutralizing among all variants of spike ECDs. These data demonstrated the immunosuppressive effects and notable individual differences in KT.
Figure 2. Surrogate neutralizing against multiple spike ECDs in HC and KT after two doses of vaccines. HC and KT with 2 doses of vaccines were analysed for the surrogate neutralizing using the CoVariant arrays. Surrogate neutralizing against SARS-CoV-2 (a) wild-type ECD, (b) alpha ECD, (c) beta ECD, (d) gamma ECD, (e) delta ECD, and (f) omicron ECD. HC-AZ2 n = 15, KT-AZ2 n = 17, HC-M2 n = 15, KT-M2 n = 23. The data were analysed using one-way ANOVA, followed by Tukey’s post-hoc testing, with significance set at p < 0.05, denoted by distinct letters.
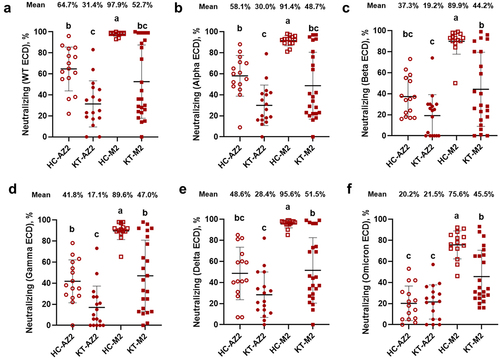
In HC groups, surrogate neutralizing was elevated in HC-AZ2 and gradually decreased over the variants, from 64.7% in wild-type to 20.2% in Omicron ECD (). Similarly but with a slighter degree in HC-M2, from 97.9% in wild-type to 75.6% in Omicron ECD. The differences between vaccines regarding surrogate neutralizing were higher in HC-M2 than HC-AZ2 among all spike ECD variants. In KT groups, surrogate neutralizing was elevated in KT-AZ2 and decreased into baseline after the Beta variant, from 31.4% in wild-type, 19.2% in Beta, to 21.5% in Omicron ECD. Similarly but with a slighter degree in KT-M2, from 52.7% in wild-type to 45.5% in Omicron ECD. Compared to KT-AZ2, KT-M2 showed similar surrogate neutralizing in wild-type and Alpha ECDs but higher in Beta, Gamma, Delta, and Omicron ECDs. The result suggested that M2 would be a better option for KT and HC to fight against emergent SARS-CoV-2 variants.
To gain a deeper understanding of the humoral immune response to SARS-CoV-2 and its variants after KT vaccinations, it is essential to analyse the vaccine-induced antibody productions. We detected all the major antibody isotypes in both KT and HC after vaccinations using Cy3-labelled anti-human IgG+IgA+IgM (). Compared to HC-AZ2, KT-AZ2 inhibited antibodies against wild-type, Alpha, Beta, and Gamma ECDs but had no difference in Delta and Omicron ECDs. Compared to HC-M2, KT-M2 suppressed antibodies against all ECDs, except Delta ECD. These data demonstrated the immunosuppressive effects not only in neutralizing but also total antibodies in KT.
Figure 3. Profiling the serum antibody responses against multiple spike ECDs in HC and KT after two doses of vaccines. Vaccinated serums from HC and KT were used to quantify the total antibodies against various spike ECD by using the CoVariant arrays. Total antibodies against SARS-CoV-2 (a) wild-type ECD, (b) alpha ECD, (c) beta ECD, (d) gamma ECD, (e) delta ECD, and (f) omicron ECD. HC-AZ2 n = 15, KT-AZ2 n = 17, HC-M2 n = 15, KT-M2 n = 23. The data were analysed using one-way ANOVA, followed by Tukey’s post-hoc testing, with significance set at p < 0.05, denoted by distinct letters.
