ABSTRACT
RhoA, Rac1 and CDC42 are small G proteins that play a crucial role in regulating various cellular processes, such as the formation of actin cytoskeleton, cell shape and cell migration. Our recent results suggest that MLL is responsible for maintaining the balance of these small Rho GTPases. MLL depletion affects the stability of Rho GTPases, leading to a decrease in their protein levels and loss of activity. These changes manifest in the form of abnormal cell shape and disrupted actin cytoskeleton, resulting in reduced cell spreading and migration. Interestingly, their chaperone protein RhoGDI1 but not the Rho GTPases, is under the direct transcriptional regulation of MLL. Here, we comment on the possible implications of these observations on the signalling by Rho GTPases protein network.
Actin cytoskeleton formation is under direct control of Rho GTPases, RhoA, Rac1 and CDC42. Rho GTPases are small G proteins that play a significant role in regulating the formation of the actin cytoskeleton, which is responsible for cell shape, cell migration and cytokinesis. The activity of Rho GTPases is maintained by Rho-specific guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs), while their stability is maintained by a protein called as guanine nucleotide dissociation inhibitor (RhoGDI) [Citation1]. RhoGDIs act as chaperones, masking Rho GTPases prenyl group and inhibiting their activation and inactivation cycling [Citation2]. So far, the only regulatory mechanism known for controlling RhoGDI activity is by modulating affinity of Rho GTPases and RhoGDI towards each other by post-translational modifications. We have recently shown that mixed lineage leukemia (MLL or KMT2A) plays a role in the regulation of small Rho GTPases via transcriptional regulation of RhoGDI1 (or ARHGDIA). In contrast to the local effect exerted by the Rho GTPase–RhoGDI interaction regulation via post-translational modifications, this regulation has far reaching affects as discussed below.
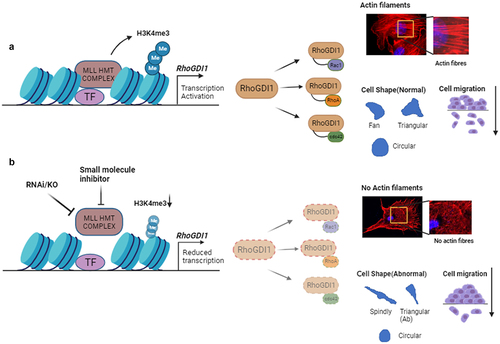
In brief, in our study, we observed that the loss of MLL diminishes the cellular pool of RhoA, Rac1 and CDC42 proteins, leading to abnormal cell shape and impaired cell spreading and migration, due to a disrupted actin cytoskeleton () [Citation3]. Depletion of MLL reduced the stability and activity of Rho GTPases, and this was dependent on its catalytic SET domain, but it appears that these Rho GTPases were not direct targets of MLL transcriptionally. Rather, MLL regulated the transcript levels and hence protein levels of the RhoGDI1. We demonstrated that MLL binds directly to RhoGDI1 promoter to deposit histone 3 lysine 4 (H3K4) trimethylation marks. Overexpression of RhoGDI1 in MLL-depleted cells rescued some of the defects observed above. We tested the therapeutic potential of MLL inhibition by using the triple-negative breast cancer (TNBC) cell line, MDA-MB-231, which has high expression of RhoGDI1, demonstrating that depletion or inhibition of MLL using small molecules reduced tumour progression in nude mice.
Figure 1. MLL regulates transcription of RhoGDI1 to regulate the levels of Rho GTPases. (a) MLL HMT complex is involved in the active transcription of RhoGDI1. RhoGDI1 acts as a chaperone for the small Rho GTPases, RhoA, Rac1 and CDC42, ensuring their stability in the cell. These Rho GTPases regulate the cell shape, cell migration and actin cytoskeleton formation. (b) Inhibition of MLL using RNAi or small molecule inhibitors in cells reduces the transcription of RhoGDI1, which further affects the stability and function of small GTPases and results in the abnormal cell shape and cell migration, most likely as a consequence of reduced actin cytoskeleton.
