Abstract
Two known furanocoumarins, namely, 6ˊ, 7ˊ-dihydroxybergamottin (1) and oxypeucedanin hydrate (2), were isolated from Kaffir lime (Citrus hystrix DC) fruit peel using an assay of nitric oxide scavenging effect-guided fractionation. The inhibitory activities of isolated coumarins on the production of nitric oxide (NO) and inducible nitric oxide synthase (iNOS) were studied in mouse macrophages RAW264.7 cells, whereas their inhibitory effect on cyclooxygenase-2 (COX-2) production was investigated using HT-29 and HCT116 cells. The results demonstrated that 1 inhibited lipopolysaccharide-interferon gamma-induced NO and iNOS production in RAW264.7 cells and COX-2 production in HT-29 and HCT116 cells with IC50 values of 16.16 ± 1.08, 18.63 ± 1.42, 18.19 ± 0.95 and 17.53 ± 0.88 µg/mL, respectively; 2 showed IC50 values of 18.23 ± 1.25, 22.54 ± 1.56, 22.27 ± 1.14 and 23.24 ± 1.05 µg/mL, respectively. Additionally, the positive control curcumin exhibited these activities with IC50 values of 12.52 ± 0.63, 15.55 ± 1.34, 14.29 ± 0.58 and 16.14 ± 0.65 µg/mL, respectively. The inhibitory effect of 1 on the production of iNOS and COX-2 was significantly stronger than that of 2. This study indicated that furanocoumarins from Kaffir lime fruit peels have potency for further development as an anti-inflammatory drug.
PUBLIC INTEREST STATEMENT
A sustained inflammation is a pathological condition. Non-steroidal anti-inflammatory drug is a drug of choice to treat inflammation, but many people suffer from their side effects. Nowadays, many researchers try to investigate the natural compounds to be developed as new drugs for treating inflammation. Curcumin is well-known natural compound used as therapeutic agents for inflammation-associated diseases. Citrus hystrix DC is an edible plant widely used in Thailand. Its leaf and fruit have been used as an ingredient in Thai foods. Its fruit is used as traditional medicine for headache, flu, fever, sore throats, bad breath, digestion, tonic and blood purifier. This fruit is rich in compounds showing various pharmacological effects. In this study, active furanocoumarins were isolated from C. hystrix fruit peel and showed a potential to develop as anti-inflammatory drug since these compounds presented similar inhibition on NO, iNOS and COX-2 productions to those of curcumin.
Competing Interests
The authors declare no competing interests.
1. Introduction
Inflammation, expressed in the form of swelling, heat, redness, fever and pain, is a normal reaction of living tissue to injury (Libby, Citation2007; Mequanint, Makonnen, & Urga, Citation2011). The inflammation process starts with the release of numerous chemical mediators from immune cells to the damage area. Two interesting chemical mediators, prostaglandins E2 (PGE2) and nitric oxide (NO), are produced to induce symptom-like pain, fever, redness and swelling (Vliet, Eiserich, & Cross, Citation2000). These two mediators are also associated with the pathogenesis of several diseases such as rheumatoid arthritis, acute gout and atherosclerosis (Vliet et al., Citation2000). The inhibition of NO, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) production are, therefore, an interesting option for reducing inflammation and associated diseases (Alderton et al., Citation2001; Surh et al., Citation2001).
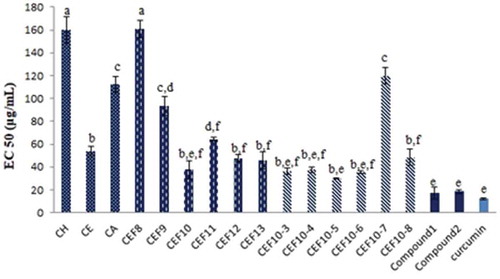
Figure 2. The in vitro NO-scavenging activity of the fractions, sub-fractions and compounds 1 and 2.
The data were expressed as mean ± SD; n = 3. The different letters following the data show the significant content differences at p ≤ 0.05.