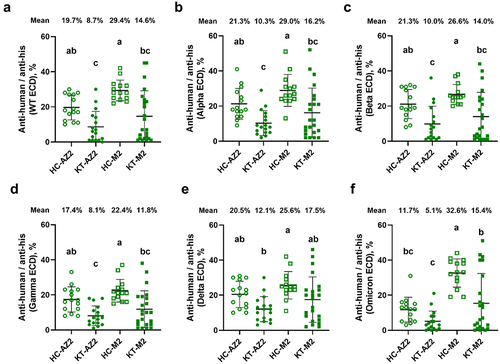
In the HC-AZ2 group, the antibody maintained within a certain range from 19.7% in wild-type to 20.5% in Delta but decreased to 19.7% in Omicron ECD (). In the HC-M2 group, the antibody slightly decreased over the variants from 29.4 % in wild-type to 25.6% in Delta but slightly increased to 32.6% in Omicron ECD. The differences between vaccines regarding total antibodies were higher in HC-M2 than HC-AZ2 in Omicron ECD but not obvious in other variants. In KT groups, the differences between vaccines also showed higher total antibodies in KT-M2 than KT-AZ2 in Omicron ECD but no other variants. The results from the total antibodies also suggested that M2 would be a better option for KT and HC to fight against Omicrons.
Although the spike RBD is a short domain containing ~ 220 amino acids, it is the key domain that interacts with human ACE2 and leads to invasion. In this study, we also immobilized a set of spike RBDs and analysed the surrogate neutralizing (Figure S1). Compared to HC-AZ2, KT-AZ2 suppressed surrogate neutralizing in Omicron but had no effects on other RBDs. Compared to HC-M2, KT-M2 exhibited lowered surrogate neutralizing among all variants of spike RBD. In HC groups, surrogate neutralizing was elevated in HC-AZ2 and gradually decreased over the variants, from 50.5% in wild-type to 14.7% in Omicron RBD. Similarly but with a slighter decay in HC-M2, from 96.5 % in wild-type to 72.4% in Omicron RBD. In HC groups, the differences between vaccines regarding surrogate neutralizing were elevated in HC-M2 than HC-AZ2 among all spike RBD variants. In KT groups, the surrogate neutralizing was similar in KT-M2 and KT-AZ2 among all spike RBD variants.
Along with the surrogate neutralizing, we reported the total antibodies against multiple spike RBDs (Figure S2). In the HC-AZ2 group, total antibodies gradually decreased after the Alpha, from 20.2% in wild-type to 11.5% in Omicron RBD. In the HC-M2 group, total antibodies were maintained within a certain range between 29–38% among all variants. In HC groups, the differences between vaccines regarding total antibodies were elevated in HC-M2 than HC-AZ2 among all spike RBD variants. In KT groups, the total antibodies were similar in KT-M2 and KT-AZ2 among all spike RBD variants, except for higher KT-M2 in Omicron RBD.
To understand the relationship between the surrogate neutralizing and total antibodies, we performed correlation analysis in multiple spike ECDs or RBDs. The results demonstrated significant and moderate to high correlations between binding antibodies and surrogate neutralizing in multiple spike ECDs, e.g. R-square 0.793 for wild-type, 0.630 for Alpha, 0.537 for Beta, 0.536 for Gamma, 0.441 for Delta, and 0.624 for Omicron ECDs (Figure S3). As for RBDs, the relationships were significant with low to moderate correlations between binding antibodies and surrogate neutralizing, e.g. R-square 0.520 for wild-type, 0.503 for Alpha, 0.533 for Beta, 0.423 for Gamma, 0.391 for Delta variants, and 0.010 in omicron RBD (Figure S4).
Discussion
SARS-CoV-2 variants have demonstrated more significant contagion and pathogenicity than their original counterparts [Citation15]. KT faces heightened infection risks due to suppressed immune systems, and these variants might be less susceptible to vaccine-induced protection. Our research sought to chart the antibody responses of KT post-COVID-19 vaccination. The study employed the CoVariant microarrays, incorporating both ECD and RBD spike protein domains, to provide an in-depth analysis of humoral immunity post-two vaccine doses for KT. Although immune profiling showed a marked decline in surrogate neutralizing across various variants in both KT-AZ2 and KT-M2, the KT-M2 still outperformed the KT-AZ2 across many variants.
To counteract potential rejections in KT, patients are often prescribed long-term immunosuppressive drugs [Citation16,Citation17]. The prevalent immunosuppressive medicines are Rituximab, Tacrolimus, and others [Citation18–20]. Tacrolimus plays a pivotal role in staving off acute transplant rejections by inhibiting T-cell activities. Previous studies have underlined the correlation between T cells and neutralizing antibodies [Citation21–23]. Our observations further spotlight a notable reduction in the neutralizing capability among KT on Tacrolimus, a trend mirrored in other studies [Citation24,Citation25].