How does MLL regulate cell shape and maintain actin cytoskeleton?
Cell shape is governed by the interaction of cells with each other and with the extracellular matrix. Out of the three cytoskeletal components, the actin filaments, microtubules and intermediate filaments, actin cytoskeleton is a major cell shape determiner. Profound changes in cell shape due to changes in microenvironment are well known and largely depend on the dynamic nature of actin cytoskeleton [Citation4]. The signalling by Rho GTPases protein network drives the formation and maintenance of actin cytoskeleton. Microinjections of constitutively active Rho GTPases, RhoA, Rac1 and CDC42 gave rise to distinct actin containing structures stress fibres, lamellipodia and filopodia, respectively [Citation5–7]. However, formation of these structures is more complex and often requires activities of more than one Rho GTPase at a given point of time. Rho GTPases, as described earlier, are known to activate two different types of actin nucleators, WASP/WAVE complex and diaphanous-related formins, that synthesize actin polymers. WASP/WAVE complex proteins stimulate the actin polymerization through the Arp2/3 complex, which uses the existing actin filament to form a new branch of actin filament, whereas the formins (Dia1, −2 and −3) induce the formation of un-branched actin filaments [Citation8]. Our results indicated that U-2OS cells devoid of MLL showed dramatic reduction in actin stress fibres, similar to what is observed upon depletion of RhoA, CDC42 and to some extent Rac1. Although the pathways were not experimentally dissected, the loss of actin stress fibres and active levels of Rho GTPases in MLL-depleted cells is so striking that it is safe to speculate that both pathways are affected here.
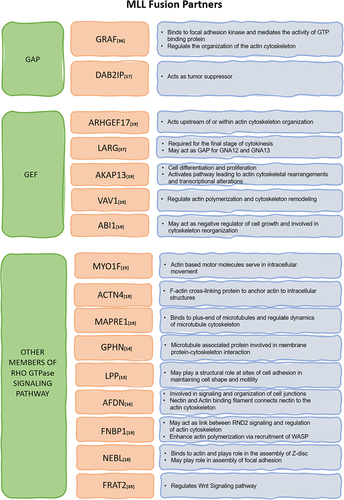
We observed that MLL-deficient cells displayed shape defects. Previous siRNA screening in MDA-MB-231 cells depleted of various proteins involved in Rho GTPase signalling displayed numerous phenotypes but particularly exhibited five peculiar cell shapes, namely ‘elongated or spindly’, ‘large spread’, ‘triangular’, ‘fan shaped’ and ‘small round’ [Citation9]. Notably, treating U-2OS cells with MLL siRNA exhibited prominently two of the above five shapes, spindly shape, i.e. elongated cells having two protrusions and triangular shape with cells having three protrusions, with one protrusion anomalously elongated (). These results indicated MLL’s involvement in Rho GTPase signalling pathway. We also observed cells with a large number of filopodia after MLL depletion. Previous literature indicates that explosive filopodia formation occurs due to a number of reasons including depletion of ARP2/3 complex [Citation10]. MLL has also been shown to affect the expression of a sizable number of proteins involved in regulation of actin cytoskeleton [Citation11,Citation12]. Thus, there is an overlap between MLL targets and Rho GTPase signalling pathway. Additionally, at least 18 of all the leukaemogenic fusion partners of MLL are implicated in Rho GTPases signalling () [Citation13–19]. A significant number of fusion partners belong to the GEFs and GAPs categories, which have a direct role in regulating the functions of Rho GTPases [Citation20]. This emphasizes the crucial cross talk between MLL and Rho GTPase signalling in cancer.
Figure 2. MLL fusion partners from Rho GTPase signalling pathway – many Rho GTPase regulators like GAPs and GEFs as well as other proteins functioning in Rho GTPase signalling act as fusion partners for MLL as depicted in the table. They may dysregulate the cell cytoskeleton by changing the expression or perturbing functions of genes that play important role in actin, microtubule and intermediate filament regulation.
