Abstract
Teak, known for its high-quality timber and economic value, holds significant potential as the major component of an agroforestry system to combat climate change and support sustainable development goals. The challenge of slow teak growth can be addressed by introducing fast-growing teak varieties amenable to vegetative propagation. Furthermore, the improvement of teak through somatic cell genetics, such as inducing polyploidy, can result in more vigorous, faster-growing, and drought-tolerant trees. While a report exists on a tetraploid teak accession with enhanced less water tolerance, information on the induction and characterization of tetraploid teak is lacking. In this study, we report the induction of polyploid teak by immersing 1 – 1.5 cm nodal stem segments and shoot tips obtained from teak shoot cultures in a modified MS liquid medium containing 5 − 30 µM oryzalin for one week, followed by subculture for 6 passages. The resulting shoots were acclimatized and grown in the nursery for 10 months. Based on flow cytometry for ploidy analysis of pre-selected plants having morphologies typical of polyploid followed by chromosome counting, we identified two tetraploid plants from one accession and 18 mixoploid teak plants from four accessions. Chromosome counting analysis confirmed that a diploid plant had a chromosome number of 2n = 2x = 36, tetraploid plant had a chromosome number of 2n = 4x = 72, and mixoploid plant exhibited both chromosome number of 2n = 2x = 36 and 2n = 4x = 72 in root tip cells. Morphological characterization revealed that tetraploid and mixoploid teak plants displayed typical polyploid characteristics, such as larger, thicker, and greener leaves, increased root diameter, increased cell size in root tip region, and larger stomata size but with reduced density. The development of tetraploid teak holds the potential to provide faster-growing and more drought-tolerant varieties, facilitating breeding efforts, including the development of triploid varieties.
Introduction
Teak (Tectona grandis L.f.) is a tree species belonged to the Lamiaceae and is found in tropical forests in India, Myanmar, Laos, Thailand, and Indonesia (Kertadikara and Prat Citation1995). This tree bears significant economic value due to its high-quality timber. Teak wood is known for its durability, strength, and resistance to insect pests (Gupta et al. Citation1980; Kertadikara and Prat Citation1995). Teak wood is primarily used in various construction applications, such as building houses, making ships and furniture (Srinivasan et al. Citation2012).
Conventional teak accessions grow very slowly, taking over 40 years to reach a harvestable size of 30–40 cm in diameter (Palanisamy et al. Citation2009). However, there have been commercially viable clones of fast-growing teak in Indonesia since the mid-1990s, introduced either through new initiatives by multinational corporations or selected from existing germplasms. Some of these new clones were introduced as part of plantation investment packages, while others were made available as planting material to the public. However, the initial enthusiasm for investing in teak plantations waned due to disparities between the promised and actual growth rates. More recently, the introduction of elite teak clones in Indonesia has garnered significant attention, as these plants exhibit rapid growth and produce straight boles. This new introduction is a relatively recent selection from Sabah, Malaysia (Goh and Monteuuis Citation2012). Eight clones from this selection were brought to Indonesia, commonly referred to as “Salomon teak.” This new introduction appears to align more closely with people’s expectations. Young trees, aged 5–8 years, exhibit qualities comparable to conventional teak trees at about 20 years of age, particularly in terms of physical attributes like modulus of elasticity and rupture, although they have a lower specific density (Pramasari et al. Citation2014).
These fast-growing, high-quality teak clones can make significant contributions to achieving Sustainable Development Goals in several aspects. The exceptional quality of these trees, when planted individually in small numbers, attracts higher prices, often in the form of cash, which can help lift people out of poverty. Their superior timber quality and high yields can provide the resources needed for building healthy houses, promoting overall well-being. Establishing teak plantations using these clones may result in shorter harvest cycles and increased yields, which can drive innovation and rejuvenation within the timber industry. Simultaneously, this approach can enhance and benefit land ecosystems. Teak agroforestry or any teak planting utilizing these fast-growing clones can be viewed as a potent strategy for mitigating climate change. This is achieved by harvesting and sequestering atmospheric carbon dioxide into durable, high-quality timber for houses and furniture that has a long-lasting impact. Our observations on a plantation of these clones, with a population density of 600 trees per hectare over 10 years, resulted in trees reaching a height of 12 meters and an average diameter of 30 centimeters. This setup has the potential to sequester 52 tonnes of carbon. This calculation is based on the harvested timber from 600 trees, each 8 meters long, with a wood diameter of 25 centimeters, a 50% specific density, and a 45% carbon content. This level of carbon sequestration is notably higher when compared to traditional teak plantations of 20 years, which yield 81 tonnes of carbon per hectare, including the root bole (Kongmeesup and Boonyanuphap Citation2019).
These elite teak clones could be further enhanced through somatic cell genetics, such as mutation breeding and chromosome doubling, aimed at obtaining tetraploid teak. Induced mutation of tissue-cultured shoots using gamma irradiation has been reported (Ahmad Parlaongan et al. Citation2022). Experiments on the induction of tetraploid teak have also been conducted by a few authors, but no tetraploid plants were successfully recovered (Rahayu Citation2014; Fauzan et al. Citation2017). A tetraploid teak accession has demonstrated increased tolerance to drought treatment in a greenhouse setting (Ridwan et al. Citation2018) however, the process by which this tetraploid teak clone was developed has not been reported.
Inducing tetraploidy in tree or timber species offers several advantages. Tetraploid plants often exhibit a faster growth rate and larger organs in timber trees (Zhang et al. Citation2023). Tetraploid plants can also produce timber with superior wood quality by increasing trunk diameter (Diallo et al. Citation2008). Additionally, tetraploidy induction reduces the number of inflorescences and seeds (Vleugels et al. Citation2014). It also decreases male and female fertility in timber trees by delaying flowering times (Oates et al. Citation2012), which can help reduce the invasiveness of the species in non-native ecosystems. Inducing tetraploidy can enhance genetic variation, which is useful for breeding programs aimed at developing new varieties or hybrids with specific traits (Madani et al. Citation2021). Tetraploid trees may also improve adaptability to environmental conditions, including tolerance to abiotic stress factors like drought (Xu et al. Citation2019) and resistance to biotic stress factors such as pests and diseases (Li et al. Citation2019). However, not all changes resulting from tetraploidy induction are beneficial. For instance, tetraploidy induction can lead to decreased photosynthetic rates and reduced synthesis and decomposition ability of carbohydrates (Xu et al. Citation2020).
This paper reports, for the first time, the successful induction of tetraploid teak in several accessions using oryzalin in in vitro shoot cultures. The tetraploidy of these plants was confirmed through various methods, including flow cytometry, determination of root tip chromosome numbers, measurement of root tip cell length, assessment of stomatal density and size, and evaluation of overall plant morphology.
