Abstract
Many host species of avian brood parasites have evolved to recognize and reject foreign eggs and chicks in the nest. Yet, other hosts accept and care for parasitic young, despite the fitness losses associated with raising non-kin. It has been suggested that nest predation upon host nests by brood parasites could select for coevolved acceptance by hosts, even when their cognitive and motor traits allow for the successful rejection of brood parasitism. Using a modeling approach, I analyzed the conditions that favor the evolution of two predatory strategies by parasites and the acceptance of parasitism in the presence of predatory parasites. The Mafia strategy represents retaliatory parasites that punish rejecter hosts by depredating their nests. In contrast, the Farmer strategy represents farming parasites which depredate advanced stage host nests. Both predatory strategies benefit when hosts become available for future parasitism by renesting. The modeling showed that higher rates of parasitism and rejection, and lower rates of discovery of renests by Farmer parasites, favor the Mafia strategy over Farmer. Host acceptance of parasitism never yielded greater fitness payoffs over the rejection of parasitic eggs by hosts, implying that lack of host rejection in the presence of predatory brood parasites should not be taken as evidence of coevolution yielding an evolutionary equilibrium. Further experimental and empirical work should concentrate on documenting the frequency and context in which parasites discover and prey upon host nests, to better predict the conditions under which different strategies of predatory parasites are favored.
Introduction
Nesting birds typically remove fallen litter, broken eggshells, and dead nestlings from nests to secure the safe and efficient incubation of eggs and growth of nestlings (Guigueno & Sealy Citation2012). It, therefore, remains an evolutionary puzzle why many host species of obligate avian brood parasites accept discordant eggs and distinctive young of heterospecifics in their nests, and pay the fitness costs associated with raising genetically unrelated progeny (Hauber et al. Citation2004; Peer & Sealy Citation2004). Perhaps even more surprising is that some other hosts have evolved fine-tuned recognition mechanisms to reject parasitic eggs (Bán et al. Citation2013), even when these closely match in size and color their own eggs (Stevens et al. Citation2013), yet many of these same hosts do not reject distinctive parasitic nestlings upon hatching, even after these parasites have expelled or out-competed the hosts’ own eggs and young (Lotem Citation1993; Lawes & Marthews Citation2003).
There are several feasible evolutionary paths and mechanisms that explain the non-ejection of unrelated eggs and young, including an evolutionary lag in the appearance and spread of anti-parasitic adaptations in host populations recently invaded by parasites, or an evolutionary equilibrium between rejection benefits and rejection costs, including recognition errors (Rothstein Citation1990). In general, however, the coevolutionary dynamics between hosts’ and parasites’ responses are understood to match only few of these theoretical scenarios (reviewed in Davies Citation2011; Kilner & Langmore Citation2011; Feeney et al. Citation2012), and there is a need to both generate new theoretical models for host–parasite coevolutionary dynamics and to collect new experimental data to test the predictions of these alternative hypotheses (e.g. Servedio & Hauber Citation2006, followed by Hauber et al. Citation2013).
Increasing amounts of anecdotal, observational, and experimental evidence suggest that brood parasitic birds may act as predators on host nests (Dow Citation1972; Arcese et al. Citation1996; Smith et al. Citation2002; Briskie Citation2007; Dubina & Peer Citation2013). For example, field observations and video recordings at potential hosts of the brown-headed cowbird Molothrus ater have directly documented depredation of eggs and nestlings (Elliott Citation1999). Correlative data on predation rates of host nests suggest that non-parasitized nests of reed warblers Acrocephalus scirpaceus, song sparrows Melospiza melodia, and northern cardinals Cardinalis cardinalis are subjected to higher rates of predation, potentially by their respective brood parasitic species, than are the parasitized nests of the same hosts (reviewed in Ortega Citation1998). Parasite density may also show a positive correlation with predation rates of non-parasitized nests over time, suggesting a direct or indirect effect of the presence of parasites regarding the predation rates of hosts nests (Arcese et al. Citation1996, but see McLaren & Sealy Citation2000). Finally, in several experimental studies, host nests, from which parasitic eggs were removed by researchers, suffered significantly higher rates of partial predation than control nests (Soler et al. Citation1995; Hoover & Robinson Citation2007; Hauber Citation2009, but see Canestrari et al. Citation2014). That predation of non-parasitized host nests is a behavior specially adapted to the parasitic breeding biology of brown-headed cowbirds, for example, is also suggested by observations that female cowbirds often remove but do not always act as predators to consume eggs and chicks of hosts (Sealy Citation1994).
