?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Cancer is one of the leading causes of deaths and health debts worldwide. Novel plant extracts with anticancer properties can alleviate this disease burden. The aim of this study was to screen for new therapeutic agents with anticancer properties against a panel of four cancer cell lines. Cytotoxicity screening of aqueous and methanolic extracts from bark, fruits and leaves of Bersama engleriana was carried out on A546 cell line and the best fractions from the methanolic extract of fruits (BEfr: methanolic extracts of fruits) were performed on four cancer cell lines. Methanolic extracts from the root of Bersama engleriana gave the highest yield (21.55%). Phytochemical analysis showed that the tested methanolic extracts contained polyphenols. The F3, a fraction from the methanolic extract of fruits exhibited potent antiproliferative activities with IC50 values of 60 ± 0.91, 53.73 ± 0.79, 50.91 ± 0.46 µg/ml on U-87MG, MG-63 and MIAPaCa-2 cells, respectively. The F3 fraction also caused death to 50% (CC50) of normal HFF cells, i.e. at 527.4 ± 0.81 µg/ml. Selectivity indexes (SI = CC50/IC50) of 10.52, 8.79, 9.81 and 10.25 were obtained for A549, U-87MG, MG-63 and MIAPaCa-2 cells, respectively. These results confirmed the anticancer potential of Bersama engleriana.
Graphical Abstract:
PUBLIC INTEREST STATEMENT
Globally, amongst the non-communicable diseases, cancer is the second leading cause of death, after cardiovascular disease. Chemotherapy is routinely used for cancer treatment. The Antimicrobial and Biocontrol Agents Unit (Cameroon), and the Cancer Biology and Inflammatory Disorder Division (India), in collaboration with the Departments of Biotechnology and Medical Biosciences of the University of the Western Cape have been actively screening plant-derived natural products for anticancer properties. Bersama engleriana, a tree occurring in forests of tropical Africa, is a typical example of such phytotherapeutic potential that can be harnessed in this way. However, the increasing application of natural-product-based anticancer therapies are raising concerns regarding the biosafety of their applied concentrations in humans. Our in-vitro cell-based studies provide further evidence that plant products may have potent antitumor properties with relatively few side effects. Therefore, the indigenous use of plants as herbal remedies for the treatment of cancer has a scientific basis.
Competing Interests
The authors declares no competing interests.
1. Introduction
Globally, 9.6 million cancer deaths have been reported worldwide in 2018 (Bray et al., Citation2018). The cancer mortality rate is exacerbated by the majority of cancer patients being diagnosed at an advanced stage of the disease (Miller et al., Citation2019; Siegel, Miller, & Jemal, Citation2019). The efficacies of several classes of anticancer agents (e.g. alkylating agents, antimetabolites, antibiotics and hormones) are often limited by adverse effects such as cardiotoxicity, nephrotoxicity, ototoxicity, neurotoxicity and drug resistance (Fung et al., Citation2018; Mannheimer, Duval, Prasad, & Gustafson, Citation2019). Antitumor drugs have also been associated with the development of secondary malignancies (Benjamini et al., Citation2015). All of the drawbacks presently associated with cancer chemotherapy-induced side effects are thus an impetus for the search for newer, more efficacious and better-tolerated drugs.
An intuitively plausible way to achieve this objective is to study plant-derived natural products with anticancer properties (Huang, Ju, Chang, Reddy, & Velmurugan, Citation2017). Currently, ~ 60% of all approved anticancer drugs are of plant-derived natural origin (Ngo, Okogun, & Folk, Citation2013; Thomford et al., Citation2018). Drugs like Vinca alkaloids, taxanes and camptothecins derived from the Madagascan periwinkle plant Catharantus roseus, the Pacific yew Taxus brevifolia and the Chinese tree Camptotheca acuminata, have all improved the chemotherapeutic outcome of some cancers (Jain & Jain, Citation2011; Talib & Mahasneh, Citation2010). Bersama engleriana (Melianthaceae), a tree occurring in forests of tropical Africa, is traditionally used in the treatment of cancer, spasms, infectious diseases, male infertility and diabetes (Amit, Vikas, Vaibhav, Vikash, & Siddhartha, Citation2010; Watcho, Makemdjio, Nguelefack, & Kamanyi, Citation2007). Several studies have shown that methanolic extracts from various parts of Bersama engleriana possess antioxidant and antimicrobial activity (Amit et al., Citation2010; Kuete et al., Citation2008). However, the cytotoxic activity of Bersama engleriana on cancer cell lines has not been extensively evaluated. Thus, this study aimed to evaluate the in vitro antiproliferative potential of the aqueous and the methanolic extracts and the derived fractions of the methanolic extract from Bersama engleriana against a panel of four cancer cell lines (A549, MIAPaCa-2, U-87MG and MG-63). In addition, we also evaluated whether there is a difference in response to extracts between tumor and non-cancerous human cell lines (HFF).
