ABSTRACT
With the increasing elderly population an increase in the number of bony fractures associated to age-related diseases such as osteoporosis also follows. The relatively high stiffness of the acrylic bone cements used in these patients has been suggested to give raise to a suboptimal load distribution surrounding the cement in vivo, and hence contribute to clinical complications, such as additional fractures. The aim of this study was to develop a low-modulus bone cement, based on currently used, commercially available poly(methyl methacrylate) (PMMA) cements for vertebroplasty. To this end, acrylate end-functionalized oligo(trimethylene carbonate) (oTMC) was incorporated into the cements, and the resulting compressive mechanical properties were evaluated, as well as the cytotoxic and handling properties of selected formulations. Sixteen wt%oTMC was needed in the vertebroplastic cement Osteopal V to achieve an elastic modulus of 1063 MPa (SD 74), which gave a corresponding compressive strength of 46.1 MPa (SD 1.9). Cement extracts taken at 1 and 12 hours gave a reduced MG-63 cell viability in most cases, while extracts taken at 24 hours had no significant effect on cell behavior. The modification also gave an increase in setting time, from 14.7 min (SD 1.7) to 18.0 min (SD 0.9), and a decrease in maximum polymerization temperature, from 41.5°C (SD 3.4) to 30.7°C (SD 1.4). While further evaluation of other relevant properties, such as injectability and in vivo biocompatibility, remains to be done, the results presented herein are promising in terms of approaching clinically applicable bone cements with a lower stiffness.
Introduction
Bone cements based on poly(methyl methacrylate) (PMMA) have been in orthopaedic use since the 1960s when they were first introduced for the fixation of joint prostheses.Citation1 They commonly consist of a liquid and a powder component that are mixed together at the time of implantation, creating a liquid or a paste that can be injected or packed into place and then left to harden in situ. These types of acrylic cements have been used in a variety of orthopaedic applications including implant fixation, screw augmentation and spinal augmentation procedures such as vertebroplasty. Vertebroplasty is a minimally invasive procedure, which consists in the percutaneous injection of bone cement into a fractured vertebra in order to achieve pain relief and fracture stabilization. These vertebral fractures can be caused by different pathologies such as hemangioma,Citation2 multiple myeloma,Citation3 osteolytic metastasesCitation4 and primary or secondary osteoporosis.Citation5 While being a successful treatment in terms of quick pain relief, new fractures may occur after a vertebroplasty, in untreated vertebrae. These may partly be due to the underlying disease, but a disproportionately large number of new fractures have been observed next to the vertebra treated with PMMA.Citation6,7 Also, adjacent fractures have been found to occur sooner than other new fractures.Citation8 A recent study reported a high hazard ratio (3.53) for fracture of a vertebra if it lay adjacent to a treated one.Citation9 One of the reasons for the higher incidence of adjacent fractures has been hypothesized to be the high stiffness of the injected bone cement in comparison to the surrounding bone. Most commercial acrylic bone cements have an elastic modulus in the range of 1700-3700 MPa,Citation10,11 while the elastic modulus of cancellous bone is typically in the range of 10-900 MPa.Citation12-14 In fact, several experimental studies have focused on developing novel PMMA-based cements with a lower elastic modulus,Citation15-20 but none of these cements are currently available on the market. Boger et al.Citation16,17 introduced macroporosity into the cements through the use of a hydrogel phase. However, excessive particle release from the modified cements was found.Citation15 An organic plasticizer, N-methyl-pyrrolidone, has also been used to substitute some of the liquid phase in order to reduce the elastic modulus,Citation18 but to the author's knowledge, no data on the cytocompatibility of this formulation is currently available. Recently, we introduced the use of a triglyceride oil as a method of reducing the stiffness of PMMA-based bone cements.Citation20 However, relatively high amounts of the oil were required, which had a negative effect on the cytocompatibility of the cements. A similar outcome was reported by Lam et al.,Citation19 who modified their cements with strontium-substituted hydroxyapatite-nanoparticles and linoleic acid. Modifications of PMMA using only linoleic acid in very small amounts, have, however, shown promising results, although the in vivo biocompatibility is yet to be confirmed.Citation21-23
In this study, we suggest another approach to reduce the modulus, by copolymerizing PMMA with a more flexible polymer. Poly(trimethylene carbonate) (PTMC) is used in biomedical applicationsCitation24 and could be an excellent candidate for this purpose, due to its low modulus and high ductility.Citation25 The current study hence aimed to investigate the possibility of obtaining low-modulus bone cements through the modification of PMMA-based cements with acrylate end-functionalized low molecular weight PTMC, termed oligo(trimethylene carbonate) (oTMC). The developed cements were evaluated in terms of their mechanical properties and selected formulations were further assessed in terms of their in vitro cytotoxicity, setting time and peak polymerization temperature.
