ABSTRACT
Excessive deposit of epicardial adipose tissue (EAT) were recently shown to be positively correlated with cardiovascular disease (CVD). This study aims to investigate the thickness of EAT and its association with the components of metabolic syndrome among multi-ethnic Malaysians with and without acute coronary syndrome (ACS). A total of 213 patients were recruited, with the thickness of EAT were quantified non-invasively using standard two-dimensional echocardiography. EAT thickness among the Malaysian population was prompted by several demographic factors and medical comorbidities, particularly T2DM and dyslipidaemia. ACS patients have significantly thicker EAT compared to those without ACS (4.1 mm vs 3.7 mm, p = 0.035). Interestingly, among all the races, Chinese had the thickest EAT distribution (4.6 mm vs 3.8 mm), with age (p = 0.04 vs p < 0.001), and overall diastolic blood pressure (p = 0.028) was also found to be associated with EAT thickness. Further study is warranted to investigate its role as a cardiovascular risk marker among Malaysians with ACS.
Introduction
Epicardial adipose tissue (EAT) is a form of visceral fat depot accumulated between the visceral pericardium and the myocardium, without any fascia separating it from the myocardium and the epicardial vessels. EAT contributes to 15% of total cardiac mass, which surrounds the ventricles and the surfaces of the heart as it increases in volume and thickness [Citation1]. Nourished by the coronary arteries, EAT has a variable distribution, being more prominent in the atrioventricular and interventricular grooves and right ventricular lateral wall [Citation2].
Histologically, EAT is a white adipose tissue that has brown fat-like and beige fat-like features [Citation3]. The original neonatal brown adipocytes of EAT will evolve and transition into white or beige adipocytes as human mature and age. This transition is a hallmark of EAT in adult population. In comparison to brown adipocyte that involves in preserving thermogenesis and provide cardio-protective effects [Citation4], white adipocytes function to regulate lipid synthesis and lipolysis. White adipocytes are also known to incite pathogenesis of several cardiovascular disorders such as atrial fibrillation, coronary artery disease (CAD) and congestive heart failure [Citation5].
Physiologically, the EAT layer serves to regulate vascular flow, protects the myocardium and the coronaries from inflammatory and pathogenic substances, provides mechanical protection, and serves as local source of fatty acids for the myocardium during increased energy demand [Citation6]. Similar to other visceral fat compartments, EAT also actively secretes both inflammatory cytokines such as tumour necrosis factor–α, monocyte chemoattractant protein–1, interleukin-6, nerve growth factor, resistin, as well as anti-inflammatory adipokines such as adiponectin and adrenomedullin [Citation7].
The paracrine effects of pro-inflammatory mediators released by EAT on coronary arteries and the myocardium are now being extensively studied, given their anatomic proximity [Citation7]. Cytokines and fatty acids are disseminated locally through microcirculation and vasa vasorum. Evidently, the concentration of perivascular cytokines was shown to be higher in EAT compared to subcutaneous fat and was shown to locally accelerate the atherosclerotic process by causing endothelial dysfunction, and local proliferation of smooth muscle cells, increased oxidative stress, plaque instability and neovascularization [Citation2]. In addition, patients with CAD are documented to have thicker EAT, with areas containing atherosclerotic lesion showing increased EAT depot, compared to the area with no atherosclerotic lesion. Consequently, the thickness of EAT is now proposed to be more relevant cardiovascular risk estimation than other visceral fat depot [Citation8].
Despite its potential implications in CAD, EAT is also associated with other known factors, such as age, ethnicities, adiposity status and several chronic comorbidities, including diabetes mellitus and hypertension, which makes the interpretation of its role as an independent risk marker intricate. More significantly, measures of total body fat and depot-specific adiposity reveal distinct ethnic and sex patterns, which may portend different health implications [Citation9].
The extension of the population study on the impact of EAT thickness on demographic and cardiometabolic factors has been previously established in several countries. For example, EAT thickness was strongly associated with Body Mass Index (BMI), age, baseline creatinine and glucose among 438 ACS patients in Austria [Citation10]. Race and ethnicity were reportedly to be a useful predictor for EAT thickness, in which Caucasian have higher amounts of EAT depot than African-Americans [Citation11]. On the contrary, some studies demonstrated no correlation between EAT with demographic and cardiometabolic parameters among Iranian ACS patients, including gender, height, blood pressure, glucose, total cholesterol level and ejection fraction [Citation12].