Cell spreading on extracellular matrices is another complex process which depends on activity of Rho GTPases. Spreading of a cell takes place, when it makes contacts with the extracellular milieu of tissues through integrin called as the nascent focal adhesions, which after maturation attach with actin filaments and microtubules [Citation21]. As a consequence of maturation, downstream signalling leads to a cascade after which more actin polymerization happens, resulting in spreading of cells on substrates. Impairment in Rho GTPase signalling in terms of spreading manifests itself by reduction of the area occupied by spread cells on extracellular matrix coated surface. In line with this fact, MLL-depleted cells occupied significantly lesser area as compared to the controls. Moreover, this reduction of area of spread of cells was again similar to that observed upon depletion of Rho GTPases. Taken together, our results indicated that MLL maintained actin cytoskeleton and its loss gave rise to numerous defects which phenocopied loss of Rho GTPases and, together, pointed to a central role of MLL in regulation of Rho GTPases.
MLL maintains homoeostasis of Rho GTPases via the RhoGDI1 axis
We show that MLL is responsible for maintaining the stability of RhoA, Rac1 and CDC42 proteins. Loss of MLL caused a reduction of total and active protein levels of Rho GTPases. This observation is solely a post-transcriptional phenomenon, as the relative expression of all of these genes remained unaltered after MLL depletion. Stability of these Rho GTPases is maintained by GDIs. Three Rho GDIs are known to express in mammals, RhoGDI1, RhoGDI2 and RhoGDI3. RhoGDI1 is ubiquitously expressed and has highest affinity for the above-mentioned GTPases. RhoGDI2 is expressed only in haematopoietic cells and binds with a lower affinity to Rho GTPases, whereas RhoGDI3 is mostly functional in golgi and interacts with RhoB and RhoG [Citation22]. RhoGDI1 is the most studied among GDIs, and its knockdown has been shown to reduce protein stability of RhoA, Rac1 and CDC42 [Citation23]. Our experiments show that knockdown of MLL causes reduction in relative gene expression and, thus, the overall protein levels of RhoGDI1. MLL knockout cells also showed reduced protein levels of RhoGDI1.
Interestingly, it has been observed that excessive RhoGDI1 can be harmful to certain cells, but conversely, it is also frequently found overexpressed in many cancers and is linked to the cancer cell’s ability to metastasize, invade and resist drugs. Taken together, these observations suggest that maintaining the correct level of RhoGDI1 expression in cells is of utmost importance. Regulation of RhoGDI activities is mostly studied in terms of modulating its binding with Rho GTPases. Modulation of RhoGDI binding to Rho GTPases is brought by its interactions with lipids and proteins or post-translational modifications on both RhoGDIs and Rho GTPases [Citation22]. The transcriptional regulation of RhoGDI expression is still not well understood. Besides our study, decrease of H3K4 trimethylation on Arhgdib (ortholog of human RhoGDI2) promoter and concomitant reduction in its expression has been reported in MLL knockout mouse embryonic fibroblasts (MEFs) [Citation24]. In this study however, the authors did not find any significant changes in methylation status at the promoter of Arhgdia (ortholog of human RhoGDI1) [Citation24]. It can be concluded that MLL regulates RhoGDIs differently in humans and mice. As not much is known about the function of mouse Arhgdib, further experiments are required to understand whether the difference in this MLL-mediated regulation has functional implications.