2. Materials and methods
2.1. Plant material
Fresh parts of Bersama engleriana were harvested in December 2015 in Bamendjou, Cameroon, and a voucher specimen (reference: 24725/HNC) was identified at the National Herbarium in Yaoundé. The plant parts were washed thoroughly with fresh water, shade-dried for three weeks until constant weight was attained and ground to fine powders which were stored at room temperature until used.
2.2. Preparation of extracts
One hundred grams of each plant organ powder were weighed and separately placed into 1000 ml each of absolute methanol or double distilled water, for 72 hours accompanied by occasional stirring at 25ºC. The resultant solutions were sieved and filtered through Whatman N° 1 filter paper. The process was repeated three times and after each 72 hours period, the filtrates were concentrated by evaporation using a rotary evaporator (Büchi 011, Flawil, Switzerland) at 60ºC (for methanol) under reduced pressure and aqueous extracts were lyophilized using a Martin Christ Beta 2–8 lyophilizer (Germany). The methanolic concentrates were air-dried until constant weights were achieved. The final masses of the extracts were recorded and used to calculate the yield. The residues from the extracts were collected and stored at 4°C.
2.3. Phytochemical screening analysis
Phytochemical screening of the crude extracts was carried out to identify alkaloids, flavonoids, glycosides, saponins, tannins, anthocyanins, anthraquinones, polyphenols and triterpenes (Harborne, Citation1998).
2.4. Fractionation of active crude extracts (BEfr) using column chromatography
Column chromatography was used to fractionate the active crude extract (50 g). Different solvent systems of increasing polarity comprising hexane, ethyl acetate, methylene chloride and methanol served as mobile phases. The stationary phase consisted of adsorbent silica gel (100–200 mesh, Merck Chemicals). Column chromatography fractions were pooled according to their TLC profiles.
2.5. Cell lines and culture media
Human lung carcinoma epithelial cells (A549), human gliomas cells (U-87MG), human osteosarcoma cells (MG-63), pancreatic ductal adenocarcinoma cells (MIAPaca-2) and human foreskin fibroblasts (HFF) were selected for antiproliferative activities and the cells lines purchased from the American Type Culture Collection (ATCC) (Manassas, Virginia, USA). The U-87MG and MG-63 were grown in IMDM (Sigma, New Delhi, India), MIAPaca was grown in RPMI-1640 medium (Sigma, New Delhi, India), whilst A549 and HFF were grown in DMEM (Sigma, New Delhi, India). The cells lines were chosen based on the high incidence of these cancers over the 200 different types of cancer that afflict humans and are the most frequent cancer diagnosed and the leading cause of cancer death among males following by prostate cancer. Furthermore, there are the most available cancer cell lines as the cells grow faster and easy to maintain, especially MG-63, A549 and HFF cells.
These growth media were supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO), phenol red (Thermo Scientific, New Delhi, India) and 1% penicillin-streptomycin (Sigma, New Delhi, India) and were maintained at 37ºC in a humidified atmosphere containing 95% air and 5% CO2.
2.6. Assessment of cell viability
The MTT cell proliferation assay was performed using 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide. In brief, cell lines at 80% confluence were detached by trypsinization, and the cells were seeded at a density of 7 × 103 cells/well in a 96-well plate for each cell line and the plate incubated for 24 h at 37°C in a 5% CO2 incubator. Stock solutions of the extracts (10 mg/ml) were prepared in 10% dimethyl sulfoxide (DMSO) and serially diluted (0–1000 µg/ml) and added to the 96-well plate containing the various cell lines. Finally, 96-well plates with the treated cells were incubated for 48 h at 37°C in a 5% CO2 incubator. Thereafter, 100 µl of MTT for cancer cells or MTS (3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) for normal HFF cells was then added to fresh cell-culture media and incubated for an additional 3 hours. The formazan crystals formed were dissolved with DMSO (100 μl/well) and the optical density measured using a microplate reader (Thermo Scientific, USA) at 550 nm and 490 nm for MTT and MTS, respectively. The control treatment wells contained only cells. All experiments were performed in triplicate.