Nowadays, it is desirable to use natural products as alternative or complementary medicines for the prevention and treatment of some diseases. Curcumin, for example, is a well-known natural compound used as a therapeutic agent for inflammatory-related diseases (Julie & Jurenka, Citation2009). In Thailand, some medicinal plants have been reported to be valuable sources of anti-inflammatory agents such as Terminalia chebula Retz. (Gall), which exhibited a potent inhibitory activity on lipopolysaccharide-induced NO production in RAW264.7 cells (Kakatum, Jaiarree, Makchucit, & Itharat, Citation2012). Various natural compounds with potential anti-inflammatory activity through the inhibition of NO production were isolated from plants in the Rutaceae family. Citrusin XI was isolated from the fruit of Citrus unshiu (Noh et al., Citation2015), essential oil from the seeds of Zanthoxylum myriacanthum wall.ex. Hook.f. (Li et al., Citation2014) and coumarins from the leaves of Z. avicennae (Cho et al., Citation2012). Interestingly, an ethyl acetate extract prepared from the leaves of Glycosmis parva inhibited COX-2 production (Buranabunwong, Ruangrungsi, Chansriniyom, & Limpanasithikul, Citation2015).
Previous reports led us to question the inhibitory effect of Kaffir lime (C. hystrix DC), an edible plant found throughout the Southeast Asia region, including Thailand. Its fruit has traditionally been used to treat abscesses, fever and coughs (Srisukha et al., Citation2012). Moreover, its fruit peel has been reported to be a component of a traditional Thai medicine called Prasaplai, which is active in the remedy of primary dysmenorrhea (Sriyakul, Kietinun, Pattaraarchachai, & Ruangrungsi, Citation2012). The chemical constituents found in this plant are alkaloids, flavonoids, phenolics and tannins (Sri Tunjung, Cinat, Michaelis, & Smales, Citation2015). Coumarins and glycosides were also isolated and identified (Seeka et al., Citation2016). The biological activity in the fruit peel of this plant is reported to have antimicrobial (Srisukha et al., Citation2012), anticholinesterase (Seeka et al., Citation2016) and antioxidant effects (Hutadilok, Chaiyamutti, Panthong, Mahabusarakam, & Rukachaisirikul, Citation2006). Furthermore, coumarins isolated from this fruit peel have demonstrated anti-inflammatory activity through their inhibitory effect on NO production (Murakami et al., Citation1999), but there are still no reports on their inhibitory effect on COX-2 production. In the present study, the fractionation of a hydroalcoholic extract of Kaffir lime fruit peel using a NO-scavenging effect-guided technique was performed. The isolated active compounds were accordingly investigated for a potential anti-inflammatory agent through their inhibitory effects on the production of NO and iNOS in macrophages RAW 264.7 cells and on COX-2 production in HT-29 and HCT116 cells.
2. Materials and methods
2.1. Plant material
The C. hystrix DC fruits were purchased from a local market in Chiang Mai, Thailand, in July 2017 and authenticated by Wannaree Charoensup, a botanist from the Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University. Voucher specimen No.023233 was deposited in the Herbarium at the Faculty of Pharmacy, Chiang Mai University, Thailand.