Various MLLs proteins regulate cell motility
Rho GTPases, as discussed above, play a very prominent role in actin cytoskeleton formation and cell migration. RhoA, Rac1 and CDC42 all give rise to different actin-rich structures essential for movement of cells and perform different tasks during migration. Numerous reports have identified MLL and its core-complex proteins, such as WDR5 and Dpy30, crucial for migration [Citation25–27]. In addition, WDR5 has been shown to affect the plasticity of nucleus, which gives selective advantage to the cells migrating in 3D environments [Citation28]. Moreover, MLL depletion causes downregulation of a considerable number of genes that play role in regulation of actin cytoskeleton [Citation11,Citation12]. MLL2 knockdown has also been shown to downregulate many genes that are essential for cell spreading, adhesion and maintaining polarity, resulting in spreading and migration defects [Citation29]. Contrasting results were observed in oesophageal squamous cell carcinoma where overexpression of MLL3 caused migration and proliferation defects [Citation30]. Collectively, these studies demonstrate how MLL family members and their complex proteins affect cancer cell motility and survival through a variety of targets. Our findings underscore MLL’s role in regulating actin dynamics and cell migration through the stabilization of Rho GTPases, which ultimately affects the actin cytoskeleton. Various structures of actin form depending on the activities of Rho GTPases and determine speed and direction of cell movement. In a moving cell, these structures form, reorient and disintegrate rapidly giving rise to visual dynamics of actin. These structures form and push against the plasma membrane and determine the shape of cells [Citation31]. In our experiments, live U-2OS cells, in a steady state, displayed well-formed lamellipodia with dynamic ruffling. In contrast, the MLL-depleted cells showed a sharp reduction of actin dynamics. This result confirmed the role of MLL protein in regulating actin dynamics. We also observed reduction in cell migration upon MLL loss, which could not be alleviated even with external stimulus like thrombin which can increase cell migration [Citation32,Citation33]. The overall reduction in migration displayed by MLL-depleted cells can be attributed to the collective effect of all the phenotypes we observed upon loss of MLL in the cells. However, we do report a sizable but not complete rescue of cell migration in MLL-depleted cells by overexpressing RhoGDI1. Overexpressing of RhoGDI1 in control cells had opposing effects on its migration as its overexpression has been reported to cause migration defects [Citation34]. This yet again indicates that the appropriate expression of RhoGDI1 within cells is paramount.
Relationship between MLL fusion proteins and Rho GTPase signalling in driving cancer
Leukaemia caused by chromosomal translocations in mll gene has been very well documented. Fusion partners of MLL in 90% of acute myeloid leukaemia (AML) and 70% acute lymphoid leukaemia (ALL) cases are proteins that function in superelongation complex of RNA Polymerase II [Citation35]. Thus, the fusion proteins hijack the transcription elongation and deregulate the transcription of target proteins which is thought to lead to the development of leukaemia. It is important to note that various MLL fusion proteins deregulate Rho GTPase signalling pathway. For instance, Rac1 activation via MLL-ENL fusion seems to be triggered by Frat2, which takes part in Wnt signalling and interacts with GSK-3β. It has been suggested that this activation promotes neoplastic transformation and cell proliferation, and thus, the MLL fusion protein, Frat2 and Rac activation together drives cancer [Citation36]. In addition to the proteins involved in transcription, many MLL fusion partners belong to the GEFs and GAPs categories. For instance, the human GRAF (GTPase Regulator Associated with Focal Adhesion Kinase) gene encodes a protein that acts as a GAP of Rho. Interestingly, GRAF gene translocation and fusion with MLL disrupt both alleles of GRAF in some patients, leading to myelodysplastic syndrome/acute myeloid leukaemia (MDS/AML) with the deletion of genetic material on chromosome 5q. The fusion of GRAF with MLL might lead to abnormal activation of Rho GTPase signalling pathways, contributing to the oncogenic transformation of haematopoietic cells. This could probably enhance the invasive behaviour of leukaemic cells and promote disease progression [Citation37]. MLL fused with the Rho GEFs has also been shown to cause malignancy. A gene located at 11q23 that encodes LARG (leukaemia-associated Rho GEF) protein has been found to be fused with the mll gene in cases of AML. The GEF-encoding gene fused with MLL causes abnormal Rho GTPase activation and haematopoietic cell oncogenic transformation [Citation38]. Components of the Rho GTPase signalling pathway, including GAPs and GEFs, have emerged as potential therapeutic targets in cancer treatment. Understanding their mode of action could offer insights into targeted therapeutic strategies for AML.