The variability in results on the distribution of EAT provided a significant perspective for ethnically diverse countries, such as Malaysia. There are presently no studies demonstrating the relationship between EAT and ethnicity as well as metabolic syndrome indicators in patients with and without ACS in the country. Therefore, this study aims to describe the EAT thickness among the multi-ethnic Malaysian population presented with and without ACS with its association with metabolic syndrome components.
Methodology
This was a retrospective, cross-sectional study of 213 patients who went for echocardiography in Cardiology Unit at University Malaya Medical Centre (UMMC), Malaysia from 1 January of 2019 until 30 September 2022. Patients’ registration number and demographic data were obtained from the Cardiology Unit using the National Cardiovascular Disease Database (NCVD)-ACS Registry Notification Form, while the patient’s medical record including blood pressure, BMI and blood biomarkers (total cholesterol, triglycerides, high-density lipoprotein; (HDL), low-density lipoprotein (LDL) and glycated haemoglobin (HbA1C) was obtained from the medical record unit.
Inclusion and exclusion criteria
Malaysian patients with and without ACS aged 18 to 75 years old who underwent echocardiography within the study period at UMMC were included in the study. Patients were excluded from the study based on the following exclusion criteria: incomplete demographic information and medical record pertaining to the metabolic syndrome components, patients with cerebrovascular disease, patients with heart, hepatic or renal failure and patients with poor quality of echocardiogram images. A pilot study involving 30 echocardiogram images was done to optimize the quantification of epicardial fat thickness from echocardiogram images.
Identifications of patients with metabolic syndrome
Lab values of fasting plasma blood glucose level, triglycerides and HDL-cholesterol level were obtained within the days of hospitalization, with echocardiogram data obtained within 3 months from the admission date or from the date when the laboratory test was being done. Patients were categorized with cardiometabolic syndrome if presented with central obesity (waist circumference ≥90 cm in males, ≥ 80 cm in females (for South Asian population), or BMI (>30 kg/m2) along with any two of the following four factors: triglycerides ≥150 mg/dL (1.7 mmol/L); HDL-cholesterol <40 mg/dl (1.03 mmol/L) in males, < 50 mg/dL (1.29 mmol/L) in females; blood pressure, systolic blood pressure (BP) ≥ 130 or diastolic BP ≥85 mmHg or fasting glucose ≥5.6 mmol/L (100 mg/dl) [Citation13]. The cardiometabolic parameters were outlined in accordance to the Malaysia Clinical Practice Guideline on Management of Obesity 2nd Edition (2023) and the Malaysia Clinical Practice Guideline on Management of Type 2 Diabetes Mellitus (T2DM) 6th Edition (2020) in which the overweight and obesity are corresponded to ≥23 kg/m2 and ≥27.5 kg/m2, respectively.
Echocardiographic evaluation
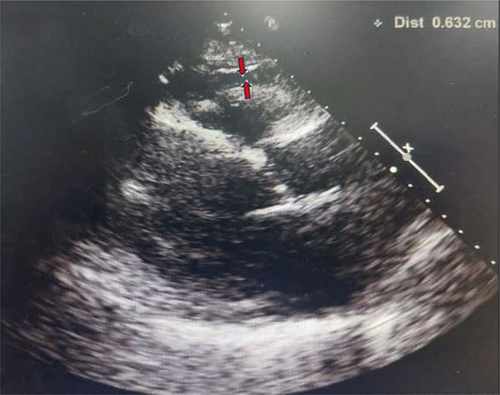
EAT can be visualized and measured non-invasively using standard two-dimensional echocardiography as described in the literatures [Citation14,Citation15]. Using Philips Xcelera R3.3 software, EAT can be observed as an echo-free space between the outer layer of myocardium and the visceral layer of pericardium (Image 1). The image was paused to view the EAT and the EAT thickness was measured at the parasternal long axis on the free wall of the right ventricles after 3 cardiac cycles of systole. Area of the EAT was also recorded. Three readings were taken and the mean value was obtained for analysis. Ejection fraction was also measured using echocardiogram to evaluate patient’s cardiac function, as per described by the literatures [Citation16,Citation17]. These measurements were performed by the sonographers who were blinded to the clinical and quantitative analysis data.