Results
The elastic moduli, compressive strengths and yield strains of the unmodified and modified cements are summarized in . The strength was determined according to the ASTM F-451 standard, which specifies the strength as the stress at the 2.0% offset (2.0% proof stress), upper yield point, or at fracture, whichever occurs first. None of the samples exhibited a clear fracture, only increasing deformation. For the unmodified cements, the 2.0% proof stress and the upper yield point usually coincided, whereas the modified cements exhibited no clear upper yield point (the stress increased continuously, although with a different slope). The 2.0% proof stress was hence reported as the compressive strength (yield strength).
Table 1. Compressive mechanical properties of the tested cements. Mean values are shown, with standard deviations. N = number of specimens tested per group.
The effect of oTMC on the elastic modulus was greater for Osteopal V than for Simplex P; statistically significant differences were found between all formulations except between the two controls and between Osteopal V-16%oTMC vs. Simplex P-16%oTMC and Osteopal V-16%oTMC vs. Simplex P-18%oTMC and Simplex P-16%oTMC vs. Simplex P-18%oTMC. Hence, OsteopalV-16%oTMC and Simplex P-18%oTMC were chosen for further testing as these formulations also showed moduli of approximately 900 MPa, which corresponds to the upper limit for cancellous bone reported in the literature.Citation12-14
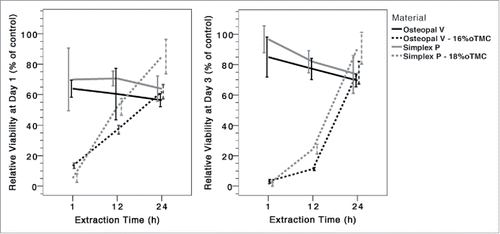
shows the relative viability of cells cultured in extraction media from different cements for 1 and 3 d. Compared to the control group cement extracts generally caused a decrease in cell viability. The extent of this effect was different among the materials and the incubation times. After 1 d the 1 h extraction media of the modified cements had an increased cytotoxic effect compared to media obtained from unmodified cements (p < 0.003). However, although there was a trend for increased cytotoxicity also for the 12 h modified cement extracts, there was no statistically significant difference between any of the modified cements and their respective base cement for 12 or 24 h extracts (p > 0.086). After 3 d of incubation even lower cell viabilities were detected for the 1 h and 12 h groups of the modified samples compared to the 1 d incubation, and for this incubation time the differences were statistically significant also for the 12 h extracts compared to the base cements (p < 0.048). Nevertheless, no statistically significant difference was found between any of the cement extracts at 24 h (p > 0.117).
Figure 1. Relative cell viability of MG-63 cells after 1 (left) and 3 (right) d of contact with cement extraction media, taken at different time points after commencement of cement preparation.
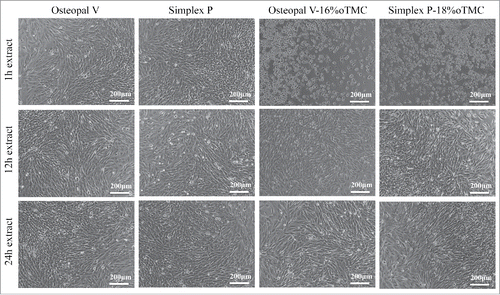
Morphological investigations confirmed the initial cytotoxic effects on the cells as described before. After 1 d of incubation, cells of the 1 h extraction media of the modified cements exhibited an apoptotic, rounded cell morphology (). In contrast, cells cultured in the 12 h and 24 h extraction media were more fibroblastic, similarly to the control (not shown) and unmodified cements. On the other hand the cell density was lower, explaining the lower relative viability compared to the control group. This was also observed for all extraction time points of the unmodified cements. Prolonged incubation time (3 d) had no effect on cell morphology. Cells in the 1 h extracts of the modified cements were apoptotic whereas cells of all other groups showed a normal cell morphology and increased cell density compared to day 1.