The inhibition percentages were determined using the formula:
The 50% inhibition concentrations (IC50) for cancer cells and the 50% cytotoxicity concentration (CC50) for normal cells were determined using GraphPad Prism version 8.0.0 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com.
2.7. Selectivity index (SI) of F3 fraction
The degree of selective toxicity of the F3 fraction towards cancer cell lines relative to the non-cancerous cell line (HFF) was expressed as the selectivity indexes (SI) as outlined previously (Boyom et al., Citation2014).
2.8. Statistical analysis
Data were analyzed using two-way ANOVA using GraphPad Prism Data are expressed as mean ± SD of experiments performed in triplicate. Error bars represent the SD and *p < 0.0001, denotes significant differences between the means of the untreated and treated cells or two test groups.
3. Results and discussion
3.1. Yields of extraction
This study assessed the in vitro anticancer potential of extracts and derived fractions from Bersama engleriana. The plant extracts were fractionated using column chromatography. The yields of extraction varied from 14.24% (BEfr) to 21.55% (BEe), according to plant parts and extraction solvent, with the methanol extract of the stem bark giving the best yield 21.55% (BEe) compared to the other parts (Table ). Generally, the yield of methanolic extracts was the highest and it could be due to the high capacity of methanol to extract secondary metabolites in plant materials (Parekh, Jadeja, & Chanda, Citation2005). It is known that methanol is a solvent which has a high extraction potential relative to its ability to weaken the membranes in order to extract the greatest number of secondary metabolites.
Table 1. Yields of aqueous and methanol extracts and fractions obtained from the active extract of Bersama engleriana
3.2. Phytochemical analysis tools
Anthraquinones, flavonoids, saponins, tannins, polyphenols and triterpenes were previously reported to be present in Bersama engleriana (Kuete et al., Citation2008; Tapondjou, Miyamoto, & Lacaille-Dubois, Citation2006). The current results showed that all the tested extracts contained polyphenols. Flavonoids and tannins were also present in several extracts while none of the tested extracts contained anthocyanins and triterpenes (Table ). This difference may be due to the composition of secondary metabolites at the harvest sites (Sampaio, Edrada-Ebel, & Da Costa, Citation2016).
Table 2. Phytochemical screening of aqueous and methanolic extracts from Bersama engleriana
The Column chromatography fractions were pooled according to their TLC profiles. Based on TLC spot pattern analysis (Supplementary material, Figure S1), 36 fractions were grouped into 14 sub-fractions and low yield fractions were not tested.
3.3. Antiproliferative screening of extracts and fractions against A549 (human lung carcinoma epithelial cells)
The methanolic and the aqueous extracts and fractions from the methanolic extract of fruits were initially screened against A549 cancer cell line, using the MTT assay. Only the methanolic crude extract of fruits (BEfr) and one of its most active fraction, F3 (Table ) were represented because BEfr and F3 activities were the greatest for both the crude extract and the tested fractions to illustrate the concentration dependency (Supplementary material, Figures S2 and S3). The other extracts from methanolic and aqueous extracts of either parts of the plants (Table ) have poor antiproliferative activities when tested on the A549 cancer cell line (Table ). Nonetheless, IC50 values for all the tested samples ranged from 68.95 ± 1.04 to 198.6 ± 1.29 µg/ml and 50.11 ± 1.63 to 249.9 ± 0.242 µg/ml for crude extracts and fractions derived from BEfr, respectively. BEfr was the best active extract (IC50 = 68.95 ± 1.04 µg/ml), while the F3 a fraction from the methanolic extract of fruits (IC50 = 50.11 ± 1.63 µg/ml) was more active than the BEfr extract (Table ).