MLL inhibitors as a potential therapeutic for RhoGDI1 cancers
[Citation35]In addition to the leukaemia, recent study indicates that fusion proteins containing MLL can drive sarcomas as well [Citation39]. Further, a large number of solid tumours have also been found to contain mutants of MLL not formed due to chromosomal rearrangements. These solid tumours are derived from a plethora of cancers like lung, breast, colon endometrial and bladder cancers. Many of the mutations are non-sense resulting in truncated MLL protein [Citation40]. This highlights the importance of MLL in cellular homoeostasis and its role in driving cancer.
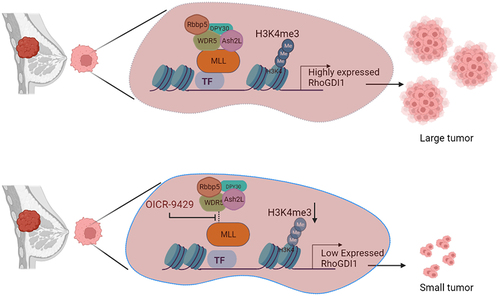
The tumour cells in triple-negative breast cancers do not express receptors for oestrogen, progesterone or ERBB2 protein. These cancers form at least 15% of total breast cancers cases. TNBCs are characterized as displaying high proliferative rate, greater brain metastases formation propensity with early onset and poor prognosis [Citation41]. TNBCs are resistant to conventional hormone treatment regime. Therefore, currently there is no definite treatment available for treating these cancers. Tissues and cell lines obtained from specific breast cancers display increased levels of RhoGDI1 expression and, as a result, exhibit high metastatic activity [Citation42]. One of these triple-negative breast cancer cell lines that shows high RhoGDI1 expression is MDA-MB-231 and is commonly used as an in vivo model for breast cancer research. Our xenograft studies indicate that MLL and RhoGDI1 depletion in MDA-MB-231 cells results in smaller tumours in nude mice. Even though MLL fusion proteins cause significant disruption to the gene expression profile of the transformed cells, the maintenance of leukaemogenesis still relies on one functional allele of MLL. As a consequence, targeting the catalytic activity of MLL in the transformed cells has led to significant progress in cancer treatment in mouse models [Citation43] Consequently, several small molecule inhibitors of both peptide and non-peptide origin have been identified that aim to disrupt the MLL/WDR5 interaction and impede its methyl transferase activity [Citation44] We decided to use OICR-9429, a non-peptide inhibitor of MLL-WDR5 interaction that has previously demonstrated efficacy in treating different types of cancers in mouse models [Citation45,Citation46]. It is important to note here that MDA-MB-231 carries wild type MLL. However, by treating MDA-MB-231 cells with OICR-9429, we observed a substantial decrease in the relative gene expression of RhoGDI1 (). Additionally, intravenous administration of OICR-9429 to nude mice significantly reduced the size of xenografts formed by MDA-MB-231 cells, providing further support for the effectiveness of this treatment. Our findings suggest that the catalytic activity of MLL can be a novel target for treating TNBC using small molecule inhibitors. We also believe that a similar approach could be utilized for treating other types of cancers that display overexpression of Rho GTPases and RhoGDI.
Figure 3. Inhibition of MLL can reduce tumours in xenografts. MDA-MB-231 cells are triple-negative breast cancer cells with high RhoGDI1 expression, which promotes tumour formation. MLL with its core-complex proteins promotes the transcription of RhoGDI1 by methylating the RhoGDI1 promoter. Treatment with OICR-9429, a non-peptide inhibitor of MLL-WDR5 interaction, reduces the activity of MLL at RHOGDI1 promoter, thus reducing the expression of RhoGDI1 gene. This results in tumour regression. Thus, inhibition of MLL can be used as a potential therapeutic in tumours showing high expression of RhoGDI1.