Image 1 Measures of EAT thickness marked by red arrows.
Statistical method
Data collected were analysed using Statistical Package for Social Science (SPSS) version 28. Categorical data were expressed as number and percentage (%), while continuous data were represented by mean ± standard deviations. Comparison of the mean epicardial fat thickness and metabolic syndrome components between ethnic and gender were performed. Spearman’s correlation test was used to assess the association between the epicardial fat thickness with metabolic syndrome components. Statistical significance was set at p-value <0.05.
Results
Baseline characteristics
A total of 213 patients (125 patients presented with ACS and 88 patients presented without ACS) who underwent echocardiography from 2019 until 2022 were included in the study. The baseline demographics of enrolled patients are described in . The mean age of patients was 59.4 years old and 70.0% were predominantly by male, in which 72.8% was diagnosed with ACS and 65.9% was without ACS. In relation to ACS stratification, the patients with ACS were more likely presented with non-ST segment myocardial infarction (NSTEMI) and unstable angina (UA), in which corresponding to 35.2% and 32.8% respectively, while patients presented with ST-Elevation Myocardial Infarction (STEMI) are 23.2%.
Table 1. Patient demographic profile, cardiometabolic and medication data.
Malays comprised the highest populations in both groups with and without ACS (45.6% and 50%), followed by Indians (38.4% and 27.3%) and Chinese (16.0% and 20.5%), respectively. Compared to the non-ACS group, patients with ACS were presented with significant comorbidities, including dyslipidaemia (75.2% vs 42.0% respectively) and T2DM (92.8% and 71.6%, respectively). Average BMI among patients with ACS were equivalent to patients without ACS, with BMI of 26.9 kg/m2. Patients with ACS were predominantly overweight (47.5%), and 40.2% were in the obese category with the remaining 12.3% were with normal BMI. Majority of non-ACS patients recruited were predominantly obese, (43.2%), followed by those that are overweight (42.0%) and 14.8% of patients were with normal BMI.
All components of cardiometabolic parameters were slightly higher in patients without ACS, with systolic and diastolic blood pressure values ranging from 139.6 ± 25.84 mmHg and 78.0 mmHg. Blood biomarkers such as cholesterol, LDL, FBG and HbA1C values were 4.8 mmol/L, 3.0 mmol/L, 7.8 mmol/L and 8.2%, respectively. There is a slight decrease in HDL level in male patients with ACS (0.9 mmol/L), as well as female patients without ACS (1.1 mmol/L). However, there were no statistical differences noted for other cardiometabolic parameters, with exception of cholesterol level, in which corresponded to p = 0.039.
Echocardiographic evaluation of EAT thickness
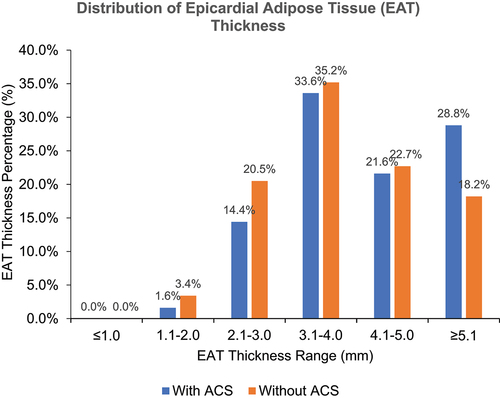
Average EAT thickness was measured at 4.0 mm. Interestingly, EAT was significantly thicker among patients with ACS compared to those without (4.1 mm vs 3.7 mm, respectively, p = 0.035). Mean ejection fraction was 54.2 ± 16.96%, and ejection fraction among ACS patients was significantly lower as compared to those without ACS (551.7 ± 17.50% vs 57.8 ± 15.56%, respectively, p = 0.010) (). The EAT thickness range for both groups were in the range of 3.1 to 4.0 mm (33.6% and 35.2%, respectively) ().
Table 2. EAT thickness and ejection fraction among patients with and without ACS.