Table 3. Effect of crude extracts and fractions from fruits of Bersama engleriana on drug-resistant A549 human lung carcinoma epithelial cells
The inhibition observed with extracts and fractions of B. engleriana may be attributed to endogenous phenolic compounds (Table ). A previous study revealed that the bark and leaves possessed flavonoids, phenols, anthraquinones and saponins which may be responsible for the observed activity (Kuete et al., Citation2008). Flavonoids and tannins have been shown to possess antimutagenic and anticancer activity (Hirano, Oka, & Akiba, Citation1989). The association between polyphenols and reduced cancer risk has been reported in studies that showed a decrease in cancer risk with consumption of vegetables and fruits rich with polyphenols (Ferguson, Kurowska, Freeman, Chambers, & Koropatnick, Citation2004).
3.4. Antiproliferative potential of selected fractions against other drug-resistant cancer cells and non-cancerous HFF cells
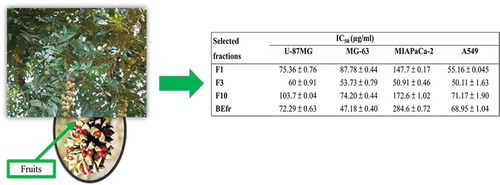
The spectrum of cytotoxicity of selected fractions from the methanolic extract of fruits (F1, F3 and F10) and the methanolic extract of fruits (BEfr) was determined in a panel of three cancer cell lines mainly U-87MG, MG-63 and MIA-PaCa2 (Supplementary material, Figures S4–S7). The F3 fraction, a fraction from the methanolic extract of fruits was also tested against normal HFF cells (Supplementary material, Figure S8). IC50 values ranged from 47.18 ± 0.40 to 274.7 ± 1.47 µg/ml (Table ). The F3 fraction was the most active (IC50 of 60 ± 0.91, 53.73 ± 0.79 and 50.91 ± 0.46 µg/ml on U-87MG, MG-63 and MIAPaca-2 cancer cells, respectively, while the crude BEfr, was less active on MIAPaca-2, with a high IC50 of 284.6 ± 0.72 µg/ml. The extract was most active on MG-63 with an IC50 of 47.18 ± 0.40 µg/ml (Table ).
Table 4. Cytotoxic effects of selected fractions against U-87MG, MG-63, MIA-PaCa2 and A549 cancer cell lines, and the cytotoxic effects of F3 against HFF cells and their selectivity index for F3
As previously described in Table with the A549 cancer cells, the results reported in Table confirm that the F3 fraction from BEfr is more active than the other extracts (Table ). Moreover, the F3 fraction demonstrated the best anticancer activity against the four cancer cell lines tested (Table ; Supplementary material, Figure S6). The fractionation of plant extracts may result in improved or loss of activity (Nwodo, Iroegbu, Ngene, Chigor, & Okoh, Citation2011). An increase in F3 activity was noticed when compared to the crude extracts (Table ). The difference could be explained by the fact that fractionation induced and increased the higher concentration of the principal active compounds, which presumably are responsible for the observed activity. In addition, the bioactive secondary metabolites in F3 might have been more concentrated, preserving and increasing the anticancer potency of the original extract. The increasing applications of natural-product-based anticancer therapies are raising concerns regarding the biosafety of their applied concentrations in humans (Mushtaq, Abbasi, Uzair, & Abbasi, Citation2018; Thomford et al., Citation2018). Therefore, the safety of fraction F3 was checked on normal HFF cells.
The CC50 of 527.4 ± 0.81 µg/ml was obtained and the selectivity indexes of 8.78, 9.81, 10.35 and 10.52 of the F3 fraction were obtained with U-87MG, MG-63, MIAPaCa-2 and A549 (Table ). We could confirm that the 6% DMSO used here as the positive control was more toxic to all cancer cell lines than the various plant extracts and fractions tested. Furthermore, the tested cancer cell lines are less viable than the untreated cancer cell lines, but there are more viable than the cancer cell lines treated with the positive control, in all the experiments conducted (Supplementary material Figures S2–S8). The differences in cell viability show that the aqueous and the methanolic extracts from all parts of the B. engleriana, and mostly the fractions from the methanolic extract of fruits from B. engleriana could contribute a good anticancer compound.