Material and methods
Plant material
The plant materials used for polyploidy induction consisted of teak shoot cultures from in vitro cultures, aged 4–6 weeks, including 5 mutant clones, namely MK10, MK17, MK44, MK61, and MK71, and the KSP clone as a diploid control for flow cytometry analysis. The cultures were maintained in a semi-solid modified MS formulation (Murashige and Skoog Citation1962) containing (in mg L−1, except stated otherwise) modified macro salts (1200 NH4.NO3, 3030 KNO3, 65.99 CaCl2.2H2O, 170 KH2PO4, 370 MgSO4.7H2O), micro salts (25.35 MnSO4.H2O, 12.9 ZnSO4.7H2O, 9.30 H3BO3, 1.245 KI, 0.375 Na2MoO4.2H2O, 0.0375 CuSO4.5H2O, 0.0375 CoCl.6H2O, 55.05 FeNaEDTA), 2.0 Thiamin HCl, 4.0 Calcium Panthotenate, 100 Myo-inositol, 0.1 BA, 30000 sugar, and 8000 agar.
Meanwhile, to determine the optimum time for the metaphase stage in cytological analysis, 192 teak seedlings of KSP clone obtained from in vitro culture were used. These in vitro shoots were acclimatized and grown and maintained in the nursery as described for plant regeneration in the nursery sub section. After 10 months, the teak seedlings, that we referred in this paper as teak plants had an average stem height of 40.3 cm, stem diameter of 0.8 cm, and a leaf number 8, with a leaf length of 35.8 cm and a leaf width of 16.7 cm.
Polyploidy induction with oryzalin
Polyploidy induction in teak was performed using shoot tip and nodal stem inocula from in vitro teak shoot cultures. Each inoculum was approximately 1−1.5 cm in length, consisting of one node, and each node has two opposite leaves. The shoot tip and nodal stem inocula were immersed and shaken in a shaker for 7 days in a liquid modified MS medium with the addition of oryzalin at concentrations of 0 µM, 1.25 µM, 2.5 µM, 5 µM, 7.5 µM, 15 µM, 30 µM, and 60 µM. Each treatment consisted of 5 replications and each replication comprised 5 inocula. The inocula were rinsed in liquid modified MS medium without oryzalin and then planted in semi-solid modified MS medium described above with a pH of 5.7−5.8, with a total of 5 inocula per bottle. The shoot cultures were placed in a room with a temperature ranging from 25−27 °C, humidity between 60–70%, and light intensity provided by two fluorescent lamps, each 40 watts, for 16 h per day. To separate chimeras, the shoot cultures were subsequently subcultured every 4 weeks up to 6 passages with an inoculum of one node stem segment or shoot tips measuring 1−1.5 cm. Each culture flask was filled with 5 inocula.
Plant regeneration in the nursery
The sixth subculture’s shoots, aged 4 weeks, were then acclimatized and subsequently planted in the nursery as it was described above. The shoots were immersed for 2 min in a mixture solution of fungicide (Masalgin, containing active ingredient of 50.4% benomyl) and bactericide (Agrept, containing 20% streptomycin sulfate) each at a dose of 2 gL−1. Afterward, they were planted in a plastic container measuring 37 cm x 30 cm x 12 cm filled with a growth medium composed of a mixture of sand, soil, cocopeat, and husk in a ratio of 2:1:1:2. The growing medium was steamed for 4 h for pasteurization before use. The shoots were watered regularly and once a week with a 2 g L−1 solution of Growmore fertilizer (15 N-15P-15K) using a sprayer. The plastic containers containing the teak shoots were covered with a clear plastic sheet, tied with rubber strips, and placed in a greenhouse with shade ranging from 50–75%.
After 6 – 8 weeks, the acclimatized rooted teak shoots were planted in a nursery medium, which was a mixture of soil, husk, and manure in a ratio of 10:1:1, supplemented with 5 grams of lime and 2 grams of NPK (15-15-15) fertilizer per kg of nursery medium. The nursery medium was put into polybags measuring 21 cm x 25 cm for the growth of seedlings from the acclimatized rooted shoots. The seedlings were placed in plots with 50% shade and watered daily to prevent drying out. These methods of acclimatization, growing and maintaining plant in the nursery was also applied for clone KSP of which the regenerated plants would be used for mitotic analysis.
Ploidy analysis based on flow cytometry
Plant ploidy analysis using flow cytometer was conducted to some of 10-month-old plants with specific traits that exhibit indication of polyploid traits, such as longer, wider, thicker, stiffer, greener leaves with wavy leaf margin. Leaf samples for ploidy analysis using flow cytometry and stomata observation, as well as root tips for cytological observation and root tip cell diameter, were taken from these plants.
The ploidy level of teak seedlings was analyzed using a PartecCyFlowSpace flow cytometer using ‘CystainTM PI absolute P’ reagent kit (Sysmex 05-05022). From the group of plants with specific traits, the uppermost fully opened and green leaves were collected and washed with running water, and then the water on the leaves dried with tissue paper. The leaves measuring 0.5−1.0 cm2 are finely chopped and immersed in 250−500 μL Nuclease Extraction buffer (Sysmex). The mixture was then sieved using a cellTricsTM 30 μm filter, followed by the addition of 750−1000 μL staining solution which contain 2 mL Staining buffer, 12 μL Propidium Iodide and 6 μL RNAse A (Sysmex 05-05022). Readings for diploid control plants were positioned at a channel of around 200; therefore, tetraploid plant will be indicated with readings at a position of around 400. The results of the flow cytometry analysis were displayed on a computer screen in the form of graphs using the FlowMax software version 2.81. Some of the plants showing polyploidy were reanalyzed for their ploidy level 2–3 times with the flow cytometer and subsequently confirmed through chromosome counting analysis, cell diameter measurement at the root tips as well as characterized through stomatal observation.
Chromosome counting
Chromosome observation was carried out using the squash method, as this method can be used as an alternative to ensure good physical separation of chromosomes (Fukui Citation1996). The procedure followed (Manton Citation1950) modified by Praptosuwiryo and Mumpuni (Citation2018). The root tips from the sample plants with specific morphology were cut to a length of 1 cm and then washed with water to remove soil or dirt on the root. The root tips were placed in a dark bottle containing a cold 0.002 M solution of 8-hydroxyquinoline and stored in a refrigerator at a temperature of 4 °C for 3–5 h. The root tips were rinsed again with water, the root cap was removed, and then the root tips were fixed in 45% acetic acid for 10 min. The root tips were transferred to a mixed solution of 1 N HCl and 45% acetic acid in a 3:1 (v/v) ratio at a temperature of 60 °C for 1 min and 20 s. The root tips were immersed in 2% aceto-orcein for 3–5 min for the purpose of chromosome staining. The root tips were cut to a length of 1–2 mm, then placed in a drop of 2% aceto-orcein on a microscope slide, covered with a cover slip, gently tapped with a rubber pencil, and slightly heated over a Bunsen burner to allow the aceto-orcein stain to penetrate the cell and stain the chromosomes. After cooling, the surface of the cover slip was gently pressed, and the edges of the cover slip were sealed with clear nail polish. The slide was observed under a light microscope at magnification of 1000x using immersion oil.
Before using this method of chromosome counting to confirm level of ploidy of the plants, we studied the effect of root tip sampling time on the phases of mitosis to obtain optimal time for the metaphase stage. Plants in the nursery of clone KSP of 10-month-old were the source of the root tips samples. Root tips were sampled every 30 min between 5:30 AM and 11:30 AM for microscopic slide preparation. From each plant, 2–3 root tips were taken, and from each sample of root tips, 6 fields of view were observed for chromosome analysis. The number of chromosomes was observed in cells that were in the metaphase stage. For each plant, a total of 10 metaphase cells were observed.