Correlation of EAT thickness with age, gender and ethnicities
Age was found to be significantly correlated with EAT thickness, in both groups of patients with ACS (p = 0.040) or without ACS (p < 0.001) (). Thicker EAT was noted among male ACS patients (4.3 mm (IQR 1.8)) compared to female ACS patients (3.9 mm (IQR 1.3)). Interestingly, EAT was thicker among female non-ACS patients (4.2 mm (IQR 2.2)) when compared to male non-ACS patients (3.6 mm (IQR 1.3)). Among all races, Chinese are presented with thicker EAT of 4.6 mm (IQR 2.1) among the ACS group and those without ACS 3.8 mm (IQR 1.2). However, genders and ethnicities were reported to be statistically insignificant.
Table 3. The mean thickness of epicardial fat by age, gender and ethnicity.
Associations of EAT thickness with cardiometabolic parameters
Diastolic blood pressure was found to be negatively correlated with EAT thickness for the overall patients in the study cohort (r = −0.15, p = 0.028) (). Other than diastolic blood pressure, epicardial fat thickness was not found correlated with any other components of metabolic syndrome, regardless of ACS diagnosis.
Table 4. Correlation between EAT thickness with metabolic syndrome component.
Discussion
EAT, a type of visceral adipose tissue that surrounds the heart is quantifiable, modifiable and multifaceted tissue that has both local and systemic effects. Enlarged EAT contributes to atherosclerotic cardiovascular disease [Citation18], and the thickness of EAT is increasingly recognized as a potential biomarker for ACS, parallel to its association with metabolic syndrome [Citation19]. Measures of total body fat and depot-specific adiposity reveal distinct ethnic patterns [Citation20], which may portend different health implications particularly among multi-ethnics Malaysian.
In our current study, patients with ACS are presented with significantly reduced ejection fraction and exhibited thicker EAT thickness. Our findings are consistent with the reports from the neighbouring Asian countries including Korea, Japan and India, Singapore and Indonesia where patients with underlying cardiovascular disease including CAD and heart failure with reduced ejection fraction are associated with increased EAT thickness [Citation21–26]. Evidently, EAT is a metabolically active visceral depot and serves as an important source for both pro-inflammatory adipokines including tumour necrosis factor-α, interleukin 1, interleukin 6, nerve growth factor as well as anti-inflammatory adipokines production. Adipose inflammation, which is present in metabolic diseases, is closely linked to detrimental changes in the structure and function of the cardiovascular system [Citation27]. Excessive deposition of epicardial increase secretion of pro-inflammatory adipokines and induce compositional changes in the inner layer of intima through paracrine and vasocrine pathways and promote the development of CAD [Citation28,Citation29]. Additionally, individuals with increased EAT are also play in the development, progression and vulnerability of coronary vessel plaque, indicating its key role in the progression of CAD [Citation8].
Interestingly, our findings indicate that EAT distribution is lower in our study cohort, particularly those with ACS but within the normal Asian range of between 1.5 to 4.4 mm30 [Citation29] for the non-ACS patients. Caucasian ACS patients in general have 15% thicker EAT compared to the Asian population [Citation30] with the variability in EAT distribution among different ethnic groups can be hypothesized by racial variation in visceral fat distribution [Citation31]. Indeed, most global studies reported the remarkable differences in EAT thickness between races and ethnicity of subjects with and without CAD [Citation11,Citation32,Citation33]. Notably, the current study demonstrated that the Malaysian Chinese population has a higher epicardial fat thickness, in both ACS and non-ACS group. Contrary to our findings, the recent National Health and Morbidity Survey 2019 indicates that abdominal adiposity is highest among Indians. However, the current findings are in agreement with another Malaysian study, whereby Chinese ethnicity was regarded as one of the risk factors for left ventricular diastolic dysfunction in T2DM in Malaysian populations [Citation34]. It is known that EAT is associated with impaired left ventricular (LV) function, even if cardiovascular disease is absent [Citation35].