Figure 2. Representative light micrographs of cells after 1 d of incubation in extraction media of unmodified and modified cements. Cells in 1 h extraction media from modified cements showed signs of apoptosis, i.e. cell shrinkage and round cell morphology. Cell morphology in 12 h and 24 h extracts was comparable to extracts of the unmodified cements as well as the control group (the latter is not shown).
The handling properties were evaluated for the vertebroplastic cement as the base, i.e. for Osteopal V and Osteopal V modified with 16%oTMC, and are shown in . A statistically significant difference was found between the 2 materials for all handling properties (p < 0.02, t-test).
Table 2. Handling properties of Osteopal V and Osteopal V-16%oTMC. Error values indicate standard deviations.
Discussion
In this study, a telechelic acrylate-functionalized oligo(trimethylenecarbonate) (α,ω-oTMC-diacrylate)Citation26 was used as partial MMA monomer substitute in PMMA bone cements, with the aim of enhancing relative chain mobility and flexibility and hence decrease the modulus of the cements. The resulting materials were studied in terms of mechanical properties, cytocompatibility and handling properties.
The desired decrease in stiffness, specified as an elastic modulus of approximately 900 MPa,Citation12-14 was achieved with 16% oTMC in Osteopal V and 18% oTMC in Simplex P. Larger amounts of oTMC were generally needed in Simplex P compared to Osteopal V to reach similar moduli. This followed from the relative amounts of cement components: in order to reach a similar oTMC to monomer ratio, a larger weight percentage of the total cement was needed in Simplex P than in Osteopal V (see Materials and Methods section). The strength of the modified Osteopal V and Simplex P cements was still substantially higher, 46.1 ± 1.9 MPa and 34.6 ± 1.2 MPa, respectively, than that of the potentially surrounding trabecular bone (0.1–15 MPaCitation12-14). In previous studies on low-modulus bone cements, formulations with similar moduli (872-1000 MPa) also exhibited strength values above those of the trabecular bone, but varying between 17–45 MPa.Citation16,17,20-22 However, the lowest value, 17 MPa, was observed after compression tests where the specimens were subjected to cyclic preconditioning (i.e., loaded repeatedly within the elastic region) before testing to failure, which could result in a higher modulus than for specimens directly tested to failure.Citation21 In the study by Lam et al., no cement with a similar modulus was reported, but it appeared that a higher strength could be reached in the low-modulus cements than in the current study.Citation19 This could be due to a strengthening effect of the linoleic acid-functionalized hydroxyapatite particles, which were added to the cement besides the linoleic acid substitution of part of the monomer.
While the elastic modulus of the cements decreased with an increased amount of oTMC, the yield strain also decreased (), but was still well above that found in trabecular bone, which commonly lies below 1%.Citation27
While cells in contact with extracts from modified Osteopal V and Simplex P, taken 1 h and 12 h after mixing the cements, showed a decreased viability (although not statistically significant for 12 h extracts after 1 day of contact), cement extracts taken at 24 h induced no change in cell viability compared to the unmodified material extracts. Light micrographs also indicated little effect on cell morphology, except for the 1 h group. This may be attributed to the quasi-dynamic extraction method used. During sampling the extracts were replaced by fresh culture medium. Thus, the different extracts correlate to different stages of cement curing. The results hence suggest that the release of cytotoxic components was increased for the modified cements, especially during the first hour(s) of the setting reaction. This may be attributed to the increased setting time of these samples () and/or to a higher diffusion rate of residual monomer in the low-modulus, higher molecular mobility cements. Once the polymerization is completed the cytotoxicity of the modified materials is comparable to their unmodified counterparts. This is in accordance with a previous study on modified PMMA cements,Citation23 where both a modification with castor oil, as well as a customized PMMA formulation (components acquired from Sigma-Aldrich) induced a cytotoxic effect at early time points, but not with 24 h extracts.