Using different modeling approaches, Pagel et al. (Citation1998), Soler, Møller et al. (Citation1998), Robert et al. (Citation1999) and Chakra et al. (Citation2014) studied the fitness consequences of acceptance and rejection by hosts in the presence of retaliatory parasites, referred to as the “Mafia” strategy (Zahavi Citation1979). The Pagel et al. (Citation1998) model showed, for example, that acceptance might be a stable strategy when retaliation rates are high, the cost of raising a parasitic chick is small, and parasitism rate is low on the subsequent nesting attempts of accepting hosts. These authors suggested, following Wyllie (Citation1981), that retaliation by parasites against rejecter hosts, may have evolved as an extension of the parasite’s behavior to depredate host nests that have advanced too far for parasitic laying to be successful (Soler et al. Citation1999). Depredated hosts will typically renest and the renesting attempt will then be available for future parasitism. In agreement with this suggestion, Pagel et al. (Citation1998) considered documented examples of predatory acts by brown-headed cowbirds and common cuckoos Cuculus canorus to support the existence of retaliation by brood parasites.
Thus, there is extensive evidence that parasites are predators (Dow Citation1972; Briskie Citation2007; Dubina & Peer Citation2013) and that experimental removal of parasite presence/access to non-parasitized host nests results in decreased predation (Hoover & Robinson Citation2007; Zanette et al. Citation2011). These data are consistent with the Farming, as well as the Mafia hypotheses, and two specific studies experimentally provide direct evidence for predictions of the Mafia strategy per se (Soler et al. Citation1995; Hoover & Robinson Citation2007). In contrast, it remains unsupported empirically whether selection for acceptance in hosts by retaliatory parasites may provide a general explanation for the lack of rejection in many host species. Using a modeling approach, I consider here the fitness payoffs for the parasites and the effects upon the hosts’ responses to the two different predatory strategies of brood parasites, Mafia and Farmer, in the context of coevolutionary arms races. This is the first modeling study to simultaneously assess whether coevolved acceptance may be a stable strategy by the host in the presence of Mafia and/or Farmer.
Methods and results
The evolution of different predatory strategies by brood parasites
Using an asymmetrical game-theoretical approach, Pagel et al. (Citation1998) demonstrated that in the presence of brood parasitism, natural selection may favor the evolution of rejection of parasitic eggs and young in hosts. In turn, in the presence of rejection behavior, natural selection may favor the evolution of retaliatory parasites if, among other conditions, renesting attempts of rejecter hosts are likely to be discovered and parasitized.
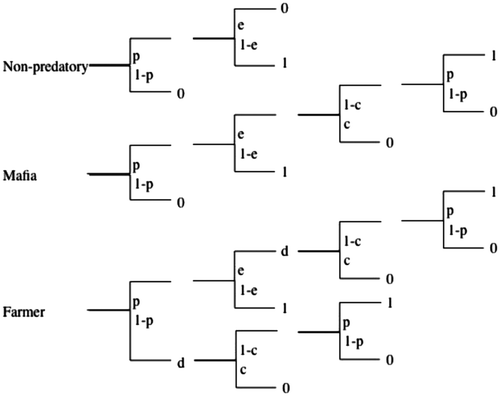
Employing a similar analytical approach, I consider here the alternative scenarios where hosts are parasitized during each of their nesting attempts with a probability p (Figure ). Hosts defend against parasitism on their first breeding attempt by ejecting the parasitic egg (or chick) with a given probability e but they accept parasitism on their renesting attempt which always follows when the first nest is depredated by parasites; that is, the probability of renesting is 1.0 following predation. Note that rejection followed by universal acceptance during a second nesting attempt is an assumption that is unlikely to be upheld generally, although there is evidence that adaptive tolerance of brood parasitism by hosts occurs when temporally variable resources, such as nest sites or time left to successfully raise and fledge a brood, are limited (Petit Citation1991, but see Lindholm Citation2000; Hoover Citation2003; Krüger Citation2011). In addition, several studies report that hosts may switch from rejection to acceptance with repeated parasitism, thus justifying the model’s assumption for at least some host taxa (e.g. Soler, Soler et al. Citation1998; Samas et al. Citation2011).
In this scenario of hosts, that are rejecters then accepters, parasites are modeled to engage in one of three strategies (Figure ):
Non-predatory parasites lay their eggs with the constant probability p in any given host nests, and then they move onto seeking other host nests which are suitable for parasitism.
Retaliatory, or Mafia, parasites lay their eggs with a constant probability in any given host nest. They then return to the parasitized host nests to monitor them. They depredate a proportion of 1 − c of those monitored nests, from which the parasitic egg had been ejected at a rate of e, and c is the relative cost of predation to the parasite. Once rejecter nests are retaliated against, hosts always renest, making their nesting attempt available for further parasitism at the constant probability p.