Chromosome observation was carried out under a microscope with 1000x magnification. Chromosome counts were carried out from metaphase plates of mitotic division cells in which individual chromosomes were clearly distinguishable. An Olympus microscope U-TV0, 5XC-3 5H12344, connected to a computer was used to document the pictures. The chromosome image was adjusted to enhance the quality of image by using Gimp 2.10.32. The number of chromosomes was manually counted by dividing the chromosomes visible in the photo into four parts. The counted chromosomes were those visible in the region, and the total number of chromosomes was summed up from the four counted parts.
Chromosome counting was conducted to verify the results of ploidy determination using flow cytometer. Therefore, only samples of plants totaling 29 from flow cytometer identified diploid, tetraploid and mixoploid plants were analyzed and presented in this paper.
Observation of the length of root tip cells
Observation of cell length at the root tip was carried out on 2 tetraploid plants, 5 mixoploid plants, and 4 diploid plants that had been confirmed through flow cytometry and chromosome counting analysis. Three slides were prepared from each plant sample for this observation. The measurement of cell length at the root tip was conducted under a microscope at a total magnification of 1000x, using immersion oil, with a total of 6 observation fields per preparation and 3 cells in each field.
Observation of stomata
Stomatal observation was also conducted on 3 tetraploid plants, 2 mixoploid plants, and 4 diploid plants, as in the observations described above. Stomatal observation was performed by applying transparent nail polish to the lower leaf surface taken from the third leaf from the tip of the stem. After the nail polish dries, the lower leaf surface was stamped with a transparent tape, and then it was peeled off and affixed to a microscope slide, with 6 slides per plant. Stomatal cells were observed under a light microscope at a magnification of 400x. Stomatal density was observed in three randomly selected observation fields from each stomata preparation, with each observation field measuring 350 µm × 250 µm. Then, 3 stomata were randomly selected from each field of view for measuring their length and width.
Data analysis
To determine whether there is a significant difference in the size and density of stomata as well as the diameter of root cells between tetraploid, mixoploid, and diploid plants, a one-way Anova test was conducted at a 5% significance level using SPSS 15.0 software. Determination of the means and standard error as well as the creation of graphs were performed using Sigma Plot 15.0.
Results
Polyploidy induction with oryzalin
Our experiment aimed to induce polyploidy in teak, resulting in numerous seedlings or young plants in plastic bags within the nursery, which we refer to as ‘teak plants.’ These plants had underwent a series of steps, including induction by immersing nodal shoot and shoot tips inoculum in an anti-mitotic agent, shoot proliferation in tissue culture, acclimatization, and growth in the nursery. Throughout these processes, many shoots were affected by contamination and did not survive, and many died in the acclimatization and in the nursery. Despite these challenges, we successfully recovered 1.059 plants from five different accessions and eight levels of oryzalin concentration. Of these, 203 plants originated from the control treatment with 0 µM oryzalin, leaving 856 plants from oryzalin treatments.
Among these, 205 plants exhibited morphological characteristics typical of polyploids (), which were having thicker, larger, and darker green leaves, taller stature, and greater root diameters. From these preselected plants, using flow cytometer we determined the ploidy level of 158 plants and found 138 plants to be diploid, 16 plants to be mixoploid and only 4 plants to be tetraploid (). Further chromosome counting of those plant that had been identified with flow cytometer showed that flow cytometer results may not be accurate especially for diploid determination. For example, for MK44 treatment of 5 and 7.5 oryzalin resulted in each diploid and tetraploid plants, while chromosome counting revealed that there was a mixoploid from the supposedly diploid plants. The tetraploid plants of this treatment were not included in chromosome counting. Likewise, the treatment of MK61 at 7.5 µM oryzalin showed 6 diploids and 1 mixoploid after flow cytometer analysis, but chromosome counting revealed that one of the diploids was in fact mixoploid. False diploid identification from flow cytometer analysis of mixoploid plants according to chromosome counting also occurred in two other treatments and accessions such as MK17, oryzalin 7.5 µM, and MK71 oryzalin 7.5 µM. We confirmed the tetraploid determination of flow cytometer with chromosome counting for 2 plants derived from treatment of accession MK17 with 7.5 µM oryzalin (Tabel 1).
Table 1. Summary of the effects of clones and oryzalin doses on the plant recovery, identification of suspect polyploid plants, and identification of plant ploidy using flow cytometry, and their limited confirmation with chromosome counting.
The varying number of plants recovered for each treatment did not seem to be influenced by the oryzalin concentrations. Instead, it appeared that different levels of oryzalin, ranging from 1.25 to 60 µM, did not result in plant mortality but rather allowed for the induction of polyploidy, albeit with a low frequency. Nevertheless, we managed to obtain tetraploid and mixoploid plants from four out of five genotypes that we tested (). We did not confirm the ploidy level of plants from the induction experiment that did not show ploidy indications. This strategy may not be the best, as not all the plants that displayed indications of polyploidy turned out to be polyploidy.
Ploidy analysis based on flow cytometry
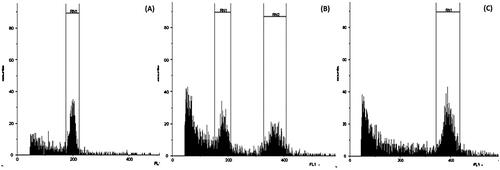
For flow cytometer analysis, we set the diploid control samples of diploid teak at channel 200 (RN1). Therefore, samples that fell in channel 400 (RN2) indicated as tetraploid, and samples with those two channels, indicated as mixoploid. However, our analysis showed that the channel or peak of the graph indicating relative DNA content was at 198.48 for the diploid control, which fell a little outside the designated channel of RN1, and peaked at 391.79 for the tetraploid, which was a little outside the channel 400 of RN2. However, both had high CV values of 4.97% and 5.15%, respectively (). The peak of the mixoploid samples was slightly more off than those of diploids and tetraploids. For example, a clone of MK61 mixoploid had a relative DNA content that peaked at channel 181.35 and 369.10 with CV values of 7.78% and 5.31%, respectively ().
Figure 1. Flow cytometry analysis of preselected plants from oryzalin treatment compared to the untreated control: (A) a diploid clone of KSP in the untreated control produced a peak at 198.48 with a coefficient of variation (CV) of 4.97%, (B) a mixoploid of MK61 produced two peaks at 181.35 and 369.10 with CVs of 7.78% and 5.31%, respectively, and (C) a tetraploid of clone MK17 peaked at channel 391.79 with a CV of 5.15% (CV).