4. Conclusion
In conclusion, we demonstrated the cytotoxic potential of the aqueous and the methanolic extracts of fruits and the fractions of the methanolic extract of fruits from Bersama engleriana against A549, MIAPaca-2, MG-63 and U-87MG cancer cell lines. We also showed the ability of the derived F3 fraction to be non-toxic to normal HFF cells as compared to the positive control. The constituents of the methanolic extract of fruits and their derived fractions, especially F3, are potentially cytotoxic and analytic purification of F3 to identify the active constituents is imperative. The significance of these preliminary findings can serve as the basis for further studies to delineate the anticancer potential of Bersama engleriana. This study supports the ethnobotanical use of Bersama engleriana for possible anticancer activity. To the best of our knowledge, the demonstrated anticancer activities of the aqueous and the methanolic extracts and the methanolic fractions of fruits in this study are being reported here for the first time. Further investigation should attempt to integrate multifaceted modes of anticancer action of the interested compounds.
Conflict of interest
The authors declare no conflict of interest.
Supplemental Material
Download MS Word (2.2 MB)Acknowledgements
The authors acknowledge the Cameroon National Herbarium (Yaoundé) for the plant identification. The authors express their gratitude to the CSIR-Indian Institute of Chemical Biology, Kolkata-West Bengal for access granted in their institution. We thank Mr Asish Mallick for help with the cell culture work.
Supplementary material
The supplemental data for this article can be accessed here.
Additional information
Funding
Notes on contributors
Michèle Stella Majoumouo
Michele Stella Majoumouo, is a PhD candidate at the Antimicrobial & Biocontrol Agents Unit, Laboratory for Phytobiochemistry and Medicinal Plants Studies, Department of Biochemistry, University of Yaoundé 1 Cameroon. She is also awarded the OWSD fellowship to carry part of her PhD project at the DST/Mintek Nanotechnology Innovation Centre (NIC) – Biolabels Node, Department of Biotechnology at University of Western Cape She has also been awarded many fellowships, such as the ‘Coimbra Group Short Stay Scholarship Programme for young researchers from Sub-Saharan Africa, 2015, CNC – “Center for Neuroscience and Cell Biology UC Biotech Building”, Portugal (2015); “Research training fellowship” for developing country scientists (RTF-DCS), 2015, CSIR-Institute of Chemical Biology, India (2015).She is using plants derived products to target multi-resistant cancer cell lines and some pathogenic strains. She is also focused on the green synthesis of nanoparticles from plant extracts, to test their biology activities on cancer cell lines and infectious diseases.
References
- Amit, L., Vikas, G., Vaibhav, T., Vikash, K., & Siddhartha, G. (2010). Phytochemistry and pharmacological activities of Bersama engleriana Guerke-An overview. International Research Journal of Pharmacy, 1(1), 89–10.
- Benjamini, O., Jain, P., Trinh, L., Qiao, W., Strom, S. S., Lerner, S., … Keating, Z. (2015). Second cancers in patients with chronic lymphocytic leukemia who received frontline fludarabine, cyclophosphamide and rituximab therapy: Distribution and clinical outcomes. Leukemia & Lymphoma, 56(6), 1643–1650. doi:10.3109/10428194.2014.957203
- Boyom, F. F., Fokou, P. V., Tchokouaha, L. R., Spangenberg, T., Mfopa, A. N., Kouipou, R. M., … Zollo, P. H. (2014). Repurposing the open access malaria box to discover potent inhibitors of Toxoplasma gondii and Entamoeba histolytica. Antimicrobial Agents and Chemotherapy, 58(10), 5848–5854. doi:10.1128/AAC.02541-14
- Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., & Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424. doi:10.3322/caac.21492
- Ferguson, P. J., Kurowska, E., Freeman, D. J., ., Chambers, A. F., & Koropatnick, D. J. (2004). A flavonoid fraction from cranberry extract inhibits proliferation of human tumor cell lines. The Journal of Nutrition, 134(6), 1529–1535. doi:10.1093/jn/134.6.1529
- Fung, C., Dinh, P., Jr., Ardeshir-Rouhani-Fard, S., Schaffer, K., Fossa, S. D., & Travis, L. B. (2018). Toxicities associated with cisplatin-based chemotherapy and radiotherapy in long-term testicular cancer survivors. Advances in Urology, 2018, 8671832. doi:10.1155/2018/8671832
- Harborne, J. B. (1998). Phytochemical methods: A guide to modern techniques of plant analysis. New York: Chapman and Hall.