Farmer, or Farming parasites, lay their eggs in nests of hosts with a constant probability p. They also monitor host nesting attempts, and discover the proportion d of all hosts nests that do not contain their offspring and have advanced too far for successful parasitism to take place. They depredate a proportion of 1 − c of these nests, at a cost c. Once again, all hosts renest after nest failure when depredated by the parasite, and these renests are then available for parasitism at a rate of p. For tractable modeling, there is no farming of the renesting attempts in this hypothetical predatory strategy.
Under these assumptions, the fitness payoffs for the three parasitic strategies are as follows;
for the non-predatory strategy:(1)
for the Mafia:(2)
and for the Farmer:(3)
for a constant population size of nesting hosts (i.e. it is assumed that the same number of host nests are available for all types of predatory parasites).
Solving the inequalities of each pair of respective strategies yields that for all values of c, d, e, and p that are between 0 and 1, the non-predatory strategy will always have a lower fitness than either the Mafia or the Farmer. Thus, a predatory strategy will always be favored by selection over the non-predatory strategy, unless the cost of predation is c = 1 (i.e. the parasite dies when attempting to depredate the nest) when all three strategies have the same payoff predation by brood parasites is likely to evolve. In contrast, in the absence of rejecter hosts (e = 0), the non-predatory and the Mafia strategies will have the same, and both lower fitness payoff, relative to Farmer, so predation by brood parasites should be favored prior to the onset of the first coevolutionary stage, namely the evolution of specific host rejection behaviors in response to brood parasitism. However, if the hosts are able to detect and reject foreign eggs from the nest due to pre-existing nest maintenance behaviors (e.g. Guigueno & Sealy Citation2012), then the effective value of e > 0, and so the stage is set for both Mafia and/or Farmer to be favored over the non-predatory parasite strategy (Equation1(1) ).
Regarding the relative fitness payoffs of the two predatory strategies, if c < 1 and e > 0, then Mafia would be favored over Farmer, when (Equation2(2) ) > (Equation3
(3) ), which simplifies to
(4)
This inequality, however, will be dependent on p, e, and d (Figure ), and more likely to hold for
larger values of p,
larger values of e, and
smaller values of d.
Figure 2. Relative fitness payoffs of Mafia/Farmer in response to variation in parameters d (discovery of renest by parasite), e (ejection), and p (parasitism). Values above 1 (dashed line) indicate fitness payoffs for Mafia > Farmer.
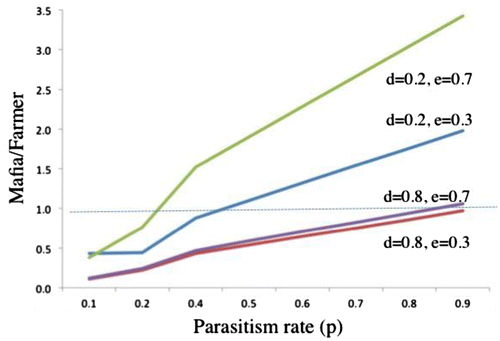
Thus, the Mafia strategy will be favored when parasitism rates are high, ejection rates are high, and the proportion of non-parasitized nests discovered by the parasite is low. Importantly, unless the cost of depredating host nests always results in the death of the parasite (c = 1, an unlikely scenario for each predation attempt), this cost does not enter into the inequality (Equation4(4) ), implying that host nest availability for parasitism, rather than the cost of nest predation to the parasite itself, will drive the evolution of the specific predatory strategy by the parasite.
All throughout this section I have assumed that the additional cognitive costs of remembering the location of previous parasitized nests are nil, and so are the costs of assessing the status of host nests as suitable or unsuitable for parasitism (i.e. by counting host eggs), so that the parasites are able to accurately evaluate the clutch-completion stage and the parasitism status of host nests (Low et al. Citation2009; Gloag et al. Citation2011; Dubina & Peer Citation2013). Cost free cognitive mechanisms are unlikely to evolve (Lotem Citation1993), and will require further modeling, with accurate empirical estimates of cognitive cost parameters, to assess their relevance and impact on the relative fitness payoffs of each of the predatory strategies by parasites.