Author contributions
A.N.C. and S.T. wrote the manuscript. S.G. designed the figures and edited the manuscript.
Acknowledgments
We thank Pushti Gandhi for her inputs to the manuscript. A.N.C. is recipients of Junior and Senior Research Fellowships of the University Grants Commission, India, toward the pursuit of a PhD degree of the Manipal University. S.G. is recipients of Junior Research Fellowship of University Grant Commission, India, toward the pursuit of a PhD degree of Regional Centre for Biotechnology.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Additional information
Funding
References
- Mosaddeghzadeh N, Ahmadian MR. ’the rho family gtpases: mechanisms of regulation and signaling’. Cells. 2021 Jul 01;10(7):1831. MDPI. doi: 10.3390/cells10071831
- Mosaddeghzadeh N, Kazemein Jasemi NS, Majolée J, et al. Electrostatic forces mediate the specificity of RHO GTPase-GDI interactions. Int J Mol Sci. 2021 Nov;22(22):12493. doi: 10.3390/ijms222212493
- Chinchole A, Lone KA, Tyagi S. MLL regulates the actin cytoskeleton and cell migration by stabilising Rho GTPases via the expression of RhoGDI1. J Cell Sci. 2022 Oct;135(20): doi: 10.1242/JCS.260042
- Small JV, Rottner K, Hahne P, et al. Visualising the actin cytoskeleton. Microsc Res Tech. 1999;47(1):3–17. doi:10.1002/(SICI)1097-0029(19991001)47:1<3:AID-JEMT2>3.0.CO;2-2
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7
- Ridley AJ, Paterson HF, Johnston CL, et al. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8
- Nobes CD, Hall A. Rho, rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81(1):53–62. doi: 10.1016/0092-8674(95)90370-4
- Tojkander S, Gateva G, Lappalainen P. Actin stress fibers – assembly, dynamics and biological roles. 2012. doi: 10.1242/jcs.098087
- Pascual-Vargas P, Cooper S, Sero J, et al. RNAi screens for Rho GTPase regulators of cell shape and YAP/TAZ localisation in triple negative breast cancer. Sci Data. 2017;4(1):1–13. doi: 10.1038/sdata.2017.18
- Dimchev V, Lahmann I, Koestler SA, et al. Induced Arp2/3 complex depletion increases FMNL2/3 formin expression and filopodia formation. Front Cell Dev Biol. 2021;9:634708. DOI:10.3389/fcell.2021.634708
- Artinger EL, Mishra BP, Zaffuto KM, et al. An MLL-dependent network sustains hematopoiesis. Proc Natl Acad Sci U S A. 2013;110(29):12000–12005. doi: 10.1073/pnas.1301278110
- Malik KK, Sridhara SC, Lone KA, et al. MLL methyltransferases regulate H3K4 methylation to ensure CENP-A assembly at human centromeres. PLoS Biol. 2023 Jun;21(6):e3002161. doi: 10.1371/journal.pbio.3002161
- Dahéron L, Veinstein A, Brizard F, et al. Human LPP gene is fused to MLL in a secondary acute leukemia with a t(3;11) (q28;q23). Genes Chromosomes Cancer. 2001;31(4):382–389. doi: 10.1002/gcc.1157
- Eguchi M, Eguchi-Ishimae M, Seto M, et al. GPHN, a novel partner gene fused to MLL in a leukemia with t(11;14)(q23;q24). Genes Chromosomes Cancer. 2001;321:212–221. doi: 10.1002/gcc.1185
- Duhoux FP, Ameye G, Libouton J-M, et al. The t(11;19(q23;p13) fusing MLL with MYO1F is recurrent in infant acute myeloid leukemias. Leuk Res. 2011;35(9):e171–e172. doi: 10.1016/j.leukres.2011.04.022