From our study, EAT was only found to be correlated with age and diastolic blood pressure. The correlation of EAT with age was statistically significant and is in agreement with several studies. It has been well established that CAD risk in middle-aged and elderly patients is correlated with increased EAT thickness, and regarded as a prominent indicator for diagnosis of CAD among this age group [Citation36]. As a person ages, the epicardial fat thickness will increase due to redistribution of the fat to the visceral and trunk [Citation37]. Additionally, the diastolic blood pressure also appears to be correlated with EAT thickness for overall populations. Indeed, a large population of our study samples are obese and are with metabolic syndrome, risk factors that are commonly associated with atrial distension. With increased of EAT thickness, the close proximity of the left atrium and left ventricle may affect the diastolic filling of the left ventricle, and later causing left atrial distension, which is a common risk factor for atrial fibrillation and other CAD complications [Citation38].
The current study has recognized medical comorbidities, such as T2DM and dyslipidaemia were highly prominent among our study cohort, particularly among our ACS group; known risk factors for the development for CAD. However, other cardiometabolic parameters such as systolic blood pressure (SBP), fasting blood glucose (FBG), BMI and lipid related markers (triglycerides (TG) and HDL) did not show any significant differences with EAT thickness. Indeed, it is essential to further evaluate these factors for a comprehensive understanding of relationship between EAT thickness and the risk of CAD. A more in-depth exploration of these factors with EAT in a randomized trials or prospective observational study can offer a more valuable insights into the complex connections between EAT changes, metabolic elements and the overall risk of coronary artery disease in populations with high comorbidities.
Although the current study provides valuable insights into the relationship between epicardial fat thickness and ACS diagnosis among different ethnic groups in Malaysia, it is important to acknowledge that this retrospective, cross-sectional study has several limitations that may have influenced the results. One of the limitations is the potential confounding effect of lifestyle factors, which were not thoroughly assessed in this study. Other factors that may have influenced the results include the dose and duration of the treatment administered to the patients. Furthermore, the small sample size (N = 213) limits the generalizability of the findings to the broader population. Despite these limitations, the study provides a foundation for future research to investigate the role of epicardial fat thickness as a potential cardiovascular risk marker in Malaysians with ACS.
Conclusions
Despite the limitations of this study, EAT thickness among Malaysian populations appears to be influenced by several risk factors, including age, diastolic blood pressures and underlying concomitant comorbidities, particularly T2DM and dyslipidaemia. This is the first Malaysian study that provides a preliminary point of view on thickness of epicardial fat across the multi-ethnicities in patients presenting with and without ACS. Further study is needed to determine the role of EAT thickness as a cardiovascular risk marker in patients presented with and without ACS.
Ethical approval
Data collection was initiated upon approval by the Medical Ethics Committee of Universiti Malaya Medical Centre (UMMC) – Ethics number: 2016117–4586.
Author contributions
Conceptualization, A.H.A.J, W.A.S.M, H.Z.H and W.A.W.A; Methodology, A.H.A.J, W.A.S.M, H.Z.H, A.S.M.Z and W.A.W.A; Data curation, W.A.S.M, A.H.A.J., K.N.I, and N.B.; Formal Analysis, W.A.S.M, A.H.A.J., K.N.I. and N.B.; Investigation, W.A.S.M. and A.H.A.J.; Resources, W.A.S.M, A.H.A.J, S.S, H.Z.H, A.S.M.Z. and W.A.W.A.; Writing – Original Draft, W.A.S.M. and A.H.A.J.; Writing – Review and Editing, W.A.S.M, A.H.A.J., K.N.I., N.A.Z.Z., H.Y.L. and K.G; Funding Acquisition S.S, A.S.M.Z, M.F.M.N, H.Z.H, and A.H.A.J.
Acknowledgments
We would like to thank the Director General of Health Malaysia for his permission to publish this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The analysed data used to support the findings of this study are included within the article.
Additional information
Funding
References
- AlZaim I, Hammoud SH, Al-Koussa H, et al. Adipose tissue immunomodulation: a novel therapeutic approach in cardiovascular and metabolic diseases. Front Cardiovasc Med. 2020;7:602088.
- Bertaso AG, Bertol D, Duncan BB, et al. Epicardial fat: definition, measurements and systematic review of main outcomes. Arq Bras Cardiol. 2013;101(1). doi: 10.5935/abc.20130138
- Sacks HS, Fain JN, Bahouth SW, et al. Human epicardial fat exhibits beige features. J Clin Endocrinol Metab. 2013;98(9):E1448–9. doi: 10.1210/jc.2013-1265
- Chen HJ, Meng T, Gao PJ, et al. The role of brown adipose tissue dysfunction in the development of cardiovascular disease. Front Endocrinol (Lausanne). 2021;12:652246. doi: 10.3389/fendo.2021.652246
- Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol. 2022;19(9):593–606.