It should be noted that previous studies assessing the cytocompatibility of PMMA-based bone cements often use diluted extracts in order to simulate physiological processes and transport phenomena of body fluids.Citation28,29 Furthermore, extracts are normally obtained using almost cured or fully cured cements. No dilutions were used in the current study and extracts were obtained in situ, i.e. from the setting cement. Hence, a worst-case scenario was applied, and a comparison with data from the literature is difficult. It can also be noted that previous in vitro cytotoxicity studies on bioactive, calcium phosphate based bone cements have also used similar dilutions, and needed 8-fold dilutions to reach similar values as the control at 1 d.Citation30
Osteopal V containing 16% oTMC had a significantly shorter doughing time than unmodified cement (2.6 ± 0.2 min compared to 4.7 ± 0.1 min), but a longer setting time (18.0 ± 0.9 min compared to 14.7 ± 1.7 min), indicating a faster formation of a pasty consistence with the addition of oTMC, which slowed down the diffusion of monomer within the paste and hence increased the setting time.Citation31 In agreement with this, the polymerization temperature significantly decreased with the addition of oTMC, suggesting that the reactions took place over a longer period of time, giving a lower peak polymerization temperature (from which the setting time is calculated, according to ASTM F-451 Citation32). A decrease in the peak polymerization temperature could be advantageous from a clinical point of view, as high temperatures may have negative effects on surrounding tissues.Citation33,34
While the results presented herein are promising, further evaluation of clinically relevant properties is needed, such as injectability and in vivo biocompatibility. The complexity involved in producing the modifying phase may also be a limiting factor to future use, when comparing these cements to other low-modulus cements, e.g. those modified using linoleic acid, where the modifying phase can be acquired ready-made, and lower amounts may be used.Citation21-23
In conclusion, it is possible to reduce the elastic modulus of PMMA bone cements by incorporating small amounts of acrylated oTMC, a reduction which could be important in order to avoid clinical complications related to biomechanical changes after surgery, especially in the increasingly larger elderly population, where low-density, low-modulus bone is more common.
Materials and methods
Acrylate-functionalized oligo(trimethylenecarbonate) (α,ω-oTMC-diacrylate, 1500 Da) was synthesized and characterized as described elsewhere.Citation26 Bulk ring-opening polymerization of trimethylene carbonate was performed using 1,4-butanediol as co-initiator (100:8 feed ratio) at 160°C under argon. In order to make it cross-linkable, the resulting hydroxyl-terminated pre-polymer (1 eq) was modified by acrylation with acryloyl chloride (2 eq) in the presence of N,N-diisopropylethylamine (2 eq) and 4-dimethylaminopyridine (0.2 eq). The product was purified by filtration through a column of activated alumina and roto-evaporation of the solvent.
Two types of commercially available bone cements were used as base cements, namely Simplex P (Stryker Orthopaedics) and Osteopal V (Heraeus Medical GmbH). While Simplex P is indicated for use in e.g., joint prosthesis fixation, Osteopal V has been developed for use in the spine and contains larger amounts of radiopacifier (45.0 wt% zirconium dioxide, ZrO2, in comparison to the 10 wt% barium sulfate, BaSO4, of Simplex P). The powder phase of Osteopal V (26 g) contains 54.6 wt% poly(methyl acrylate-co-methyl methacrylate), 45.0 wt% zirconium dioxide, 0.4 wt% benzoyl peroxide, and chlorophyll VIII. The liquid phase (10 ml) contained 92 wt% methyl methacrylate monomer, 2 wt% N,N-dimethyl-p-toluidine and 6 wt% other additives including chlorophyll VIII and hydroquinone. The powder phase of Simplex P (20 g) contains 75 wt% methyl methacrylate-styrene copolymer (in turn containing 1.7 wt% benzoyl peroxide), 15 wt% poly(methyl methacrylate) and 10 wt% BaSO4. The liquid component (10 ml) contained 97.5 vol% methyl methacrylate monomer, 2.5 vol% N,N-dimethyl-p-toluidine and small amounts of hydroquinone.