Chromosome counting analysis
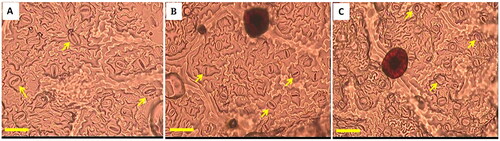
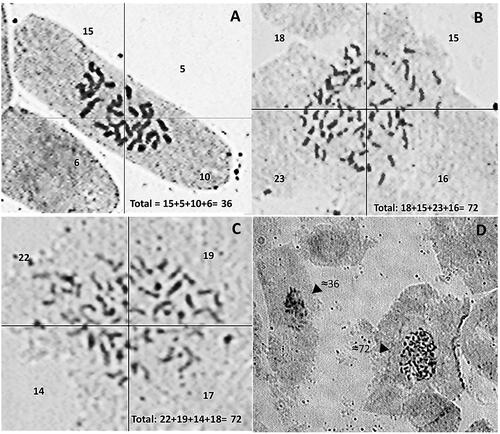
Chromosome observations of the root tips of teak plants revealed various stages of cell division i.e. mitosis, including prophase, metaphase, anaphase, telophase, and interphase (). During prophase, chromatin structures thickened and shortened to form visible chromosomes, and the nuclear membrane disappeared (). In metaphase, the chromosomes were condensed and aligned at the cell’s equatorial region and could be counted (). Anaphase was characterized by the two groups of chromosomes separated to opposite poles of the cells (). At telophase, the formation of a cell wall resulted in the separation of two sister cells (). Most of the cells were in interphase, during which the cell nucleus became apparent, and the chromosomes reverted to chromatin threads ().
Figure 2. Mitotic phases of root tip cells of teak plants: (A) prophase, (B) metaphase, (C) anaphase, (D) telophase, and (E) interphase, bar = 10 µm.
We attempted to determine the optimal time for observing metaphase in teak plants. Our observations on 192 teak plants indicated that mitotic division occurred at all sampling times from 5:30 AM to 11:30 AM. The highest percentage of plants (90%) underwent mitotic metaphase when sampled in the morning, and this percentage decreased to about 30% by 11.30 AM (). Regardless of the sampling time, all phases of mitosis were observable, but most of the cells (70−80%) were in interphase, while about 20−30% were almost evenly distributed across prophase to telophase. Prophase virtually ceased after 9.30 AM, and an hour later, metaphase started to decrease from 7% to 4%. In general, the fluctuation in the percentage of mitotic phases during the observation period from 6:30 AM to 11:30 AM was within the range of 11% for interphase, and these percentages varied among the phases of mitosis with fluctuations of approximately 4 – 5% (). However, it can be inferred that sampling and fixing teak root tips for observing metaphase chromosomes yielded the best results in the early morning, from 5:30 AM to 6:00 AM. The data indicating the highest percentages of prophase and anaphase at 6:30 AM as the earliest observation time may suggest that mitosis might have commenced even earlier. It is possible that mitosis occurs continuously as the root grows continuously.
Table 2. The mitotic activities in root tips of teak plants showed as percentage of cells underwent different stages according to time of fixation from 05:45–11:30 AM.
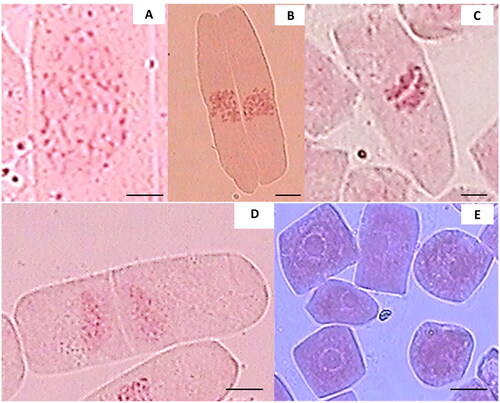
Chromosome counting was performed to confirm the results of the flow cytometric identification of polyploid teak. Our observations revealed that 8 diploids plants were confirmed to have a chromosome number of 2n = 2x = 36 (). Additionally, 2 tetraploids plants were confirmed with a chromosome number of 2n = 4x = 72 (), while 13 mixoploids plants have a chromosome number of 2n = 4x = 72 () and of 2n = 2x = 32 some of which cells could be seen in a field of view (). The mixoploid plants that showed both diploid cell with diploid chromosome and tetraploid cells with tetraploid chromosome confirmed the chimeric nature of the mixoploid plant from flow cytometry analysis.
Figure 3. Metaphase chromosome number of root tips of teak plants from polyploidy experiment, (A) control diploid MK44 (2n = 2x = 36), (B) tetraploid of MK17clone (2n = 4x = 72), (C) mixoploid of MK61 clone (2n = 2x + 4x; tetraploid cell 4x = 72), (D) chimeric tissue with a diploid cell (chromosome number of ≈36) and a tetraploid cell (chromosome number of ≈72) of accession MK61-30-101.
The length of root tips cells
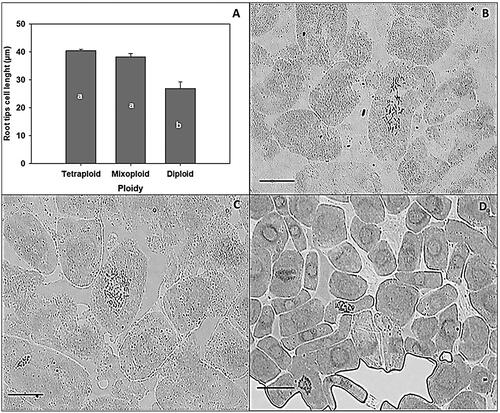
One way analysis of variance on the length of root tip cells of teak showed that induced tetraploid and mixoploid had no significant different, but both significantly different from the diploid untreated control (α = 0.004) (). The size of tetraploid and mixoploid root tip cells were almost twice of those of diploid root tips cell ().
Figure 4. The comparison of root tip cell size among tetraploid, mixoploid and diploid plants. (A) histogram of average root tip cell length, and the comparison of root cell size in an induced tetraploid (B), induced mixoploid (C), and a control diploid teak plant (D), bar = 20 µm. The same letters on figure (A) do not differ significantly according to the duncan multiple range test at a 5% significance level, where the p-value (pr > F) <0.001.
Comparison on stomata size and density among diploid and induced tetraploid and mixoploid teak
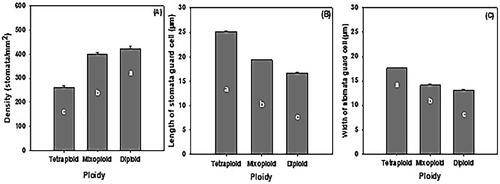
One-way analysis of variance showed significant differences among control diploid teak, tetraploid, and mixoploid for leaf stomata variables such as length (α = 0.000), width (α = 0.000), and density (α = 0.033). The average stomata length of the tetraploid was 25.19 ± 0.65 µm, which was larger than that of the mixoploids, measuring 19.32 ± 0.63 µm, and significantly larger than that of the control diploids, which averaged 16.68 ± 0.46 µm (). The average width of the stomata for tetraploids (17.62 ± 0.21 µm) was significantly wider than those of diploids (13.16 ± 0.45 µm), and mixoploids (14.16 ± 0.93 µm), although the last two did not significantly differ from each other (). Therefore, the tetraploid teak plants had stomata approximately twice as large as those of diploids, while the mixoploids were only 1.2 times larger than the diploids. The stomata density of the tetraploids (22.80 ± 5.23 cells/350 × 250 µm2) was significantly lower, at almost half the density of diploids (37.01 ± 5.31 cells/350 × 250 µm2), although these diploids did not significantly differ from the mixoploids (34.77 ± 6.03 cells/350 × 250 µm2) () The differences in size and density of stomata between induced polyploids and the diploid counterparts were visually noticeable ().