2.2. Extraction and isolation
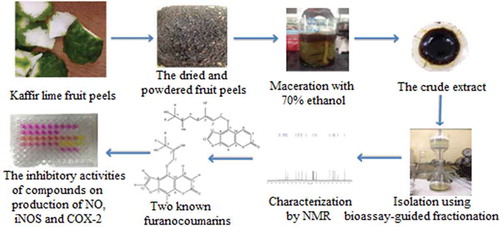
The dried and powdered fruit peel from the C. hystrix DC was macerated with 70% ethanol for 3 days (Putri et al., Citation2013) and concentrated under reduced pressure. The crude extract was dissolved in 20% ethanol (Sreejayan & Rao, Citation1997) and then partitioned with n-hexane and ethyl acetate, respectively. Each portion was concentrated under reduced pressure to obtain three fractions, n-hexane fraction (CH), ethyl acetate fraction (CE) and aqueous fraction (CA). All three fractions were assayed for in vitro NO-scavenging activity. The CE fraction, which showed the most activity, was further separated for four consequential steps of column chromatography (CC) with NO-scavenging activity-guided fractionation technique. In the first step, the CE fraction was separated to become CEF1 to CEF13. In the second step, the highest potent fraction CEF10 was separated using a mixture of hexane: chloroform (5:95) as the eluent and sub-fractions CEF10-1 to CEF10-8 were obtained. In the third step, fraction 10–5 was selected for further separation by using a mixture of chloroform: methanol (99:1) to give 12 sub-fractions. In the fourth step, sub-fraction CEF10–5–5 was separated by using a mixture of n-hexane: ethyl acetate (1:1) as the eluent. Finally, sub-fraction CEF-10-5–5–5 was purified by the preparative thin layer chromatography (TLC) to obtain compound 1. Another active fraction CEF 10–6 was also further separated by CC, using a mixture of chloroform: methanol (99:1), and sub-fraction CEF10-6–3 was finally purified by the preparative TLC, using hexane : ethyl acetate (2:3) as the developing solvent to give compound 2. The isolated compounds were identified by a nuclear magnetic resonance (NMR) spectroscopic technique and determined inhibitory activity on the inflammatory mediator production using a cell-based study.
2.3. General experimental procedure
The 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded using a Bruker Biospin 400 MHz spectrometer with tetramethylsilane (TMS) as the internal standard and CDCl3 as the solvent. The chemical shifts are expressed in δ values. The analytical and preparative TLC were performed on precoated silica gel 60F254 (Merck, 0.25 or 0.50 mm thickness).
2.4. In vitro NO-scavenging activity
In vitro NO-scavenging activity using a Griess reaction was undertaken for every sample. The reaction mixture was composed of 800 μL of 6.25 mM sodium nitroprusside, dissolved in phosphate buffer saline (pH 7.4) and 200 μL of curcumin, used as a positive control or tested samples at concentrations of 10, 25, 50, 100 and 200 μg/mL. The reaction mixture was incubated at 37°C for 150 min. Then, the reaction mixture was transferred to the 96-well plate. The Griess reagent, a mixture of naphthylethylene diamine and sulphanilamide (Sreejayan & Rao, Citation1997), was added and incubated at room temperature for 5 min. The absorbance was measured at 540 nm.
2.5. Cell-based study for inhibitory activity on inflammatory mediator production
2.5.1. Cell culture and cell viability study
The cell viability of compounds 1 and 2 at concentrations of 10, 25, 50, 100 and 200 μg/mL and the positive control curcumin at concentrations of 2.5, 5, 10, 25, 50 and 100 μg/mL on RAW 264.7, HT-29, and HTC 116 cells were measured using cell proliferation regent WST-1 according to the described method (Saenjum, Chaiyasut, Chansakaow, Suttajit, & Sirithunyalug, Citation2012).
2.5.2. Inhibition of NO and iNOS production
To examine the effect of compounds 1 and 2 on NO and iNOS production in RAW 264.7 cells, a modified method (Hong et al., Citation2002; Hu et al., Citation2003; Kim et al., Citation2004; Sirithunyalug et al., Citation2018) was used. In brief, the RAW 264.7 cells were cultured with Dulbecco's Modified Eagle medium (DMEM) in a 24-well plate for 24 h. Then, the cells were replaced with a new medium containing various concentrations of the tested samples (10–100 µg/mL) and incubated for 12 h. A mixture of 10 ng/mL lipopolysaccharide (LPS) and 250 pg/mL IFN-γ was added to the cell culture and then further incubated for 72 h. The supernatants of the culture media were collected to measure for NO production using a Griessreaction, and the cell lysates were measured for iNOS using a mouse total iNOS immunoassay kit. The results were expressed as 50% inhibitory concentrations (IC50) and curcumin was used as a positive control.