The liquid components of Osteopal V and Simplex P were modified by replacing some of the MMA monomer with 16, 18 and 20 wt% (of the total cement) of oTMC, and homogenizing the mixture using a Vortex-Genie 2 (Scientific Industries). The cements were then prepared by mixing the resulting liquid with the (unmodified) powder phase in a Cap Vibrator (Ivoclar Vivadent AB), for 30 s. The resulting cements are herein referred to as “base cement – x wt% oTMC,” i.e., Osteopal V-16%oTMC refers to Osteopal V as the base cement, modified with 16 wt% oTMC.
The mechanical properties of the cured cements were determined using uniaxial compression tests in a universal materials testing machine (AGS-X, Shimadzu Corp.) according to ASTM F-451.Citation32 Cylindrical specimens of 6 mm diameter and 12 mm height were tested at a loading rate of 20 mm/min after storage in air at room temperature for 24 h.Citation32
The cytotoxicity of selected cement extracts was investigated on MG-63 cells (ECACC, 86051601) based on ISO 10993-5.Citation35 Since this is a curing system, a previously developed method for evaluating these types of materials was used.Citation21,23 In summary, the cements were mixed as previously described and approximately 5 g per sample were injected through a 20 ml syringe into sterile 50 mL Falcon tubes. At 2.5 min after start of mixing, culture medium - DMEM/F12 supplemented with 10% bovine calf serum (Thermo Scientific HyClone), 2 mM glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin (Sigma-Aldrich) - was added to obtain a 200 mg/mL sample-to-medium ratio. Extraction was carried out at 37°C. The cell culture medium of each sample was withdrawn and replaced by fresh culture medium at 1 h, 12 h, and 24 h after adding it to the cements. Sampled extracts were filtered (0.2 µm) and either stored in the fridge or used immediately after sampling. MG-63 cells were seeded in 24 well plates at a density of 30,000 cells/cm2. For each incubation time (1 and 3 d), 3 wells were used per experimental group and 6 wells for the control. After 24 h, the culture medium was aspirated and replaced by the extraction media of each group, while fresh medium was added to the control wells. Cells were cultured for 1 and 3 d, after which the media were aspirated and cells were carefully washed with DPBS twice. To assess the cell viability an alamarBlue® viability assay (Invitrogen) was applied according to the supplier's instructions. Cell morphology was evaluated using light microscopy.
The handling properties of the cements, in terms of peak polymerization temperature, doughing and setting time were evaluated for Osteopal V and a selected formulation, according to ASTM F-451,Citation32 using 10 g of cement.
IBM SPSS Statistics v. Twenty-one (IBM) was used to perform statistical analyses at a critical level of α = 0.05. Comparison of 2 groups was carried out using t-tests, whereas comparison of more than 2 groups was performed using an analysis of variance (ANOVA) or Welch's robust test of equality of means, followed by Scheffe's or Tamhane's post-hoc test (when equal variances could or could not be confirmed, respectively).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Funding from the European Union (SPINEGO PERG07-GA-2010-268134) as well as VINNOVA (VINNMER 2010-02073) is gratefully acknowledged.