Figure 5. The comparison of the size and density of stomata from control diploids, and induced tetraploid and mixoploid teak. (A) stomata density which was the number of stomata/microscope observation field (350 µm x 250 µm), (B) length of stomata guard cell, (C) width of stomata guard cell. Bars in histogram indicates standard error. The same letters on the same parameter variable do not differ significantly according to the duncan test at a 5% significance level, where the p-value (pr > f) is <0.001 for stomata density, length and width of stomata guard cells.
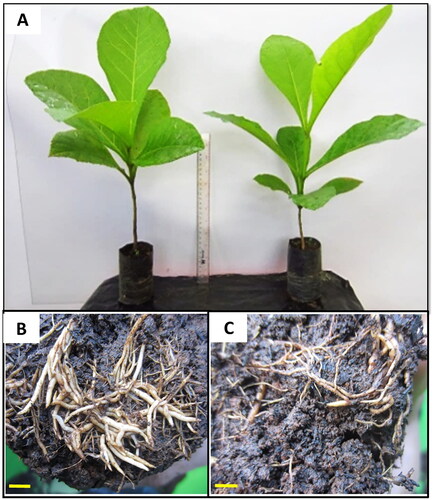
The comparison of the morphology of shoots and root systems between the control diploids and induced polyploid teaks in the nursery
While the morphology of the shoots, including the leaves of the tetraploid was generally bigger, thicker, and greener ( left) than that of the diploid ( right), it was likely influenced by the environment. On the other hand, the root system of tetraploids () was visibly larger than that of the control diploids ().
Discussion
By using inoculum consisting of nodal stem segments and shoot tips from in vitro shoots immersed for one week in a modified MS proliferation medium containing 5−30 µM oryzalin, we successfully obtained two tetraploid teak plants from one accession and 18 mixoploid teak plants from four accessions. This is the first report on the process of producing polyploid teak plants through tissue culture propagation, although tetraploid teak plants had previously been generated exhibited greater drought tolerance compared to their diploid counterparts (Ridwan et al. Citation2018). The ploidy of the tetraploid and mixoploid teak plants were confirmed by multiple analysis including flow cytometer, chromosome counting, root tip cell size, stomata size and density, and gross morphology of the plants. Barandalla et al. (Citation2007) suggested that flow cytometer analysis alone for ploidy determination was not sufficient since plant initially showed as tetraploid produced mixoploid or diploid in following clonal propagation.
The method we employed for inducing tetraploid teak plants involved immersing inocula containing axillary or apical meristems in a liquid medium supplemented with an anti mitotic agent (oryzalin), at specific concentration (1.25 − 60 µM), for a certain duration (7 days). This method has been successfully used for inducing tetraploids in bananas (Van Duren et al. Citation1996; Poerba et al. Citation2014; Citation2019a; Citation2019b). Oryzalin, as an anti-mitotic agent, has also been employed to induce tetraploidy in other plants, such as acacias (Lam et al. Citation2014), and hebes (Gallone et al. Citation2014). Oryzalin is preferred for tetraploidy induction over colchicine because it is effective at lower concentrations (Van Duren et al. Citation1996; Ganga and Chezhiyan Citation2002; Kanchanapoom and Koarapatchaikul Citation2012) and is less toxic (Ramulu et al. Citation1991; Dhooghe et al. Citation2009). The frequency of polyploidy induction, resulting in the formation of two tetraploids and 18 mixoploids out of 856 plants grown from oryzalin-induced shoot cultures, can be considered low. This low frequency is likely attributed to morphological prescreening, which selectively identifies plants displaying morphological characteristics associated with polyploidy for further ploidy confirmation using a flow cytometer. In fact, there was a weak correlation between these specific morphologies and the actual ploidy, as only 12.6% of the suspected polyploids were confirmed as polyploids through flow cytometer analysis.
Rahayu et al. (Citation2016) relying on the morphology of thicker leaves to identify polyploids from colchicine-treated P. amabilis L. Blume and P. amboinensis J.J.Sm. in vitro, found polyploid frequencies of 12.5 − 18.2% and 25.0 − 40.0% based on cytology, respectively. Ahmad et al. (2009) showed that prescreening based on stomata size increased the efficiency on tetraploid screening of two accessions of a cultivated banana “Pisang Rejang” using flowcytometer by 24.89% for accession #1 and 27.42% for accession #2 that more than twice without prescreening with 11.68% for accession #1 and 14.72% for accession #2. Therefore, the morphological prescreening methods need to be evaluated, and prescreening using stomatal size and density is considered more reliable. However, the induction of polyploids can actually reach higher percentages. Meyer et al. (Citation2009) induced polyploids in Hypericum and obtained 35% tetraploids with 30 µM oryzalin. Nevertheless, for plants that are easily propagated using tissue culture, a low induction frequency of tetraploid or mixoploid individuals can be compensated for by prolific shoot proliferation and eventual plant propagation.
Confirming the ploidy of teak plants using a flow cytometer can be somewhat problematic. According to Cires et al. (Citation2011), one of the factors that can affect the results of a flow cytometer analysis is the presence of secondary metabolites. Teak leaves are rich in secondary metabolites such as phenols, phenolic acids, flavonoids, glycosides, alkaloids, steroids, and anthraquinones (Nidavani Citation2014). The high phenolic content in the leaves can interfere with the accurate measurement of relative DNA content, leading to shifts in the intended channel. While this shift does not impact the accuracy of ploidy identification, it does reduce that the effectiveness of the machine. Additionally, addition of PVP (polyvinylpyrrolidone) and sodium metabisulfite can help inhibit phenol oxidation (Cires et al. Citation2011). Greilhuber et al. (Citation2007) mentioned that a good flow cytometry analysis should result in a histogram with a low coefficient of variation (CV) of less than 5.5%. Most of the flow cytometry analysis in this study produced histograms with a low CV of less than 5.5%. Therefore, the addition of 1% PVP and 10 mM sodium metabisulfite to the buffer solution WPB used in the study proved effective in reducing the impact of secondary metabolites in teak leaves.
Cytological analysis through chromosome counting in teak has been a challenging task. The information regarding the number of teak chromosomes is derived from Hedegart and Eigaard (Citation1965), which had been cited by many researchers, including Shrestha et al. (Citation2005). Unfortunately, the original paper by Hedegart and Eigaard (Citation1965) is not available online, making their chromosome counting method for teak inaccessible. Wulandari and Wijaya (Citation2015) tried to study teak cytology but could only identify mitotic prophase in the root tip cells. Chromosome counting in tree species is notoriously difficult due to the small size of the chromosomes and challenges associated with cell wall lysis. Nevertheless, cytological studies have been conducted on other tree species such as Mangifera indica L. (Pierozzi and Rossetto Citation2011) and Citrus sp. (Guerra et al. Citation1997).