2.5.3. Inhibition of COX-2 production
To examine the effects of compounds 1 and 2 on COX-2 production in HT-29 and HCT 116 cells, the method using a human total COX-2 immunoassay kit was modified from previous studies (Colucci, Blandizzi, Ghisu, Florio, & Del Tacca, Citation2008; Hong et al., Citation2002; Sirithunyalug et al., Citation2018). The HT-29 and HCT 116 cells (1 × 105 cells/well) were cultured with DMEM in a black 96-well plate for 24 h and then the tested substances (1–50 μg/mL) were added. Then, the cells were incubated for 12 h. The combined LPS and IFN-γ was added and continued to incubate for 72 h. The cell supernatants, which were extracted with 10 mM Tris, pH 8.0, 1% NP-40, 0.15 M NaCl and 1 mM EDTA, were collected to measure for total COX-2 levels followed the manufacturer’s protocol. The amount of DNA was quantified by a Quant-iT PicoGreen Assay (Invitrogen, P11496) according to the manufacturer’s protocols, while that of the protein produced by RAW 264.7, HT-29 and HCT116 cells was analysed using a Bradford reagent (Bradford, Citation1976; Sirithunyalug et al., Citation2018).
3. Results
3.1. Extraction and isolation
The dried and ground fruit peel of the C. hystrix (1.2 kg) was extracted with 70% ethanol to obtain a 70% hydroalcoholic extract (185 g). This extract (48 g) was then partitioned with hexane and ethyl acetate together with the evaluated in vitro NO-scavenging activity using a Griess reaction to give a hexane extract (CH, 2.6 g; EC50 160 µg/mL), ethyl acetate extract (CE, 14.9 g; EC50 53.8 µg/mL) and aqueous extract (CA, 22.8 g; EC50 112.1 µg/mL). Next, the most active CE (10.1 g) was in vitro NO-scavenging activity-guided vacuum liquid chromatography on silica gel using an n-hexane—ethyl acetate—methanol system to obtain 13 fractions (CEF1 to CEF13). The most active fraction, CEF10 (2.2 g; EC50 37.4 µg/mL), was repeatedly vacuum chromatographed on silica gel using an n-hexane—chloroform (5:95) isocratic system to obtain eight sub-fractions (CEF10-1 to CEF10-8). The results of the in vitro NO-scavenging activity showed that CEF10-5 (EC50 29.7 µg/mL) and CEF10-6 (EC50 35.1 µg/mL) are the most active sub-fractions, and they were subjected to continue separation. Sub-fraction CEF10-5 (278.7 mg) was further separated using silica gel flash CC with the isocratic system of chloroform—methanol (99:1) as the eluent to yield 12 sub-fractions (CEF10-5–1 to CEF10-5–12). Sub-fraction CEF10-5–5 (52.0 mg), which contained a major component was flash chromatographed on silica gel using an n-hexane–ethyl acetate (1:1) system to afford seven sub-fractions (CEF10-5–5-1 to CEF10-5–5-7). Then, sub-fraction CEF10-5-5-5 (41.4 mg) was finally purified by normal-phase preparative TLC with n-hexane–ethyl acetate (2:3) to yield a white crystal of compound 1 (21.2 mg; EC50 14.0 µg/mL), which was identified as 6ˊ,7ˊ-dihydroxybergamottin. Sub-fraction CEF10-6 (364.3 mg; EC50 35.1 µg/mL) was normal-phase flash chromatographed using a chloroform–methanol (99:1) system to give six sub-fractions (CEF10-6–1 to CEF10-6–6). Next, CEF10-6–3 (47.9 mg), which contained more of the target compound, was purified by normal-phase preparative TLC with n-hexane–ethyl acetate (2:3) to obtain a white crystal of compound 2 (oxypeucedanin hydrate, 42.8 mg; EC50 20.6 µg/mL). The in vitro NO-scavening activity of major fractions and isolated compounds was shown in Figure .