The evolution of acceptance by hosts in the presence of Mafia parasites
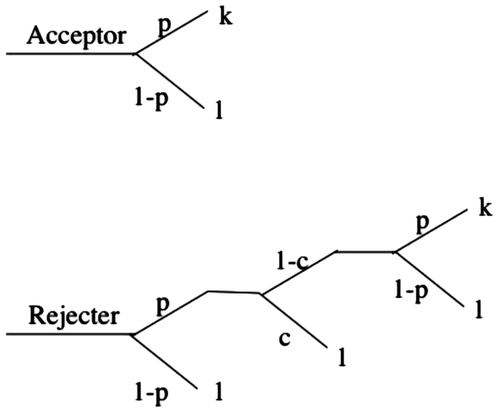
In the presence of retaliatory brood parasites, the rejection of parasitic eggs may be maladaptive if the parasites return and cause the complete loss of the host’s nests, whereas some proportion of the host’s own reproductive effort is still produced even when the clutch is parasitized (e.g. the parasite egg fails to hatch as seen in common cuckoos Øien et al. Citation1998, or the parasitic chick is raised along some of the hosts’ own young: Kilner et al. Citation2004). Using a model where hosts have two breeding attempts during the breeding season, Pagel et al. (Citation1998) showed that acceptance of parasites will be more favored when rejecters are retaliated against and then reparasitized at higher rates during the renesting attempts, compared acceptors during their regular, second breeding attempt.
In the previous section, I considered a scenario in which acceptor hosts do not regularly raise second clutches and where rejecters, whose nests were depredated by retaliatory parasites, become acceptors during their renesting attempts. Hence, unlike Pagel et al. (Citation1998), by keeping population-wide parasitism rate constant throughout subsequent breeding attempts, I assume no potential “reward” of reduced parasitism during the second clutches of acceptors; this is a reasonable assumption, because several host species of different lineages of brood parasitic birds are exposed to increased rates of parasitism during renesting attempts, compared to first nesting attempts (Hoover et al. Citation2006; Krüger Citation2011; Anderson et al. Citation2013). Thus, this alternative scenario will be used here to assess if acceptance of parasites is likely to evolve in the presence of Mafia.
Specifically, hosts are exposed to a constant level of parasitism p across repeated nesting attempts (Figure ); parasitized acceptors raise a proportion of k offspring, whereas non-parasitized hosts gain a fitness payoff of 1; rejecters are depredated by retaliatory parasites in (1 − c) proportion of nests, and renest again, where c is the cost of predation paid by the parasite. The fitness of acceptor hosts, in the presence of retaliatory parasites is, thus:(5)
and the fitness of a rejecter host is:(6)
Acceptance would then be favored if the inequality (Equation5(5) ) > (Equation6
(6) ) holds, which simplifies to:
(7)
but this cannot hold for any values of the parameters between 0 and 1, and so rejection by hosts will always be favored under any realistic values of p, c, and k. This conclusion is contrary to the findings of Pagel et al. (Citation1998) in that the Mafia predation strategy by parasites does not favor the evolution of host acceptance.
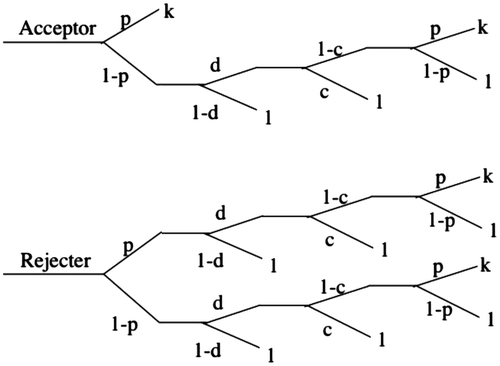
The evolution of acceptor hosts in the presence of Farmer parasites
In the presence of Farmer, all host nests at advanced stages that do not contain parasitic egg or young are subject to predation by parasites with the probability d, irrespective of whether these nests had been previously parasitized by the parasite (Figure ). Under these conditions, the fitness payoff for acceptors is(8)
The fitness payoff for rejecters is(9)
which simplifies to(10)
Selection will then favor the acceptor strategy over the rejecter strategy if the inequality (Equation8(8) ) > (Equation10
(10) ) holds, but
(11)
is not satisfied for any values of the parameters between 0 and 1, and so rejection by hosts will always be favored under any realistic parameter values. Again, the conclusion is that acceptance is not a coevolved response to predatory brood parasites.