The monitoring of root tip mitosis in diploid teak plants revealed that approximately 20% of cells underwent mitosis, and these were almost evenly distributed among all four mitotic phases: prophase, metaphase, anaphase, and telophase, while about 80% of cells were in interphase. Sampling root tips at 5:30 AM may have been late, as a high percentage of metaphase cells (8% out of the 20% mitotic cells) were observed, but 9:30 AM could be considered the latest time for sampling since no more root tip cells underwent prophase afterward.
Utilizing the results of the metaphase period determination, we were able to determine the metaphase chromosome number of diploid plants, which was 36, confirming the report by Hedegart and Eigaard (Citation1965) in Shrestha et al. (Citation2005), Yasodha et al. (Citation2018), while the number of metaphase chromosomes in tetraploids was 72, and the mixoploid showed chimeric nature of ploidy by exhibiting both cells with diploid and tetraploid chromosome. Therefore, the chimeric nature of oryzalin induced mixoploid teak manifests both in the shoot and in the roots. This is the first report on the chromosome number of a tetraploid teaks to be 72 and mixoploid teaks to be chimeric of diploid and tetraploid in the root tip cells. The chimeric plants showed diploid and teraploid cells with various percentages both in leaves and in root tip had been recovered from induced mixoploid in Echinacea purpurea (L.) Moench (Dahanayake et al. Citation2011). Chimeric plants consisting of 3 different ploidy level and combination was reported for E. purpurea (L.) Moench (Dahanayake et al. Citation2011).
Our observations of the morphology of induced tetraploid, mixoploid, and control diploid teak plants in terms of root tip cell size, stomatal size, and density, and shoot and root morphology confirmed some general phenomena. Tetraploids typically exhibited larger dimensions than diploids, and mixoploids tended to resemble tetraploids, although some traits were more similar to the diploid counterparts. For instance, we observed that tetraploids had larger stomata, but lower stomatal density compared to diploids, a pattern also documented in several other plants such as Moringa oleifera Lam. (Ridwan and Witjaksono Citation2020), Cattleya intermedia Graham ex Hook. (Silva et al. Citation2000), Anthurium andraeanum Linden ex André “Arizona” (Chen et al. Citation2009), Phalaenopsis amabilis (L.) Blume, P. amboinensis J.J.Sm. (Rahayu et al. Citation2016), and Allium x wakegi Araki (Setyowati et al. Citation2013). These results suggest that stomatal size and density could be useful for pre-screening for polyploidy.
Induced tetraploid teak plants exhibited larger, thicker, and greener leaves. Similar results were also found in rose (Kermani et al. Citation2003), P. amabilis (L.) Blume and P. amboinensis J.J.Sm. (Rahayu et al. Citation2016), Caladium Vent. ‘Tapestry’ (Cai et al. Citation2015), and Thymus persicus (Ronniger ex Rech.f.) Jalas (Tavan et al. Citation2015). The darker green leaf color in polyploid plants is likely due to a higher chlorophyll content in cells. Tetraploid Paulownia tomentosa (Thunb.) Steud. (Tang et al. Citation2010) and watermelon polyploids (Pradeepkumar Citation2011) have shown higher chlorophyll content than their diploid counterparts. The fact that tetraploid teak has a larger root diameter than diploid teak is not uncommon. Banyai et al. (Citation2010) induced tetraploids from Artemisia annua L. and found that they had a larger root system than diploids did. Setyowati et al. (Citation2013) found that induced polyploids of shallot ‘wakegi’ had a larger root diameter than diploids did.
The tetraploid teak has been proven to be more tolerant against reduced water treatment in the greenhouse (Ridwan et al. Citation2018), which suggests it may have better drought tolerance. Teak trees shed their leaves during the dry season and cease their vegetative growth. Accessions with higher drought tolerance would result in fewer leaf drops, implying increased photosynthesis and growth. However, the larger cell size associated with tetraploid teak would require monitoring of its wood quality. The results of this work would also facilitate the breeding of triploid teak, both by enabling outcrossing between tetraploid and diploid plants and within the mixoploid group.
Planting tetraploid and diploid trees intermixed or in adjacent rows might facilitate crossbreeding, potentially producing triploid teak. Planting mixoploids could also support the production of triploid trees within individual plants. Therefore, the mixoploid, although a chimera, may be valuable for breeding triploid teak. Triploid varieties may be desirable since they are seedless, potentially leading to even better biomass accumulation. The seedlessness may necessitate vegetative plant propagation for production and commercialization of clonal seedlings, which could attract interest from the private sector to further breeding efforts.
Conclusion
Using a common method to induce polyploidy by immersing in vitro shoots in liquid modified MS medium containing 5 − 30 µM oryzalin for 7 days, we have produced two tetraploids and 18 mixoploid plants from four accessions of teak mutants. The polyploid plants were preselected based on their morphology, and only 20% were confirmed to be polyploid according to flow cytometric analysis. Chromosome counting analysis using the chromosome squash technique generally confirmed the polyploidy estimation using the flow cytometer. However, there was some inaccuracy for diploid determination since some of it turn out to be mixoploid after chromosome counting. Diploids have 36 chromosomes, tetraploids have 72 chromosomes, and the mixoploids have both chromosomes count of 72, and of 36 in the root tip cells. Further characterization of the polyploid plants, as compared to the diploid counterparts, showed bigger root tip cell size, larger but less dense stomata, bigger and thicker leaves, and a greater root diameter for the tetraploids, which is common to many other induced tetraploid plants. This is the first report on the process and successful production of tetraploid and mixoploid teak plants. This result may facilitate further teak breeding for triploid varieties that could be more productive since they might be seedless.
Acknowledgement
This work was funded by the Research Center for Biomaterial, Indonesian Institute of Sciences (LIPI), under a program for Commercial Product with contract number 211/IPH.4/KP/II/2015, with Tri Handayani as the Principal Investigator. We highly appreciate the technical assistance provided by Ms. Katarina Utami Nugraheni.
References
- Ahmad F, Tambunan IR, Witjaksono. 2009. Ukuran stomata sebagai pre screening dalam identifikasi ploidi pisang dengan flow cytometer [Stomatal size as a pre-screening in the identification of banana ploidy using a flow cytometer]. Seminar Nasional Biologi XX dan Kongres Perhimpunan Biologi Indonesia XIV, Malang. 238–242. Indonesian
- Banyai W, Sangthong R, Karaket N, Inthima P, Mii M, Supaibulwatana K. 2010. Overproduction of artimisinin in tetraploid Artemisia annua L. Plant Biotechnol. 27(5):427–433. doi: 10.5511/plantbiotechnology.10.0726a.
- Barandalla L, Ritter E, Ruiz De Galarreta JI. 2007. Oryzalin treatment of potato diploids yields tetraploid and chimeric plants from which euploids could be derived by callus induction. Potato Res. 49(2):143–154. doi: 10.1007/s11540-006-9014-1.
- Cai X, Cao Z, Xu S, Deng Z. 2015. Induction, regeneration and characterization of tetraploids and variants in ‘Tapestry’ caladium. Plant Cell Tiss Organ Cult. 120(2):689–700. doi: 10.1007/s11240-014-0636-8.
- Chen WH, Tang CY, Kao YL. 2009. Ploidy doubling by in vitro culture of excised protocorms or protocorm-like bodies in Phalaenopsis species. Plant Cell Tiss Organ Cult. 98(2):229–238. doi: 10.1007/s11240-009-9557-3.