3.2. Identification of the compounds
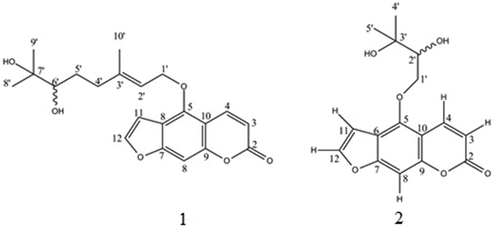
The chemical structures of the two purified compounds were successively elucidated by the 1H and 13C NMR spectroscopic techniques together with a comparison with the data from previous reports. Compound 1 was identified as a furanocoumarin namely 6ˊ,7-dihydroxybergamottin (Figure ) with a molecular formula of C21H24O6 (Murakami et al., Citation1999; Ohta et al., Citation2002). The 1H and 13C NMR data of 1 were assigned as shown in Table . In the case of compound 2, it was identified as a furanocoumarin, namely, oxypeucedanin hydrate (Figure ), with a molecular formula of C16H16O6 (Gökay et al., Citation2010). The 1H and 13C NMR data of 2 are shown in Table .
Table 1. The 1H and 13C NMR spectral data of 1 and 2 in CDCl3 (δ in ppm, J in Hz)
3.3. Cell-based assay for inhibitory activity on inflammatory mediator production
Compounds 1 and 2 showed a significant ability to inhibit the induction of NO and iNOS production in RAW 264.7 cells. Both compounds also inhibit the induction of COX-2 production in HT-29 and HCT116 cells. Table shows the IC50 values obtained from both compounds.
Table 2. The 50% inhibition concentrations of compounds 1 and 2 on NO, iNOS and COX-2 production
4. Discussion
In this study, two known furanocoumarins, namely, 6ˊ,7ˊ-dihydroxybergamottin (1) and oxypeucedanin hydrate (2), were isolated from Kaffir lime (Citrus hystrix DC) peel. 6ˊ,7ˊ-Dihydroxybergamottin is found in grapefruit juice and presented cytochrome P3A4 inhibitory activity (Ohta et al., Citation2002), whereas oxypeucedanin hydrate was isolated from various plants such as Anethum graveolens, Radix imperatoriae, Angelica dahurica and Ficus exasperata Vahl., and its biological activities have been reported to be antimicrobial, anticholinesterase, antioxidant and anti-inflammatory (Amponsah, Fleischer, Dickson, Annan, & Thoss, Citation2013; Bai et al., Citation2016; Gökay et al., Citation2010; Seo et al., Citation2013; Stavri & Gibbons, Citation2005). The results of this study found that pharmacologically active compounds 1 and 2 were isolated from a 70% ethanol extract of Citrus hystrix DC fruit peel using in vitro NO-scavenging activity-guided fractionation. Both isolated compounds demonstrated potential anti-inflammatory activity through their inhibitory effect on NO, iNOS and COX-2 production in a cell-based assay without exerting cytotoxicity. Their activities were similar to those of curcumin, a naturally anti-inflammatory compound that is widely used to compare the anti-inflammatory capacity of plant-derived anti-inflammatory compounds and which clearly has revealed a signal transduction in NO, iNOS and COX-2 inhibition in cell-based studies (Aggarwal & Harikumar, Citation2009; Fürst & Zündorf, Citation2014) (Table ). Interestingly, the inhibitory effect of 1 on the production of iNOS and COX-2 was significantly stronger than that of 2, whereas the inhibitory activity of 1 and curcumin on COX-2 production in HCT 116 cells was not significantly different. The inhibitory capacity of these compounds on NO production in RAW 264.7 cells corresponded to that of a previous report (Murakami et al., Citation1999)—that 6ˊ,7ˊ-dihydroxybergamottin showed more inhibitory activity on NO production than oxypeucedanin hydrate with IC50 values of 130 and 310 μM, respectively. Surprisingly, the inhibitory effect of both compounds on the induction of COX-2 production in HCT-116 and HT-29 cells was reported for the first time. However, some furanocoumarins were found to have drug interaction with dihydropyridine calcium channel blockers such as felodipine and nisoldipine, which are metabolized mainly via cytochrome P450, and could promote hepatotoxicity (Bailey, Arnold, Strong, Munoz, & Spence, Citation1993; Kakar, Paine, Stewart, & Watkins, Citation2004). For safety reasons, these isolated furanocoumarins should be further investigated for their effect on hepatocellular function and interference with the drugs’ metabolism.
Acknowledgements
The authors are grateful to the Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University for its support and would like to acknowledge the financial support for the cell-based study from the Cluster of Excellence on Biodiversity-based Economy and Society (B.BES-CMU), Chiang Mai University.