Discussion
Brood parasitic birds and their past and present hosts provide some of the clearest and most frequently studied examples of coevolution (Davies Citation2000; Grim Citation2007), with an intricate series of adaptations exhibited by both hosts and parasites (Davies Citation2011; Kilner & Langmore Citation2011; Feeney et al. Citation2012). Still, it remains unclear why some hosts do not discriminate against parasitic eggs and chicks by rejection. Empirical data on egg-learning constraints and parental feeding strategies indicate that evolutionary lag hypotheses may explain the apparent lack of rejection behavior observed in some hosts of the common cuckoo and the brown-headed cowbird (Rothstein Citation1990; Lotem Citation1993). Theoretical considerations, experimental evidence, and modeling approaches indicate that evolutionary equilibrium hypotheses may explain the apparent tolerance of the parasitic eggs and chicks of the retaliatory great spotted cuckoo Clamator glandarius in nests of the European magpie Pica pica (Soler et al. Citation1999) and the brown-headed cowbird in cavities of the prothonotary warbler Protonotaria citrea (Hoover & Robinson Citation2007). The results of the models presented here suggest that the different predatory strategies of brood parasites up on host nests does not select for the evolution of acceptance by hosts to pay the costs of avian brood parasitism. In turn, whether predation by brood parasites in any avian host-parasite systems is best characterized by Mafia or Farmer strategies, requires empirical, experimental assessment of alternative predictions (e.g. Hoover & Robinson Citation2007; Hauber Citation2009).
The models presented here are undoubtedly simple but, unlike some previous theoretical studies where were based on single interactions between hosts and parasites (Davies et al. Citation1996; Avilés et al. Citation2005), these calculations incorporate the iterated nature of host–parasite interactions across multiple breeding attempts and seasons (Grim et al. Citation2014). Accordingly, in several brood parasitic bird species, including common cuckoos (Moskát et al. Citation2010), brown-headed cowbirds (Hauber et al. Citation2013), and cuckoo finches (Stevens et al. Citation2013), repeated interactions between the same individual hosts and parasites occur often, resulting in multiple parasitism of host nests and sequential parasitism of the same host nests, within and between breeding seasons (Hauber et al. Citation2004; Hoover et al. Citation2006; Wagner et al. Citation2013).
The modeling outputs presented here reject the hypothesis that predation by brood parasites favors a coevolutionary response of host acceptance of costly parasitism; this is contrary to the analyses of Pagel et al. (Citation1998), Soler, Møller, et al. (Citation1998) and Chakra et al. (Citation2014) regarding the impact of the Mafia strategy favoring host acceptance; and the first such conclusion regarding the impact of the Farmer, farming strategy on the evolution of host responses. However, the new model comparisons also suggest that the relative payoffs of the two different predatory strategies for brood parasites are critically affected by the rates of parasitism, the rates of rejection by hosts, and the probability of host renesting attempts being discovered by farming parasites, assuming adequate time to renest within the same breeding season. There is clear evidence that many present and past hosts of parasitic birds are able to recognize brood parasites and frontload their antiparasitic defenses by mobbing the parasites and defending the host nests to preclude the parasites from discovering host nests in the first place (Feeney et al. Citation2012). Accordingly, observations (Figure ) and experiments with model and playback presentations of cowbirds, cuckoos, and other parasites (e.g. Kleindorfer et al. Citation2013) show that hosts respond adaptively and dynamically to the presence of parasites by mobbing it vigorously to prevent access to the nest (Henger & Hauber CitationForthcoming). In turn, from the perspective of the brood parasite, the models also revealed that some type of a predatory parasite would always be favored by selection over a non-predatory parasite. Regarding the alternative predatory behaviors, the conclusion is that the Farmer strategy is most likely to prevail over Mafia when the costs (c) of nest predation paid by the parasite are lower. However, the unmeasured but necessary sensory and cognitive costs (Lotem Citation1993) and recognition errors (Servedio & Lande Citation2003) associated with the identification of parasitic eggs in host nests in general (Farming strategy) vs. the parasite’s own eggs in particular, may tip the balance between the relative selective advantages, and must be incorporated in future modeling and empirical work.
Figure 5. Northern Cardinal attacks a brood parasitic Brown-headed Cowbird; photo credit: Bill Draker.

Yet, another implication of the models discussed here is that Mafia vs. Farmer predatory strategies must be tested simultaneously in experimental studies of predatory parasites to assess the relative contribution of each strategy to the parasites’ behaviors and the their impacts on host nest predation (e.g. by testing if parasites recognize their own vs. other parasite’s eggs in controlled laboratory, Dubina & Peer Citation2013, or field trials, Spottiswoode Citation2013). Critical tests to discriminate between Mafia and Farmer will also require direct observation of the species and the identity of individual responsible for the nest destruction/predation event and/or the experimental removal and addition of parasitic eggs to compare predation rates between naturally and artificially non-parasitized nests. Specifically: do mother parasites return and depredate nests from which their own egg was removed (but another parasite egg may have been switched or laid by another female)? For example, in a population of European magpies parasitized by great spotted cuckoos at a high rate (63.5%) Soler et al. (Citation1995) reported that nests from which parasitic eggs were removed experimentally suffered significantly higher rates of predation (16/29) than naturally non-parasitized nests (20/89, chi-square test, p < 0.001). This confirms the authors’ conclusions that these cuckoos are engaged in mostly retaliatory, rather than farming-type predation. Similar, new, and also post hoc analyses are needed with data from previously published studies, for example, on managed hosts of the brown-headed cowbirds where parasitic eggs were systematically removed for conservation purposes (Hauber Citation2009; Zanette et al. Citation2011).