- Wu Y, Zhang A, Hamilton D, et al. Epicardial Fat in the Maintenance of Cardiovascular Health. Methodist Debakey Cardiovasc J. 2017;13(1):20. doi: 10.14797/mdcj-13-1-20
- Iacobellis G, Malavazos AE, Corsi MM. Epicardial fat: from the biomolecular aspects to the clinical practice. Int J Biochem Cell Biol. 2011;43(12):1651–1654. doi: 10.1016/j.biocel.2011.09.006
- Talman AH, Psaltis PJ, Cameron JD, et al. Epicardial adipose tissue: far more than a fat depot. Cardiovasc Diagn Ther. 2014;4(6):416. doi: 10.3978/j.issn.2223-3652.2014.11.05
- Staiano AE, Broyles ST, Gupta AK, et al. Ethnic and sex differences in visceral, subcutaneous, and total body fat in children and adolescents. Obesity. 2013;21(6):1251–1255. doi: 10.1002/oby.20210
- Tscharre M, Hauser C, Rohla M, et al. Epicardial adipose tissue and cardiovascular outcome in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Eur Hear Journal Acute Cardiovasc Care. 2017;6(8):750–752. doi: 10.1177/2048872616680609
- Salami SS, Tucciarone M, Bess R, et al. Race and epicardial fat: the impact of anthropometric measurements, percent body fat and sex. Ethn Dis. 2013;23(3):281–285.
- Toufan M, Azarfarin R, Sadati B, et al. The association between epicardial adipose tissue and coronary artery disease: an echocardiographic cut-off point. J Cardiovasc Thorac Res. 2012;4(2):31–316. doi: 10.5681/jcvtr.2012.008
- Alberti KGMM, Zimmet P, Shaw J. The metabolic syndrome - a new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi: 10.1016/S0140-6736(05)67402-8
- Hirata Y, Yamada H, Kusunose K, et al. Clinical utility of measuring epicardial adipose tissue thickness with echocardiography using a high-frequency linear probe in patients with coronary artery disease. J Am Soc Echocardiogr. 2015;28(10):1240–1246.e1. doi: 10.1016/j.echo.2015.07.006
- Yagi S, Hirata Y, Ise T, et al. Canagliflozin reduces epicardial fat in patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2017;9(1). doi: 10.1186/s13098-017-0275-4
- Kim MK, Kim B, Lee JY, et al. Tissue doppler-derived e/e′ ratio as a parameter for assessing diastolic heart failure and as a predictor of mortality in patients with chronic kidney disease. Korean J Intern Med. 2013;28(1):35–44. doi: 10.3904/kjim.2013.28.1.35
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7(2):79–108. doi: 10.1016/j.euje.2005.12.014
- Villasante Fricke AC, Iacobellis G. Epicardial adipose tissue: clinical biomarker of cardio-metabolic risk. Int J Mol Sci. 2019;20(23):1–13. doi: 10.3390/ijms20235989
- Wang CP, Hsu HL, Hung WC, et al. Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus an association with metabolic syndrome and severity of coronary atherosclerosis. Clin Endocrinol. 2009;70(6):876–882. doi:10.1111/j.1365-2265.2008.03411.x
- Nazare JA, Smith JD, Borel AL, et al. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the international study of prediction of intra-abdominal adiposity and its relationship with cardiometabolic risk/intra-. Am J Clin Nutr. 2012;96(4):714–726. doi: 10.3945/ajcn.112.035758
- Verma B, Katyal D, Patel A, et al. Relation of systolic and diastolic epicardial adipose tissue thickness with presence and severity of coronary artery disease (the EAT CAD study). J Fam Med Prim Care. 2017;6(2):169–170.