The immune response magnitude among KT can differ based on the type of immunosuppressive therapy, with only 4–6% showcasing antibody development post-vaccination [Citation26–28]. A study showed that KT received one dose of the mRNA vaccine for the mRNA vaccine, and seroconversion was extremely low. Nearly 40% of patients were unresponsive after a dose of the mRNA vaccine but developed antibodies after a second dose [Citation29]. Notably, the homologous mRNA vaccine regimen displayed superior safety and tolerance compared to its heterologous vector-based vaccination strategy [Citation30]. In this study, we focused on two doses of mRNA-1273 and AZD1222 in KT. To further point out the subjects with negative vaccine responses, we summarized the negative surrogate neutralizing against multiple spike ECDs in both KT and HC (Table S3). Compared to HC-AZ2, KT-AZ2 had more negative individuals on surrogate neutralizing activity against Beta and Gamma ECDs. There were no significant differences between HC-M2 and KT-M2. Therefore, the seroconversion in KT after homologous mRNA-1273 was quite successful.
The CoVariant array facilitates high-throughput antibody testing against various SARS-CoV-2 variants. The findings indicate that kidney transplant (KT) status impacts the vaccine responses, especially to the emergent SARS-CoV-2 variants. Since KT patients are more vulnerable to SARS-CoV-2 infections, administrating vaccines and monitoring vaccine responses are advised. Future endeavours should involve long-term studies to evaluate the immune response of kidney transplant recipients post-SARS-CoV-2 vaccination, offering valuable insights into sustained immunity. Additionally, investigating the efficacy of booster types or doses will provide more insights for KT. As global variants continue to evolve, such high throughput platforms are crucial for guiding clinical decisions and responding to the dynamic landscape of the pandemic.
Author contributions
P.-X.D., S.-S.C., T.-S.H., P.-S.T., and G.-D.S. performed the experimental work. P.-X.D., S.-S.C., H.-C.S., and G.-D.S. contributed to the manuscript preparation. G.-D.S. contributed expertise and supervision to the entire project.
20240425 Supplementary data R7.docx
Download MS Word (546 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated in this study are available from the corresponding author upon reasonable request.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21505594.2024.2351266.
Additional information
Funding
References
- Tonelli M, Wiebe N, Knoll G, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11(10):2093–8. doi: 10.1111/j.1600-6143.2011.03686.x
- Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382(25):2475–2477. doi: 10.1056/NEJMc2011117
- Jager KJ, Kramer A, Chesnaye NC, et al. Results from the ERA-EDTA registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98(6):1540–1548. doi: 10.1016/j.kint.2020.09.006
- Caillard S, Chavarot N, Francois H, et al. Is COVID-19 infection more severe in kidney transplant recipients? Am J Transplant. 2021;21(3):1295–1303. doi: 10.1111/ajt.16424
- Babu TM, Kotton CN. Immunizations in chronic kidney disease and kidney transplantation. Curr Treat Options Infect Dis. 2021;13(2):47–65. doi: 10.1007/s40506-021-00248-7
- Wang SC, Rai CI, Chen YC. Challenges and recent advancements in COVID-19 vaccines. Microorganisms. 2023;11(3):11. doi: 10.3390/microorganisms11030787
- Rincon-Arevalo H, Choi M, Stefanski AL, et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6(60):6. doi: 10.1126/sciimmunol.abj1031
- Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131(14):131. doi: 10.1172/JCI150175
- Caillard S, Thaunat O. COVID-19 vaccination in kidney transplant recipients. Nat Rev Nephrol. 2021;17(12):785–787. doi: 10.1038/s41581-021-00491-7
- Kuo HC, Kuo KC, Du PX, et al. Profiling humoral immunity after mixing and matching COVID-19 vaccines using SARS-CoV-2 variant protein microarrays. Mol & Cell Proteomics. 2023;22(4):100507. doi: 10.1016/j.mcpro.2023.100507
- Su WY, Du PX, Santos HM, et al. Antibody profiling in COVID-19 patients with different severities by using spike variant protein microarrays. Anal Chem. 2022;94(17):6529–6539. doi: 10.1021/acs.analchem.1c05567
- Ho TS, Du PX, Su WY, et al. Development of SARS-CoV-2 variant protein microarray for profiling humoral immunity in vaccinated subjects. Biosens Bioelectron. 2022;204:114067. doi: 10.1016/j.bios.2022.114067
- Du P-X, Chou Y-Y, Santos HM, et al. Development and application of human coronavirus protein microarray for specificity analysis. Anal Chem. 2021;93(21):7690–7698. doi: 10.1021/acs.analchem.1c00614
- Du PX, Chou YY, Santos HM, et al. Development and application of human coronavirus protein microarray for specificity analysis. Anal Chem. 2021;93(21):7690. doi: 10.1021/acs.analchem.1c00614
- Natekar JP, Pathak H, Stone S, et al. Differential pathogenesis of SARS-CoV-2 variants of concern in human ACE2-expressing mice. Viruses. 2022;14(6): 1139. doi: 10.3390/v14061139
- Alexander J, Liu Z, Eng KY, et al. P75 Vaccine-induced antibody responses against influenza are diminished in infliximab and tofacitinib-treated IBD patients and correlate with responses to COVID-19 vaccination. Gut. 2023;72:A88–A.