Critically, these experimental studies must pay attention to whether naturally parasitized sites are simply safer from predators because of the microhabitat or parental traits, and whether differential predation occurs during the egg stage vs. the nestling stage (Hauber Citation2000, Citation2009; Hoover & Robinson Citation2007). For example, parasite chicks may produce odorous excretions aimed at deterring predation, resulting in the recently discovered pattern from great spotted cuckoos parasitizing carrion crows Corvus corone, also in Spain, that both naturally and experimentally parasitized nests suffer less predation during the nestling stage than non-parasitized nests and broods from which the cuckoo was experimentally removed (Canestrari et al. Citation2014).
Another specific prediction of the models presented here is that, all else (i.e. c, d, and e) being equal, increasing rates of parasitism will favor Mafia over Farmer. This prediction is intuitive because with increasing rates of parasitism, the number of non-parasitized nests available for predatory farming will decrease. Therefore, most nests that do not contain parasitic eggs would be nests of rejecters and predation of these nests would be classified as retaliation, although it might not rely on the parasite’s memory of the location and status of parasitized host nests. This positive relationship between the rate of parasitism and the likelihood that Mafia is selected over Farmer suggests that these two types of predatory behaviors may be favored and displayed by the same parasite species in different populations, or even across different years, depending on the variation and extent of the current density of parasites and the resulting rate of parasitism, and represent alternative tactics (sensu Gross Citation1996). Thus, from the perspective of the hosts’ experience, these two strategies of retaliation and farming may, in fact, represent a continuum with each other, or an epiphenomenon of a general predatory strategy displayed by parasites (Pagel et al. Citation1998) and would be seen in the presence of mostly parasitized hosts nests (this study). Future studies should address experimentally and comparatively the prevalence and the type of parasitic nest predation strategies across diverse, evolutionary independent avian host-brood parasite systems.
Acknowledgements
I thank D. W. Winkler for drawing my attention to this topic. The project also benefited from further discussions with P. Arcese, P. Brennan, S. Emlen, M. Clinchy, W. Feeney, R. Gloag, J. Hoover, P. Brennan, A. Lotem, K. Reeve, S. Rothstein, S. Sealy, P. Sherman, D. Swan, L. Zanette, and many other colleagues. Previous versions of the manuscripts were improved by comments from M. Andrade, R. Safran, P. Starks, and the referees of this journal. MEH was supported during the writing of this manuscript by a Howard Hughes Medical Institute Predoctoral Fellowship, the National Science Foundation Doctoral Dissertation Improvement Grant program, and the Miller Institute for Basic Research in Science at the University of California, Berkeley, as well as the Human Frontier Science Program, the President’s Office of Hunter College, and the PSC-CUNY grant scheme.
References
- Anderson MG, Gill BJ, Briskie JV, Brunton DH, Hauber ME. 2013. Latitudinal differences in the breeding phenology of Grey Warblers covary with the prevalence of parasitism by Shining Bronze-Cuckoos. Emu. 113:187–191.
- Arcese P, Smith JNM, Hatch MI. 1996. Nest predation by cowbirds, and its consequences for passerine demography. Proc Nat Acad Sci USA. 93:4608–4611.
- Avilés JM, Rutila J, Møller AP. 2005. Should the redstart (Phoenicurus phoenicurus) accept or reject cuckoo eggs? Behav Ecol Sociobiol. 58:608–617.
- Bán M, Moskát C, Barta Z, Hauber ME. 2013. Simultaneous viewing of own and parasitic eggs is not required for foreign egg rejection by a cuckoo host. Behav Ecol. 24:1014–1021.
- Briskie JV. 2007. Direct observations of shining cuckoos (Chrysococcyx lucidus) parasitising and depredating grey warbler (Gerygone igata) nests. Notornis. 54:15–19.
- Canestrari D, Bolopo D, Turlings TCJ, Röoder G, Marcos JM, Baglione V. 2014. From parasitism to mutualism: unexpected interactions between a cuckoo and its host. Science. 343:1350–1352.