- Manara E, Baron E, Tregnago C, et al. MLL-AF6 fusion oncogene sequesters AF6 into the nucleus to trigger RAS activation in myeloid leukemia. Blood. 2014;124(2):263–272. doi: 10.1182/blood-2013-09-525741
- Von Bergh ARM, Wijers PM, Groot AJ, et al. Identification of a novel RAS GTPpase-activating protein (RASGAP) gene at 9q34 as an MLL fusion partner in a patient with De Novo acute leukemia. Genes Chromosomes Cancer. 2004 Apr;39(4):324–334. doi: 10.1002/gcc.20004
- Cóser VM, Meyer C, Basegio R, et al. Nebulette is the second member of the nebulin family fused to the MLL gene in infant leukemia. Cancer Genet Cytogenet. 2010 Apr;198(2):151–154. doi: 10.1016/j.cancergencyto.2009.12.013
- Marschalek R. ‘Classification of mixed-lineage leukemia fusion partners predicts additional cancer pathways’. Ann Lab Med. 2016 Mar 01;36(2):85–100. Seoul National University. doi: 10.3343/alm.2016.36.2.85
- Meyer C, Burmeister T, Gröger D, et al. The MLL recombinome of acute leukemias in 2017. Leukemia. 2018 Feb;32(2):273–284. doi: 10.1038/leu.2017.213
- Burridge K, Guilluy C. Focal adhesions, stress fibers and mechanical tension. Exp Cell Res. 2016;343(1):14–20. doi: 10.1016/j.yexcr.2015.10.029
- Garcia-Mata R, Boulter E, Burridge K. The “invisible hand”: regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol. 2011;12(8):493–504. doi:10.1038/nrm3153
- Boulter E, Garcia-Mata R, Guilluy C, et al. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol. 2010;12(5):477–483. doi: 10.1038/ncb2049
- Wang P, Lin C, Smith ER, et al. Global analysis of H3K4 methylation defines MLL family member targets and points to a role for MLL1-Mediated H3K4 methylation in the regulation of transcriptional initiation by RNA polymerase II. Mol Cell Biol. 2009 Nov;29(22):6074–6085. doi: 10.1128/mcb.00924-09
- Zhang Y, Ji T, Ma S, et al. RETRACTED: MLL1 promotes migration and invasion of fibroblast-like synoviocytes in rheumatoid arthritis by activating the TRIF/NF-κB signaling pathway via H3K4me3 enrichment in the TLR4 promoter region. Int Immunopharmacol. 2020;82(71):106220. doi:10.1016/j.intimp.2020.106220
- Malek R, Gajula RP, Williams RD, et al. TWIST1-WDR5-hottip regulates Hoxa9 chromatin to facilitate prostate cancer metastasis. Cancer Res. 2017;77(12):3181–3193. doi: 10.1158/0008-5472.CAN-16-2797
- He FX, Zhang LL, Jin PF, et al. DPY30 regulates cervical squamous cell carcinoma by mediating epithelial-mesenchymal transition (EMT). Onco Targets Ther. 2019;12:7139–7147. doi:10.2147/OTT.S209315
- Wang P, Dreger M, Madrazo E, et al. WDR5 modulates cell motility and morphology and controls nuclear changes induced by a 3D environment. Proc Natl Acad Sci U S A. 2018;115(34):8581–8586. doi: 10.1073/pnas.1719405115
- Issaeva I, Zonis Y, Rozovskaia T, et al. Knockdown of ALR (MLL2) reveals ALR target genes and leads to alterations in cell adhesion and growth. Mol Cell Biol. 2007;27(5):1889–1903. doi: 10.1128/mcb.01506-06
- Xia M, Xu L, Leng Y, et al. Downregulation of MLL3 in esophageal squamous cell carcinoma is required for the growth and metastasis of cancer cells. pp. 605–613. 2015. doi: 10.1007/s13277-014-2616-3