Disclosure statement
No potential conflicts of interest were reported by the authors.
Additional information
Funding
Notes on contributors

Saisakun Kidarn
Saisakun Kidarn received her B.Sc. in Chemistry from Chiang Mai University (CMU), Thailand. Presently, she is studying M.S. in Pharmaceutical Sciences, Faculty of Pharmacy, CMU. Dr. Chalermpong Saenjum completed his B.Pharm., M.S. and Ph.D. from CMU. He is well versed in pharmaceutical chemistry research. He is now an assistant professor and a member of the Cluster of Excellence on Biodiversity-based Economy and Society (B.BES-CMU), CMU. Dr. Darunee Hongwiset completed her doctoral degree from Heinrich-Heine-Universität Düsseldorf, Germany, after receiving M.S.in Pharmaceutical Chemistry from Chulalongkorn University (CU) and B.Pharm. from CMU. Now she is a head of pharmacy service centre, Faculty of Pharmacy, CMU. Dr. Ampai Phrutivorapongkul received her B.Pharm. from CMU after that she finished doctoral degree under the Royal Golden Jubilee (RGJ) Ph.D. program from CU. She is interested in Pharmacognosy and Phytochemistry. She is now an assistant dean for graduate studies, Faculty of Pharmacy, CMU.
References
- Aggarwal, B. B., & Harikumar, K. B. (2009). Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. International Journal Biochemistry and Cell Biology, 41(1), 40–59. doi:10.1016/j.biocel.2008.06.010
- Alderton, W. K., Cooper, C. E., & Knowles, R. G. (2001). Nitric oxide synthases: Structure, function and inhibition. Biochemical Journal, 357(3), 593–615. doi:10.1042/bj3570593
- Amponsah, I. K., Fleischer, T. C., Dickson, R. A., Annan, K., & Thoss, V. (2013). Evaluation of anti-inflammatory and antioxidant activity of furanocoumarins and sterolin from the stem bark of Ficus exasperata Vahl (Moraceae). Journal of Scientific and Innovative Research, 2(5), 880–887.
- Bai, Y., Li, D., Zhou, T., Qin, N., Li, Z., Yu, Z., & Hua, H. (2016). Coumarins from the roots of Angelica dahurica with antioxidant and antiproliferative activities. Journal of Functional Foods, 20, 453–462. doi:10.1016/j.jff.2015.11.018
- Bailey, D. G., Arnold, J. M., Strong, H. A., Munoz, C., & Spence, J. D. (1993). Effect of grapefruit juice and naringin on nisoldipine pharmacokinetics. Clinical Pharmacology & Therapeutics, 54(6), 589–594. doi:10.1038/clpt.1993.195
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1), 248–254. doi:10.1016/0003-2697(76)90527-3
- Buranabunwong, N., Ruangrungsi, N., Chansriniyom, C., & Limpanasithikul, W. (2015). Ethyl acetate extract from Glycosmis parva leaf induces apoptosis and cell-cycle arrest by decreasing expression of COX-2 and altering BCL-2 family gene expression in human colorectal cancer HT-29 cells. Pharmaceutical Biology, 53(4), 540–547. doi:10.3109/13880209.2014.997830
- Cho, J. Y., Hwang, T. L., Chang, T. H., Lim, Y. P., Sung, P. J., Lee, T. H., & Chen, J. J. (2012). New coumarins and anti-inflammatory constituents from Zanthoxylum avicennae. Food Chemistry, 135, 17–23. doi:10.1016/j.foodchem.2012.04.025
- Colucci, R., Blandizzi, C., Ghisu, N., Florio, T., & Del Tacca, M. (2008). Somatostatin inhibits colon cancer cell growth through cyclooxygenase-2 downregulation. British Journal of Pharmacology, 155(2), 198–209. doi:10.1038/bjp.2008.333
- Fürst, R., & Zündorf, I. (2014). Plant-derived anti-inflammatory compounds: Hopes and disappointments regarding the translation of preclinical knowledge into clinical progress. Mediators of Inflammation, 2014, 1–9. doi:10.1155/2014/146832
- Gökay, O., Kühner, D., Los, M., Götz, F., Bertsche, U., & Albert, K. (2010). An efficient approach for the isolation, identification and evaluation of antimicrobial plant components on an analytical scale, demonstrated by the example of. Radix Imperatoriae. Analytical and Bioanalytical Chemistry, 398(5), 2039–2047. doi:10.1007/s00216-010-4153-2