- Cires E, Cuesta C, Fernández Casado MÁ, Nava HS, Vázquez VM, Fernández Prieto JA. 2011. Isolation of plant nuclei suitable for flow cytometry from species with extremely mucilaginous compounds: an example in the genus Viola L. (Violaceae). Anal Jard Bot Madr. 68(2):139–154. doi: 10.3989/ajbm.2273.
- Dahanayake N, Chen XL, Zhao FC, Yang YC, Wu H. 2011. Separation of tetraploid and diploid plant from chimeras in in-vitro cultures of purple coneflower (Echinacea purpurea L). Trop Agric Res & Ext. 13(1):11. doi: 10.4038/tare.v13i1.3131.
- Dhooghe E, Denis S, Eeckhaut T, Reheul D, Van Labeke MC. 2009. In vitro induction of tetraploids in ornamental Ranunculus. Euphytica. 168(1):33–40. doi: 10.1007/s10681-008-9876-1.
- Diallo A, Gbeassor M, Vovor A, Eklu-Gadegbeku K, Aklikokou K, Agbonon A, Abena AA, De Souza C, Akpagana K. 2008. Effect of Tectona grandis on phenylhydrazine-induced anaemia in rats. Fitoterapia. 79(5):332–336. doi: 10.1016/j.fitote.2008.02.005.
- Fauzan YSA, Supriyanto S, Tajuddin T. 2017. Growth and morphological changes as an early indication of in vitro ploidization of teak (Tectona grandis L.f). Jurpenhuttanaman. 14(2):128–139. doi: 10.20886/jpht.2017.14.2.128-139.
- Fukui K. 1996. Plant chromosomes at mitosis. In: Fukui K, Nakayama S, editors. Plant chromosome: laboratory methods. Boca Raton (BR): CRC Press; p. 1–17. doi: 10.1201/9780203743195.
- Gallone A, Hunter A, Douglas GC. 2014. Polyploid induction in vitro using colchicine and oryzalin on Hebe ‘Oratia Beauty’: production and characterization of the vegetative traits. Sci Hortic. 179:59–66. doi: 10.1016/j.scienta.2014.09.014.
- Ganga M, Chezhiyan N. 2002. Influence of the antimitotic agents colchicine and oryzalin on in vitro regeneration and chromosome doubling of diploid bananas (Musa spp.)J Hortic Sci Biotechnol. 77(5):572–575. doi: 10.1080/14620316.2002.11511540.
- Goh DKS, Monteuuis O. 2012. Behaviour of the “YSG Biotech TG1-8” teak clones under various site conditions: first observations. Bois for Trop. 311(311):5–19. http://www.ysgbiotech.com. doi: 10.19182/bft2012.311.a20511.
- Greilhuber J, Temsch EM, Loureiro JCM. 2007. Nuclear DNA content measurement. In: Dolezel J, Greilhuber J, Suda J, editors. Flow cytometry with plant cells. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; p. 67–101. doi: 10.1002/9783527610921.ch4.
- Guerra M, Pedrosa A, Silva AEBE, Cornélio MTM, Santos K, Soares Filho WS. 1997. Chromosome number and secondary constriction variation in 51 accessions of a citrus germplasm bank. Braz J Genet. 20(3):489–496. doi: 10.1590/S0100-84551997000300021.
- Gupta PK, Nadgir AL, Mascarenhas AF, Jagannathan V. 1980. Tissue culture of forest trees: clonal multiplication of Tectona grandis L. (teak) by tissue culture. Plant Sci Lett. 17(3):259–268. doi: 10.1016/0304-4211(80)90156-X.
- Hedegart T, Eigaard J. 1965. Chromosome number of teak (Tectona grandis L.f.). Hørsholm: the Arboretum.
- Kanchanapoom K, Koarapatchaikul K. 2012. In vitro induction of tetraploid plants from callus cultures of diploid bananas (Musa acuminata, AA group) ‘KluaiLeb Mu Nang’ and ‘Kluai Sa. Euphytica. 183(1):111–117. doi: 10.1007/s10681-011-0516-9.
- Kermani MJ, Sarasan V, Roberts AV, Yokoya K, Wentworth J, Sieber VK. 2003. Oryzalin-induced chromosome doubling in Rosa and its effect on plant morphology and pollen viability. Theor Appl Genet. 107(7):1195–1200. doi: 10.1007/s00122-003-1374-1.
- Kertadikara A, Prat D. 1995. Genetic structure and mating system in teak (Tectona grandis L.f.) provenances. Silvae Genet. 44:2–3.
- Kongmeesup I, Boonyanuphap J. 2019. Estimation of carbon offset for teak plantation in lower northern Thailand. Songklanakarin J Sci Technol. 41(3):580–586. doi: 10.14456/sjst-psu.2019.76.
- Lam HK, Harbard J, Koutoulis A. 2014. Tetraploid induction of Acacia crassicarpa A. Cunn. Ex Benth. using colchicine and oryzalin. J Trop for Sci. 26(3):347–354. https://www.researchgate.net/publication/266798051.
- Li W, Zhang Q, Wang S, Langham MA, Singh D, Bowden RL, Xu SS. 2019. Development and characterization of wheat–sea wheatgrass (Thinopyrum junceiforme) amphiploids for biotic stress resistance and abiotic stress tolerance. Theor Appl Genet. 132(1):163–175. doi: 10.1007/s00122-018-3205-4.
- Madani H, Escrich A, Hosseini B, Sanchez-Muñoz R, Khojasteh A, Palazon J. 2021. Effect of polyploidy induction on natural metabolite production in medicinal plants. Biomolecules. 11(6):899. doi: 10.3390/biom11060899.
- Manton I. 1950. Problems of cytology and evolution in the Pteridophyta. London: Cambridge University Press. doi: 10.5962/bhl.title.4667.
- Meyer EM, Touchell DH, Ranney TG. 2009. In vitro shoot regeneration and polyploid induction from leaves of Hypericum species. horts. 44(7):1957–1961. doi: 10.21273/HORTSCI.44.7.1957.
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 15(3):473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x.
- Nidavani RB. 2014. Teak (Tectona grandis LINN): a renowed timber plant with potential medicinal values. Int J Pharm Pharm Sci. 6(1):48–54.
- Oates KM, Ranney TG, Touchell DH. 2012. Influence of induced polyploidy on fertility and morphology of Rudbeckia species and hybrids. horts. 47(9):1217–1221. doi: 10.21273/HORTSCI.47.9.1217.
- Palanisamy K, Hegde M, Yi JS. 2009. Teak (Tectona grandis Linn.f.): a renowned commercial timber species. J for Sci. 25(1):1–24. https://www.researchgate.net/publication/264174561.
- Ahmad Parlaongan, Supriyanto, Arum Sekar Wulandari. 2022. Effects of gamma ray irradiation to induce genetic variability of teak planlets (Tectona grandis Linn.f.). J. Sylva Indonesiana. 5(01):10–21. doi: 10.32734/jsi.v5i01.6166.