- Blanchoin L, Boujemaa-Paterski R, Sykes C, et al. Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev. 2014;94(1):235–263. doi: 10.1152/physrev.00018.2013
- Azim AC, Barkalow K, Chou J, et al. Activation of the small GTPases, rac and cdc42, after ligation of the platelet PAR-1 receptor. Blood. 2000 Feb;95(3):959–964. doi: 10.1182/blood.V95.3.959.003k22_959_964
- van Nieuw Amerongen GP, van Delft S, Vermeer MA, et al. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res. 2000 Aug;87(4):335–340. doi: 10.1161/01.res.87.4.335
- Yu J, Zhang D, Liu J, et al. RhoGDI SUMOylation at Lys-138 increases its binding activity to Rho GTPase and its inhibiting cancer cell motility. J Biol Chem. 2012;287(17):13752–13760. doi: 10.1074/jbc.M111.337469
- Mohan M, Lin C, Guest E, et al. Licensed to elongate: a molecular mechanism for MLL-based leukaemogenesis. Nat Rev Cancer. 2010;10(10):721–728. doi: 10.1038/nrc2915
- Walf-Vorderwülbecke V, de Boer J, Horton SJ, et al. Frat2 mediates the oncogenic activation of rac by MLL fusions. Blood. 2012 Dec;120(24):4819–4828. doi: 10.1182/blood-2012-05-432534
- Borkhardt A, Bojesen S, Haas OA, et al. The human GRAF gene is fused to MLL in a unique t(5;11)(q31;q23) and both alleles are disrupted in three cases of myelodysplastic syndrome/acute myeloid leukemia with a deletion 5q. Proc Nat Acad Sci. 2000;97(16):9168–9173. [Online]. Available: www.pnas.org
- Kourlas PJ, Strout, MP, Becknell, B, et al. Identification of a gene at 11q23 encoding a guanine nucleotide exchange factor: evidence for its fusion with MLL in acute myeloid leukemia. Proc Natl Acad Sci USA. 2000;97(5):2145–2150. doi: 10.1073/pnas.040569197
- Yoshida A, Arai Y, Tanzawa Y, et al. KMT2A (MLL) fusions in aggressive sarcomas in young adults. Histopathology. 2019;75(4):508–516. doi: 10.1111/his.13926
- Rao RC, Dou Y. Hijacked in cancer: The KMT2 (MLL) family of methyltransferases. Nat Rev Cancer. 2015;15(6):334–346. doi: 10.1038/nrc3929
- Dangi CBS, Firodiya A. Triple-negative breast cancer and it’s therapeutic options. Int J Pharma Bio Sci. 2012;3(2):130–160.
- Cho HJ, Kim JT, Baek KE, et al. ‘Regulation of Rho GTPases by RhoGDIs in human cancers’. Cells. 2019 Sep 01;8(9):1037. MDPI. doi: 10.3390/cells8091037
- Thiel AT, Blessington P, Zou T, et al. MLL-AF9-induced leukemogenesis requires coexpression of the wild-type MLL Allele. Cancer Cell. 2010 Feb;17(2):148–159. doi: 10.1016/j.ccr.2009.12.034
- Ye X, Zhang R, Lian F, et al. The identification of novel small-molecule inhibitors targeting WDR5-MLL1 interaction through fluorescence polarization based high-throughput screening. Bioorg Med Chem Lett. 2019 Feb;29(4):638–645. doi: 10.1016/j.bmcl.2018.12.035
- Carugo A, Genovese G, Seth S, et al. In vivo functional platform targeting patient-derived xenografts identifies WDR5-myc association as a critical determinant of pancreatic cancer. Cell Rep. 2016 Jun;16(1):133–147. doi: 10.1016/j.celrep.2016.05.063
- Weinert T, Huwiler SG, Kung JW, et al. Structural basis of enzymatic benzene ring reduction. Nat Chem Biol. 2015 Aug;11(8):586–591. doi: 10.1038/nchembio.1849