- Hong, C. H., Hur, S. K., Oh, O. J., Kim, S. S., Nam, K. A., & Lee, S. K. (2002). Evaluation of natural products on inhibition of inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured mouse macrophage cells. Journal of Ethnopharmacology, 83, 153–159. doi:10.1016/S0378-8741(02)00205-2
- Hu, C., Zawistowski, J., Ling, W., & Kitts, D. D. (2003). Black rice (Oryza sativa L. indica) pigmented fraction suppresses both reactive oxygen species and nitric oxide in chemical and biological model systems. Journal of Agricultural and Food Chemistry, 51(18), 5271–5277. doi:10.1021/jf034466n
- Hutadilok, T., Chaiyamutti, P., Panthong, K., Mahabusarakam, W., & Rukachaisirikul, V. (2006). Antioxidant and free radical scavenging activities of some plants used in Thai folk medicine. Pharmaceutical Biology, 44, 221–228. doi:10.1080/13880200600685592
- Julie, S., & Jurenka, M. T. (2009). Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Alternative Medicine Review, 11, 141-153.
- Kakar, S. M., Paine, M. F., Stewart, P. W., & Watkins, P. B. (2004). 6ʹ,7ʹ-Dihydroxybergamottin contributes to the grapefruit juice effect. Clinical Pharmacology & Therapeutics, 75(6), 569–579. doi:10.1016/j.clpt.2004.02.007
- Kakatum, N., Jaiarree, N., Makchucit, S., & Itharat, A. (2012). Antioxidant and anti-inflammatory activities of Thai medicinal plants in Sahasthara remedy for muscle pain treatment. Journal of the Medical Association of Thailand, 95, 120–126.
- Kim, H. P., Son, K. H., Chang, H. W., & Kang, S. S. (2004). Anti-inflammatory plant flavonoids and cellular action mechanisms. Journal of Pharmacological Science, 96, 229–245. doi:10.1254/jphs.CRJ04003X
- Li, R., Yang, J. J., Shi, Y. X., Zhao, M., Ji, K. L., Zhang, P., … Hu, H. B. (2014). Chemical composition, antimicrobial and anti-inflammatory activities of the essential oil from Maqian (Zanthoxylum myriacanthum var. pubescens) in Xishuangbanna, SW China. Journal of Ethnopharmacology, 158, 43–48. doi:10.1016/j.jep.2014.10.006
- Libby, P. (2007). Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutrition Reviews, 65(12), 140–146. doi:10.1301/nr.2007.dec.S140-S146
- Mequanint, W., Makonnen, E., & Urga, K. (2011). In vivo anti-inflammatory activities of leaf extracts of Ocimum lamiifolium in mice model. Journal of Ethnopharmacology, 134(1), 32–36. doi:10.1016/j.jep.2010.11.051
- Murakami, A., Gao, G., Kim, O. K., Omura, M., Yano, M., Ito, C., … Ohigashi, H. (1999). Identification of coumarins from the fruit of citrus hystrix DC as inhibitors of nitric oxide generation in mouse macrophage RAW 264.7 cells. Journal of Agricultural and Food Chemistry, 47(1), 333–339. doi:10.1021/jf980523e
- Noh, H. J., Hwang, D., Lee, E. S., Hyun, J. W., Yi, P. H., Kim, G. S., … Kim, K. H. (2015). Anti-inflammatory activity of a new cyclic peptide, citrusin XI, isolated from the fruits of Citrus unshiu. Journal of Ethnopharmacology, 163, 106–112. doi:10.1016/j.jep.2015.01.024
- Ohta, T., Maruyama, T., Nagahashi, M., Miyamoto, Y., Hosoi, S., Kiuchi, F., … Tsukamoto, S. (2002). Paradisin C: A new CYP3A4 inhibitor from grapefruit juice. Tetrahedron, 58, 6631–6635. doi:10.1016/S0040-4020(02)00739-1
- Putri, H., Nagadi, S., Larasati, Y. A., Wulandari, N., Hermawan, A., & Nugroho, A. E. (2013). Cardioprotective and hepatoprotective effects of citrus hystrix peels extract on rats model. Asian Pacific Journal of Tropical Biomedicine, 3(5), 371–375. doi:10.1016/S2221-1691(13)60079-9
- Saenjum, C., Chaiyasut, C., Chansakaow, S., Suttajit, M., & Sirithunyalug, B. (2012). Antioxidant and anti-inflammatory activities of gamma-oryzanol rich extracts from Thai purple rice bran. Journal of Medicinal Plants Research, 6(6), 1070–1077.