- Park JS, Lee YH, Seo KW, et al. Echocardiographic epicardial fat thickness is a predictor for target vessel revascularization in patients with ST-elevation myocardial infarction. Lipids Health Dis. 2016;15(1):1–7. doi: 10.1186/s12944-016-0371-8
- Fukuda T, Bouchi R, Terashima M, et al. Ipragliflozin reduces epicardial fat accumulation in non-obese type 2 diabetic patients with visceral obesity: a pilot study. Diabetes Ther. 2017;8(4):851–861. doi: 10.1007/s13300-017-0279-y
- Bouchi R, Terashima M, Sasahara Y, et al. Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: A pilot study. Cardiovasc Diabetol. 2017;16(1). doi: 10.1186/s12933-017-0516-8
- Jin X, Hung CL, Tay WT, et al. Epicardial adipose tissue related to left atrial and ventricular function in heart failure with preserved versus reduced and mildly reduced ejection fraction. Eur J Heart Fail. 2022;24(8):1346–1356. doi: 10.1002/ejhf.2513
- Alkatiri AH, Bakri S, Patellongi I, et al. The relationship between epicardial adipose tissue thickness with severity of coronary artery disease in Indonesia. Int J Sci Basic Appl Res. 2016;30(5):244–253.
- Rim R, Viveiros A, Oudit GY, et al. Targeting perivascular and epicardial adipose tissue inflammation: therapeutic opportunities for cardiovascular disease. Clin Sci. 2020;134(7):827–851. doi: 10.1042/CS20190227
- Park JH, Park YS, Kim YJ, et al. Effects of statins on the epicardial fat thickness in patients with coronary artery stenosis underwent percutaneous coronary intervention: comparison of atorvastatin with simvastatin/ezetimibe. J Cardiovasc Ultrasound. 2010;18(4):121. doi: 10.4250/jcu.2010.18.4.121
- Meenakshi K, Rajendran M, Srikumar S, et al. Epicardial fat thickness: a surrogate marker of coronary artery disease - assessment by echocardiography. Indian Heart J. 2016;68(3):336–341. doi: 10.1016/j.ihj.2015.08.005
- Aprigliano G, Scuteri L, Iafelice I, et al. Epicardial adipose tissue thickness and acute coronary syndrome: a matter of how much or how? Int J Cardiol. 2015;199:8–9. doi: 10.1016/j.ijcard.2015.06.168
- Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22(12):1311–1319. doi: 10.1016/j.echo.2009.10.013
- Adams DB, Narayan O, Munnur RK, et al. Ethnic differences in coronary plaque and epicardial fat volume quantified using computed tomography. Int J Cardiovasc Imaging. 2017;33(2):241–249. doi: 10.1007/s10554-016-0982-1
- Pierdomenico SD, Pierdomenico AM, Cuccurullo F, et al. Meta-analysis of the relation of echocardiographic epicardial adipose tissue thickness and the metabolic syndrome. Am J Cardiol. 2013;111(1):73–78. doi: 10.1016/j.amjcard.2012.08.044
- Chee KH, Tan KL, Luqman I, et al. Prevalence and predictors of left ventricular diastolic dysfunction in Malaysian patients with type 2 diabetes mellitus without prior known cardiovascular disease. Front Cardiovasc Med. 2021;8(September):8–15. doi: 10.3389/fcvm.2021.676862
- Eren H, Omar MB, Ü K, et al. Epicardial fat tissue can predict subclinical left ventricular dysfunction in patients with erectile dysfunction. Aging Male. 2021;24(1):42–49. doi: 10.1080/13685538.2021.1945572
- Qian C, Sun Y, Jiang J. Diagnostic values of epicardial adipose tissue thickness with right common carotid artery elasticity and intima-media thickness for middle-aged and elderly patients with coronary heart disease. Int J Gen Med. 2021;14:633–639. doi: 10.2147/IJGM.S292426
- Kuk JL, Saunders TJ, Davidson LE, et al. Age-related changes in total and regional fat distribution. Ageing Res Rev. 2009;8(4):339–348. doi: 10.1016/j.arr.2009.06.001
- Lima-Martínez MM, Campo E, Salazar J, et al. Epicardial fat thickness as cardiovascular risk factor and therapeutic target in patients with rheumatoid arthritis treated with biological and nonbiological therapies. Arthritis. 2014;2014:1–7. doi: 10.1155/2014/782850.