- Pierozzi NI, Rossetto CJ. 2011. Chromosome characterization of two varieties of Mangifera indica L. Rev Bras Frutic. 33(spe1):546–551. doi: 10.1590/S0100-29452011000500074.
- Poerba YS, Ahmad F, Handayani T, Witjaksono. 2014. Induksidan karakterisasi pisang mas lumut tetraploid [induction and characterization of tetraploid pisang mas lumut]. J Biol Indones. 10(2):191–200. Indonesian http://ppvt.setjen.deptan.go.id/.
- Poerba Y, Martanti D, Handayani T, Witjaksono. 2019a. Morphology and reproductive function of induced autotetraploid banana by chromosome doubling. SABRAO J Breed Genet. 51(2):175–190.
- Poerba YS, Martanti D, Handayani, T, W., Witjaksono. 2019b. Induction of banana autotetraploids “Klutuk Sukun” and their reproductive function for producing triploid hybrids. Asian J of Plant Sciences. 18(2):91–100. doi: 10.3923/ajps.2019.91.100.
- Pradeepkumar T. 2011. Characterization of M1 generation of polyploids in watermelon variety “Sugar Baby. Rep-Cucurbit Genet Coop. 33(34):44–46.
- Pramasari D, Wahyuni I, Adi DS, Amin Y, Darmawan T, Dwiyanto W. 2014. Effect of age on chemical component of platinum teak wood – a fast growing teak wood from LIPI. 211–216. https://www.researchgate.net/publication/279551875.
- Praptosuwiryo T, Mumpuni M. 2018. Chromosome numbers of some species of Pteris (Pteridaceae) in Java, Indonesia. Biodiversitas. 19(6):2118–2126. doi: 10.13057/biodiv/d190618.
- Rahayu D. 2014. [ Evaluasi pertumbuhan dan karakteristik tanaman jati yang diberi perlakuan kolkisin [Evaluation of growth and characteristics of teak plants treated with colchicine ]. [[master’s thesis]]. Yogyakarta: Universitas Gadjah Mada. Indonesian. ]
- Rahayu EMD, Sukma D, Syukur, M, R. 2016. Induksi poliploidi Phalaenopsis amabilis (L.) Blume dan Phalaenopsis amboinensis J.J. Smith dengan kolkisin dalam kultur in vitro [Induction of polyploidy in Phalaenopsis amabilis (L.) Blume and Phalaenopsis amboinensis J.J. Smith with colchicine in in vitro culture]. J Agron Indonesia. 43(3):219. Indonesian. doi: 10.24831/jai.v43i3.11248.
- Ramulu K, Verhoeven HA, Dijkhuis P. 1991. Mitotic blocking, micronucleation, and chromosome doubling by oryzalin, amiprophos-methyl, and colchicine in potato. Protoplasma. 160(2–3):65–71. doi: 10.1007/BF01539957.
- Ridwan R, Handayani T, Riastiwi I, Witjaksono W., Witjaksono. Ridwan. 2018. Tetraploid teak seedling was more tolerant to drought stress than its diploid seedling. JPKW. 7(1):1–11. doi: 10.18330/jwallacea.2018.vol7iss1pp01-11.
- Ridwan R, Witjaksono W. 2020. Induction of autotetraploid moringa plant (Moringa oleifera) using oryzalin. Biodiversitas. 21(9):4086–4093. doi: 10.13057/biodiv/d210920.
- Setyowati M, Sulistyaningsih E, Purwantoro A. 2013. Induksi poliploidi dengan kolkisisina pada kultur meristem batang Bawang Wakegi (Allium x wakegi Araki) [Induction of polyploidy with colchicine in shoot meristem culture of Wakegi Onion (Allium x wakegi Araki). ]Ilmu Pertanian. 16(1):58–76. Indonesian doi: 10.22146/ipas.2526.
- Shrestha MK, Volkaert H, Van Der Straeten D. 2005. Assessment of genetic diversity in Tectona grandis using amplified fragment length polymorphism markers. Can J for Res. 35(4):1017–1022. doi: 10.1139/x05-033.
- Silva P, Callegari-Jacques S, Bodanese-Zanettini MH. 2000. Induction and identification of polyploids in Cattleya intermedia Lindl. (Orchidaceae) by in vitro techniques. Cienc Rural. 30(1):105–111. doi: 10.1590/S0103-84782000000100017.
- Srinivasan R, Selvam GG, Karthikeyan K, Chandran C, Kulothungan S, Govindasamy C. 2012. In vitro propagation of shoot and callus culture of Tectona grandis (L.). Glob J Biotechnol Biochem. 7(1):26–29. doi: 10.5829/idosi.gjbb.2012.7.1.06.
- Tang ZQ, Chen DL, Song ZJ, He YC, Cai DT. 2010. In vitro induction and identification of tetraploid plants of Paulownia tomentosa. Plant Cell Tiss Organ Cult. 102(2):213–220. doi: 10.1007/s11240-010-9724-6.
- Tavan M, Mirjalili MH, Karimzadeh G. 2015. In vitro polyploidy induction: changes in morphological, anatomical, and phytochemical characteristics of Thymus persicus (Lamiaceae). Plant Cell Tiss Organ Cult. 122(3):573–583. doi: 10.1007/s11240-015-0789-0.
- Van Duren M, Morpurgo R, Dolezel J, Afza R. 1996. Induction and verification of autotetraploids in diploid banana (Musa acuminata) by in vitro techniques. Euphytica. 88(1):25–34. doi: 10.1007/BF00029262.
- Vleugels T, Cnops G, Roldán-Ruiz I. 2014. Improving seed yield in red clover through marker assisted parentage analysis. Euphytica. 200(2):305–320. doi: 10.1007/s10681-014-1188-z.
- Wulandari AS, Wijaya TR. 2015. Analisis kromosom tanaman jati (Tectona grandis L.f.) dengan metode perwarnaan [Chromosome analysis of teak plants (Tectona grandis L.f.) using staining methods]. Jurnal Silvikultur Tropika. 6(1):49–54. doi: 10.29244/j-siltrop.6.1.%25p.
- Xu C, Zhang Y, Han Q, Kang X. 2020. Molecular mechanism of slow vegetative growth in populus tetraploid. Genes (Basel). 11(12):1417. doi: 10.3390/genes11121417.
- Xu J, Jin J, Zhao H, Li K. 2019. Drought stress tolerance analysis of Populus ussuriensis clones with different ploidies. J for Res. 30(4):1267–1275. doi: 10.1007/s11676-018-0729-z.
- Yasodha R, Vasudeva R, Balakrishnan S, Sakthi AR, Abel N, Binai N, Rajashekar B, Bachpai VKW, Pillai C, Dev SA. 2018. Draft genome of a high value tropical timber tree, teak (Tectona grandis L.f.): insights into SSR diversity, phylogeny, and conservation. DNA Res. 25(4):409–419. doi: 10.1093/dnares/dsy013.
- Zhang Z, Zhang Y, Di Z, Zhang R, Mu Y, Sun T, Tian Z, Lu Y, Zheng J. 2023. Tetraploid induction with leaf morphology and sunburn variation in Sorbus pohuashanensis (Hance) Hedl. Forests. 14(8):1589. doi: 10.3390/f14081589.