- Seeka, C., Sutthivaiyakit, P., Youkwan, J., Hertkorn, N., Harir, M., Schmitt-Kopplin, P., & Sutthivaiyakit, S. (2016). Prenylfuranocoumarin–HMGA–Flavonol glucoside conjugates and other constituents of the fruit peel of Citrus hystrix and their anti-cholinesterase activity. Phytochemistry, 127, 38–49. doi:10.1016/j.phytochem.2016.03.009
- Seo, W. D., Kim, J. Y., Ryu, H. W., Kim, J. H., Han, S. I., Ra, J. E., … Lee, J. H. (2013). Identification and characterisation of coumarins from the roots of angelica dahurica and their inhibitory effects against cholinesterase. Journal of Functional Foods, 5, 1421–1431. doi:10.1016/j.jff.2013.05.011
- Sirithunyalug, B., Saenjum, C., Charumanee, S., Sivamaruthi, B. S., Chaiyasut, C., Sirithunyalug, J., & Tipduangta, P. (2018). Development of colorectal-targeted dietary supplement tablets containing natural purple rice bran oil as a colorectal chemopreventive. Nutrients, 10(444), 1–13. doi:10.3390/nu10040444
- Sreejayan, & Rao, M. N. (1997). Nitric oxide scavenging by curcuminoids. Journal of Pharmacy and Pharmacology, 49(1), 105–107. doi:10.1111/j.2042-7158.1997.tb06761.x
- Sri Tunjung, W. A., Cinat, J., Michaelis, M., & Smales, M. (2015). Anti-cancer effect of kaffir lime (Citrus hystrix DC) leaf extract in cervical cancer and neuroblastoma cell lines. Procedia Chemistry, 14, 465–468. doi:10.1016/j.proche.2015.03.062
- Srisukha, V., Tribuddharat, C., Nukoolkarn, V., Bunyapraphatsara, N., Chokephaibulkit, K., Phoomniyom, S., … Srifuengfung, S. (2012). Antibacterial activity of essential oils from citrus hystrix (makrut lime) against respiratory tract pathogens. Science Asia, 38, 212–217. doi:10.2306/scienceasia1513-1874.2012.38.212
- Sriyakul, K., Kietinun, S., Pattaraarchachai, J., & Ruangrungsi, N. (2012). A comparative double-blinded randomized study: The efficacy of Prasaplai herbal extract versus mefenamic acid in relieving pain among primary dysmenorrhea patients. The Open Complementary Medicine Journal, 4(1), 16–21. doi:10.2174/1876391X01204010016
- Stavri, M., & Gibbons, S. (2005). The antimicrobial constituents of dill (Anethum graveolens). Phytotherapy Research, 19(11), 938–941. doi:10.1002/ptr.1758
- Surh, Y. J., Chun, K. S., Cha, H. H., Han, S. S., Keum, Y. S., Park, K. K., & Lee, S. S. (2001). Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutation Research, 480, 243–268. doi:10.1016/S0027-5107(01)00183-X
- Vliet, A., Eiserich, J. P., & Cross, C. E. (2000). Nitric oxide: A pro-inflammatory mediator in lung disease. Respiratory Research, 1(2), 67–72.