References
- Charnley J. Anchorage of the femoral head prosthesis to the shaft of the femur. J Bone Joint Surg Br 1960; 42-B:28-30; PMID:13855642
- Vinay S, Khan SK, Braybrooke JR. Lumbar vertebral haemangioma causing pathological fracture, epidural haemorrhage, and cord compression: a case report and review of literature. Journal of Spinal Cord Medicine 2011; 34:335-9; PMID:21756575; http://dx.doi.org/10.1179/2045772311Y.0000000004
- Angtuaco EJC, Fassas ABT, Walker R, Sethi R, Barlogie B. Multiple Myeloma: Clinical Review and Diagnostic Imaging. Radiology 2004; 231:11-23; PMID:14990813; http://dx.doi.org/10.1148/radiol.2311020452
- Georgy BA. Metastatic Spinal Lesions: State-of-the-Art Treatment Options and Future Trends. Am J Neuroradiol 2008; 29:1605-11; PMID:18566009; http://dx.doi.org/10.3174/ajnr.A1137
- Freedman BA, Potter BK, Nesti LJ, Giuliani JR, Hampton C, Kuklo TR. Osteoporosis and vertebral compression fractures-continued missed opportunities. Spine J 2008; 8:756-62; PMID:18343730; http://dx.doi.org/10.1016/j.spinee.2008.01.013
- Burton AW, Mendoza T, Gebhardt R, Hamid B, Nouri K, Perez-Toro M, Ting J, Koyyalagunta D. Vertebral compression fracture treatment with vertebroplasty and kyphoplasty: experience in 407 patients with 1,156 fractures in a tertiary cancer center. Pain Med 2011; 12:1750-7; PMID:22123171; http://dx.doi.org/10.1111/j.1526-4637.2011.01278.x
- Trout AT, Kallmes DF, Layton KF, Thielen KR, Hentz JG. Vertebral endplate fractures: an indicator of the abnormal forces generated in the spine after vertebroplasty. J Bone Miner Res 2006; 21:1797-802; PMID:17002575; http://dx.doi.org/10.1359/jbmr.060723
- Trout AT, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty: adjacent fractures occur significantly sooner. AJNR Am J Neuroradiol 2006; 27:217-23; PMID:16418388
- Nieuwenhuijse MJ, Putter H, van Erkel AR, Dijkstra PD. New vertebral fractures after percutaneous vertebroplasty for painful osteoporotic vertebral compression fractures: a clustered analysis and the relevance of intradiskal cement leakage. Radiology 2013; 266:862-70; PMID:23204545; http://dx.doi.org/10.1148/radiol.12120751
- Kurtz SM, Villarraga ML, Zhao K, Edidin AA. Static and fatigue mechanical behavior of bone cement with elevated barium sulfate content for treatment of vertebral compression fractures. Biomaterials 2005; 26:3699-712; PMID:15621260; http://dx.doi.org/10.1016/j.biomaterials.2004.09.055
- Hernandez L, Muñoz ME, Goñi I, Gurruchaga M. New injectable and radiopaque antibiotic loaded acrylic bone cements. J Biomed Mat Res Part B: App Biomat 2008; 87B:312-20; http://dx.doi.org/10.1002/jbm.b.31105
- Nazarian A, von Stechow D, Zurakowski D, Müller R, Snyder B. Bone Volume Fraction Explains the Variation in Strength and Stiffness of Cancellous Bone Affected by Metastatic Cancer and Osteoporosis. Calci Tissue Inter 2008; 83:368-79; PMID:18946628; http://dx.doi.org/10.1007/s00223-008-9174-x
- Morgan EF, Bayraktar HH, Keaveny TM. Trabecular bone modulus-density relationships depend on anatomic site. J Biomech 2003; 36:897-904; PMID:12757797; http://dx.doi.org/10.1016/S0021-9290(03)00071-X
- Helgason B, Perilli E, Schileo E, Taddei F, Brynjólfsson S, Viceconti M. Mathematical relationships between bone density and mechanical properties: A literature review. Clin Biomech 2008; 23:135-46; PMID:17931759; http://dx.doi.org/10.1016/j.clinbiomech.2007.08.024
- Beck S, Boger A. Evaluation of the particle release of porous PMMA cements during curing. Acta Biomater 2009; 5:2503-7; PMID:19409868; http://dx.doi.org/10.1016/j.actbio.2009.04.002