- Chakra MA, Hilbe C, Traulsen A. 2014. Plastic behaviors in hosts promote the emergence of retaliatory parasites. Scientific Reports. 4:4251. doi:10.1038/srep04251.
- Davies NB. 2000. Cuckoos, cowbirds and other cheats. London: T & AD Poyser.
- Davies NB. 2011. Cuckoo adaptations: trickery and tuning. J Zool. 284:1–14.
- Davies NB, Brooke M de L, Kacelnik A. 1996. Recognition errors and probability of parasitism determine whether reed warblers should accept or reject mimetic cuckoo eggs. Proc R Soc London B. 263:925–931.
- Dow D. 1972. The New Zealand long-tailed Cuckoo: nest parasite or predator? Emu. 72:179–180.
- Dubina KM, Peer BD. 2013. Egg pecking and discrimination by female and male Brown-headed Cowbirds. J Ornithol. 154:553–557.
- Elliott PF. 1999. Killing of host nestlings by the Brown-headed Cowbird. J Field Ornithol. 70:55–57.
- Feeney WE, Welbergen JA, Langmore NE. 2012. The frontline of avian brood parasite–host coevolution. Anim Behav. 84:3–12.
- Gloag R, Fiorini VD, Reboreda JC, Kacelnik A. 2011. Brood parasite eggs enhance egg survivorship in a multiply parasitized host. Proc R Soc London B. 279:1831–1839.
- Grim T. 2007. Equal rights for chick brood parasites. Ann Zool Fennici. 44:1–7.
- Grim T, Samaš P, Hauber ME. 2014. The repeatability of avian egg ejection behaviors across different temporal scales, breeding stages, female ages and experiences. Behav Ecol and Sociobiol. doi:10.1007/s00265-014-1688-9.
- Gross MR. 1996. Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol Evol. 11:92–98.
- Guigueno MF, Sealy SG. 2012. Nest sanitation in passerine birds: implications for egg rejection in hosts of brood parasites. J Ornithol. 153:35–52.
- Hauber ME. 2000. Nest predation and cowbird parasitism in Song Sparrows. J Field Ornithol. 71:389–398.
- Hauber ME. 2009. Does the removal of avian brood parasite eggs increase host productivity? A case study with brown-headed cowbirds Molothrus ater and song sparrows Melospiza melodia near Ithaca, New York, USA. Conserv Evidence. 6:83–88.
- Hauber ME, Samaš P, Anderson MG, Rutila J, Low J, Cassey P, Grim T. 2013. Life-history theory predicts host behavioural responses to experimental brood parasitism [Internet]. Ethol Ecol Evol. doi: 10.1080/03949370.2013.851121.
- Hauber ME, Yeh PJ, Roberts JOL. 2004. Patterns and coevolutionary consequences of repeated brood parasitism. Proc R Soc London B. 271:S317–S320.
- Henger C, Hauber ME. Forthcoming. Variation in antiparasitic behaviors of Red-winged Blackbirds in response to Brown-headed Cowbirds. Wilson J Ornithol.
- Hoover JP. 2003. Experiments and observations of prothonotary warblers indicate a lack of adaptive responses to brood parasitism. Anim Behav. 65:935–944.
- Hoover JP, Robinson SK. 2007. Retaliatory mafia behavior by a parasitic cowbird favors host acceptance of parasitic eggs. Proc Nat Acad Sci USA. 104:4479–4483.
- Hoover JP, Yasukawa K, Hauber ME. 2006. Spatially and temporally structured avian brood parasitism affects the fitness benefits of hosts' rejection strategies. Anim Behav. 72:881–890.
- Kilner RM, Langmore NE. 2011. Cuckoos versus hosts in insects and birds: adaptations, counter-adaptations and outcomes. Biol Rev. 86:836–852.
- Kilner RM, Madden JR, Hauber ME. 2004. Brood parasitic cowbird nestlings use host young to procure resources. Science. 305:877–879.
- Kleindorfer SM, Evans C, Colombelli-Négrel D, Robertson J, Griggio M, Hoi H. 2013. Host response to cuckoo song is predicted by the future risk of brood parasitism. Front Zool. 10:30–35.
- Krüger O. 2011. Brood parasitism selects for no defence in a cuckoo host. Proc R Soc London B. 22:2777–2783.
- Lawes MJ, Marthews TR. 2003. When will rejection of parasite nestlings by hosts on nonevicting avian brood parasites be favored? A misimprinting-equilibrium model. Behav Ecol. 14:757–770.
- Lindholm AK. 2000. Tests of phenotypic plasticity in reed earbler defences against cuckoo parasitism. Behaviour. 137:43–60.
- Lotem A. 1993. Learning to recognize nestlings is maladaptive for cuckoo Cuculus canorus hosts. Nature. 362:743–745.