- Hirano, T., Oka, K., & Akiba, M. (1989). Antiproliferative effects of synthetic and naturally occurring flavonoids on tumor cells of the human breast carcinoma cell line, ZR-75-1. Research Communications in Chemical Pathology and Pharmacology, 64(1), 69–78.
- Huang, C. Y., Ju, D. T., Chang, C. F., Reddy, P. M., & Velmurugan, B. K. (2017). A review on the effects of current chemotherapy drugs and natural agents in treating non-small cell lung cancer. Biomedicine (Taipei), 7(4), 23. doi:10.1051/bmdcn/2017070423
- Jain, R., & Jain, S. K. (2011). Screening of in vitro cytotoxic activity of some medicinal plants used traditionally to treat cancer in Chhattisgarh state, India. Asian Pacific Journal of Tropical Biomedicine, 1(2), S147–S150. doi:10.1016/S2221-1691(11)60144-5
- Kuete, V., Mbaveng, A. T., Tsaffack, M., Beng, V. P., Etoa, F. X., Nkengfack, A. E., … Lall, N. (2008). Antitumor, antioxidant and antimicrobial activities of Bersama engleriana (Melianthaceae). Journal of Ethnopharmacology, 115(3), 494–501. doi:10.1016/j.jep.2007.10.027
- Mannheimer, J. D., Duval, D. L., Prasad, A., & Gustafson, D. L. (2019). A systematic analysis of genomics-based modeling approaches for prediction of drug response to cytotoxic chemotherapies. BMC Medical Genomics, 12(1), 87. doi:10.1186/s12920-019-0519-2
- Miller, K. D., Nogueira, L., Mariotto, A. B., Rowland, J. H., Yabroff, K. R., Alfano, C. M., … Siegel, R. L. (2019). Cancer treatment and survivorship statistics, 2019. CA: A Cancer Journal for Clinicians, 69(5), 363–385. doi:10.3322/caac.21565
- Mushtaq, S., Abbasi, B. H., Uzair, B., & Abbasi, R. (2018). Natural products as reservoirs of novel therapeutic agents. EXCLI Journal, 17, 420–451. doi:10.17179/excli2018-1174
- Ngo, L. T., Okogun, J. I., & Folk, W. R. (2013). 21st century natural product research and drug development and traditional medicines. Natural Product Reports, 30(4), 584–592. doi:10.1039/c3np20120a
- Nwodo, U. U., Iroegbu, C. U., Ngene, A. A., Chigor, V. N., & Okoh, A. I. (2011). Effects of fractionation and combinatorial evaluation of Tamarindus indica fractions for antibacterial activity. Molecules, 16(6), 4818–4827. doi:10.3390/molecules16064818
- Parekh, J., Jadeja, D., & Chanda, S. (2005). Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turkish Journal of Biology, 29(2005), 203–210.
- Sampaio, B. L., Edrada-Ebel, R., & Da Costa, F. B. (2016). Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Scientific Reports, 6, 29265. doi:10.1038/srep29265
- Siegel, R. L., Miller, K. D., & Jemal, A. (2019). (2019). Cancer statistics. CA: A Cancer Journal for Clinicians, 69(1), 7–34. doi:10.3322/caac.21551
- Talib, W. H., & Mahasneh, A. M. (2010). Antiproliferative activity of plant extracts used against cancer in traditional medicine. Scientia Pharmaceutica, 78(1), 33–45. doi:10.3797/scipharm.0912-11
- Tapondjou, A. L., Miyamoto, T., & Lacaille-Dubois, M. A. (2006). Glucuronide triterpene saponins from Bersama engleriana. Phytochemistry, 67(19), 2126–2132. doi:10.1016/j.phytochem.2006.06.034
- Thomford, N. E., Senthebane, D. A., Rowe, A., Munro, D., Seele, P., Maroyi, A., & Dzobo, K. (2018). Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. International Journal of Molecular Sciences, 19, 6. doi:10.3390/ijms19061578
- Watcho, P., Makemdjio, A., Nguelefack, B., & Kamanyi, A. (2007). Sexual stimulation effects of the aqueous and methanolic extracts from the leaves of Bersama engleriana in adult male rats. Pharmacologyonline, 1, 464–476.