- Boger A, Bisig A, Bohner M, Heini P, Schneider E. Variation of the mechanical properties of PMMA to suit osteoporotic cancellous bone. J Biomater Sci Polym Ed 2008; 19:1125-42; PMID:18727856; http://dx.doi.org/10.1163/156856208785540154
- Boger A, Bohner M, Heini P, Verrier S, Schneider E. Properties of an injectable low modulus PMMA bone cement for osteoporotic bone. J Biomed Mater Res B Appl Biomater 2008; 86B:474-82; http://dx.doi.org/10.1002/jbm.b.31044
- Boger A, Wheeler K, Montali A, Gruskin E. NMP-modified PMMA bone cement with adapted mechanical and hardening properties for the use in cancellous bone augmentation. J Biomed Mater Res B Appl Biomater 2009; 90:760-6; PMID:19280644; http://dx.doi.org/10.1002/jbm.b.31345
- Lam WM, Pan HB, Fong MK, Cheung WS, Wong KL, Li ZY, Luk KD, Chan WK, Wong CT, Yang C, et al. In Vitro characterization of low modulus linoleic acid coated strontium-substituted hydroxyapatite containing PMMA bone cement. J Biomed Mater Res B Appl Biomater 2011; 96:76-83; PMID:21053263; http://dx.doi.org/10.1002/jbm.b.31741
- López A, Hoess A, Thersleff T, Ott M, Engqvist H, Persson C. Low-modulus PMMA bone cement modified with castor oil. Biomed Mater Eng 2011; 21:323-32
- López A, Mestres G, Karlsson Ott M, Engqvist H, Ferguson SJ, Persson C, Helgason B. Compressive mechanical properties and cytocompatibility of bone-compliant, linoleic acid-modified bone cement in a bovine model. J Mech Behav Biomed Mater 2014; 32:245-56
- Persson C, Robert E, Carlsson E, Robo C, López A, Godoy-Gallardo M, Ginebra MP, Engqvist H. The effect of unsaturated fatty acid and triglyceride oil addition on the mechanical and antibacterial properties of acrylic bone cements. J Biomater Appl 2015; 30:279-89
- Hoess A, López A, Engqvist H, Karlsson Ott M, Persson C. Comparison of a quasi-dynamic and a static extraction method for the cytotoxic evaluation of acrylic bone cements. Submitted 2016 Mater Sci Eng C Mater Biol Appl
- Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog Poly Sci 2007; 32:762-98
- Pêgo AP, Grijpma DW, Feijen J. Enhanced mechanical properties of 1,3-trimethylene carbonate polymers and networks. Polymer 2003; 44:6495-504.
- López A, Persson C, Hilborn J, Rojas R. Comparative characterization of oligomeric precursors intended for injectable implants. Polymers for Advanced Technologies 2013; 24:15-21.
- Kopperdahl DL, Keaveny TM. Yield strain behavior of trabecular bone. J Biomech 1998; 31:601-8; PMID:9796682
- Almeida T, Leite Ferreira BJM, Loureiro J, Correia RN, Santos C. Preliminary evaluation of the in vitro cytotoxicity of PMMA-co-EHA bone cement. Mater Sci Eng: C 2011; 31:658-62
- Anancharungsuk W, Polpanich D, Jangpatarapongsa K, Tangboriboonrat P. In vitro cytotoxicity evaluation of natural rubber latex film surface coated with PMMA nanoparticles. Coll Surf B Biointer 2010; 78:328-33; PMID:20392612
- dos Santos LA, Carrodeguas RG, Rogero SO, Higa OZ, Boschi AO, de Arruda AC. Alpha-tricalcium phosphate cement: “in vitro” cytotoxicity. Biomaterials 2002; 23:2035-42; PMID:11996045
- Farrar DF, Rose J. Rheological properties of PMMA bone cements during curing. Biomaterials 2001; 22:3005-13; PMID:11575475
- West Conshohocken, PA, USA. ASTM F 451-08 Standard Specification for Acrylic Bone Cement. American Society for Testing and Materials, 2008.
- Li S, Chien S, Branemark PI. Heat shock-induced necrosis and apoptosis in osteoblasts. J Orthop Res 1999; 17:891-9; PMID:10632456; http://dx.doi.org/10.1002/jor.1100170614
- Berman AT, Reid JS, Yanicko DR, Jr., Sih GC, Zimmerman MR. Thermally induced bone necrosis in rabbits. Relation to implant failure in humans. Clin Orthop Relat Res 1984:284-92; PMID:6723155
- Genève, Switzerland. ISO10993: Biological evaluation of medical devices: International Organization for Standardization, 2001.