- Low J, Burns KC, Hauber ME. 2009. Wild number sense in brood parasitic brown-headed cowbirds. Ibis. 151:775–777.
- McLaren CM, Sealy SG. 2000. Are nest predation and brood parasitism correlated in yellow warblers? A test of the cowbird predation hypothesis. Auk. 117:1056–1060.
- Moskát C, Bán M, Szekely T, Komdeur J, Lucassen RWG, van Boheemen L, Hauber ME. 2010. Discordancy or template-based recognition? Dissecting the cognitive basis of the rejection of foreign eggs in hosts of avian brood parasites. J Exp Biol. 213:1976–1983.
- Øien IJ, Moksnes A, Røskaft E, Honza M. 1998. Costs of Cuckoo Cuculus canorus parasitism to reed warblers Acrocephalus scirpaceus. J Avian Biol. 29:209–215.
- Ortega CP. 1998. Cowbirds and other brood parasites. Tucson (AZ): University of Arizona Press.
- Pagel M, Møller AP, Pomiankowksi A. 1998. Reduced parasitism by retaliatory cuckoos selects for hosts that rear cuckoo nestlings. Behav. Ecol. 9:566–572.
- Peer BD, Sealy SG. 2004. Correlates of egg rejection in hosts of the Brown-headed Cowbird. Condor. 106:580–599.
- Petit LJ. 1991. Adaptive tolerance of cowbird parasitism by prothonotary warblers: a consequence of nest-site limitation? Anim Behav. 41:425–432.
- Robert M, Sorci G, Møller AP, Hochberg ME, Pomiankowski A, Pagel M. 1999. Retaliatory cuckoos and the evolution of host resistance to brood parasites. Anim Behav. 58:817–824.
- Rothstein SI. 1990. A model system for coevolution: avian brood parasitism. Annu Rev Ecol Syst. 21:481–508.
- Samas P, Hauber ME, Cassey P, Grim T. 2011. Repeatability of foreign egg rejection: testing the assumptions of co-evolutionary theory. Ethology. 117:606–619.
- Sealy SG. 1994. Observed acts of egg destruction, egg removal, and predation on nests of passerine birds at Delta Marsh, Manitoba. Can Field Nat. 108:41–51.
- Servedio M, Hauber ME. 2006. To eject or to abandon? Brood parasite virulence and host clutch sizes interact to influence the fitness payoffs of alternative rejection strategies. J Evol Biol. 19:1585–1594.
- Servedio MR, Lande R. 2003. Coevolution of an avian host and its parasitic cuckoo. Evolution. 57:1164–1175.
- Smith JNM, Taitt MJ, Zanette L. 2002. Removing Brown-headed Cowbirds increases seasonal fecundity and population growth in song sparrows. Ecology. 83:3037–3047.
- Soler JJ, Møller AP, Soler M. 1998. Mafia behaviour and the evolution of facultative virulence. J Theor Biol. 191:267–277.
- Soler JJ, Sorci G, Soler M, Møller AP. 1999. Change in host rejection behavior mediated by the predatory behavior of its brood parasite. Behav Ecol. 10:275–280.
- Soler M, Soler JJ, Martinez JG, Møller AP. 1995. Magpie host manipulation by great spotted cuckoos: evidence for an avian mafia. Evolution. 49:770–775.
- Soler M, Soler JJ, Martínez JG, Pérez-Contreras T, Møller AP. 1998. Micro-evolutionary change and population dynamics of a brood parasite and its primary host: the intermittent arms race hypothesis. Oecologia. 117:381–390.
- Spottiswoode CN. 2013. A brood parasite selects for its own eggs traits. Biol Lett. 9:20130573.
- Stevens M, Troscianko J, Spottiswoode CN. 2013. Repeated targeting of the same hosts by a brood parasite compromises host egg rejection. Nat Commun. 4:2475.
- Wagner GF, Aidala Z, Croston R, Hauber ME. 2013. Repeated brood parasitism by Brown-headed Cowbirds (Molothrus ater) at Eastern Phoebe (Sayornis phoebe) nesting sites across non-consecutive years. Wilson J Ornithol. 125:389–394.
- Wyllie I. 1981. The cuckoo. London: Batsford.
- Zahavi A. 1979. Parasitism and nest predation in parasitic cuckoos. Am Nat. 113:157–159.
- Zanette LY, Clinchy M, Leonard ML, Horn AG, Haydon DT, Hampson E. 2011. Brood-parasite-induced female-biased mortality affects songbird demography: negative implications for conservation. Oikos. 121:1493–1500.