- Friedman MA, Curtis JR, Winthrop KL. Impact of disease-modifying antirheumatic drugs on vaccine immunogenicity in patients with inflammatory rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80(10):1255–1265. doi: 10.1136/annrheumdis-2021-221244
- Parlakpinar H, Gunata M. Transplantation and immunosuppression: a review of novel transplant-related immunosuppressant drugs. Immunopharmacol Immunotoxicol. 2021;43(6):651–665. doi: 10.1080/08923973.2021.1966033
- Benotmane I, Gautier-Vargas G, Cognard N, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99(6):1498–1500. doi: 10.1016/j.kint.2021.04.005
- Rozen-Zvi B, Yahav D, Agur T, et al. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin Microbiol Infect. 2021;27(8):.e1173.1–.e.4. doi: 10.1016/j.cmi.2021.04.028
- Belik M, Liedes O, Vara S, et al. Persistent T cell-mediated immune responses against omicron variants after the third COVID-19 mRNA vaccine dose. Front Immunol. 2023;14:1099246. doi: 10.3389/fimmu.2023.1099246
- Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23(2):186–193. doi: 10.1038/s41590-021-01122-w
- Sahin U, Muik A, Vogler I, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595(7868): 572–577. doi: 10.1038/s41586-021-03653-6
- Akimoto S, Onoe T, Morimoto H, et al. Analysis of acquisition of COVID-2019 neutralizing antibodies in organ transplant recipients. Transplant Proc. 2023;55(4):815–819. doi: 10.1016/j.transproceed.2023.04.003
- Bertrand D, Candon S. Protection against SARS-CoV-2 variants with COVID-19 vaccination in kidney transplant recipients. Clin J Am Soc Nephrol. 2022;17(1):3–5. doi: 10.2215/CJN.14881121
- Chavarot N, Ouedrani A, Marion O, et al. Poor anti-SARS-CoV-2 humoral and T-cell responses after 2 injections of mRNA vaccine in kidney transplant recipients treated with belatacept. Transplantation. 2021;105(9):e94–e5. doi: 10.1097/TP.0000000000003784
- Danthu C, Hantz S, Dahlem A, et al. Humoral response after SARS-CoV-2 mRNA vaccination in a cohort of hemodialysis patients and kidney transplant recipients. J Am Soc Nephrol. 2021;32(9):2153–2158. doi: 10.1681/ASN.2021040490
- Ou MT, Boyarsky BJ, Chiang TPY, et al. Immunogenicity and reactogenicity after SARS-CoV-2 mRNA vaccination in kidney transplant recipients taking belatacept. Transplantation. 2021;105(9):2119–2123. doi: 10.1097/TP.0000000000003824
- Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489
- Reindl-Schwaighofer R, Heinzel A, Mayrdorfer M, et al. Comparison of SARS-CoV-2 antibody response 4 weeks after homologous vs heterologous third vaccine dose in kidney transplant recipients: a randomized clinical trial. JAMA Intern Med. 2022;182(2):165–171. doi: 10.1001/jamainternmed.2021.7372

