?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Variations among the populations of Colletotrichum species from diseased coffee berries were studied. A total of 35 Colletotrichum isolates representing 24 districts from major coffee-producing regions of Ethiopia was studied on the basis of cultural, morphological, and pathological characteristics. The isolates differed significantly on their colony color, density, sector, and acervuli. Mycelial growth rate varied significantly (p < 0.05) among the isolates and ranged between 3.97 and 8.69 mm/day. Sporulation capacity, conidial length (12.3–17.7 μm) and conidia width (3.6–5.1 μm) also varied significantly (p < 0.05) among the isolates. The dominant forms of conidia were cylindrical and round at both ends followed by cylindrical acute at one and round at the other ends. Cluster analysis based on cultural, morphological, and pathological characteristics showed that isolates of Colletotrichum species associated diseased coffee berries fell into three distinct groups and were identified as C. kahawae, C. gloeosporioides, and C. acutatum. Among these fungal isolates, only C. kahawae was pathogenic to both detached coffee berries and coffee hypocotyls tested. The other Colletotrichum species exist as saprophytic or sequential colonizer of dead tissues. In conclusion, our study indicates the existence of variation in cultural, morphological characteristics and pathogenicity among the C. kahawae and the other related Colletotrichum isolates. However, molecular methods and other detail biochemical tests would provide the genetic diversity of the CBD pathogen populations in the country.
PUBLIC INTEREST STATEMENT
Analysis of variation among the Colletotrichum population primarily responsible for coffee berry disease gives information on geographical patterns, possible specialization for a specific crop and the fungus potential to change, and might, therefore, provide an input in understanding coffee berry disease management programs. It also serves as a basis for the development of disease-resistant coffee varieties through selection and breeding programs.
Competing Interests
The authors declare no competing interests.
1. Introduction
Coffee is one of the most important commodities in international trade, representing a significant source of income to several coffee-producing countries (Garedew et al., Citation2017). Ethiopia is believed to be the origin of Arabica coffee that accounts for 66% of the world coffee market (Berecha et al., Citation2014; Labouisse et al., Citation2008; Van der Vossen et al., Citation2015). Coffee plays a significant role in the country’s economy accounting for 5% of the gross domestic product (GDP), 10% of the total national income, 12% of the agricultural economy, 42% of government taxes from foreign trade and contributing to more than 26% of total export earnings (International Monetary Fund [IMF], Citation2016; Worako et al., Citation2008). It provides employment opportunity to about 25% of the population (International Monetary Fund [IMF], Citation2016). It is also the major source of rural household income and food security mainly in the coffee-producing areas of the country (Labouisse et al., Citation2008).
Ethiopia is the leading in Africa and the fifth-largest Arabica coffee-producing country and the seventh-largest coffee exporter in the world (International Coffee Organization [ICO], Citation2015). Coffee green exports from Ethiopia accounted for approximately 3.31% in value of world coffee green exports between the years 2001 and 2010 (Boansi & Crentsil, Citation2013). The major destinations for Ethiopian coffee exports as of the year 2010/2011 are Germany (32.61%), United States of America (11.43%) and Saudi Arabia (11.38%)(Boansi & Crentsil, Citation2013). The authors further remarked that Belgium, Italy, France and Sweden are also the major importer of this valuable cash crop indicating the global significance of Ethiopian coffee. Evidently, Ethiopian coffees rank high in intrinsic quality of the bean and remarkably attract both national and international markets (Bhattacharya & Bagyaraj, Citation2002).
In spite of this fact, coffee production in the country is continuously threatened by a range of pest and disease problems. For instance, coffee berry disease (CBD) caused by Colletotrichum kahawae is the most devastating Arabica coffee disease in the country since 1971 (Adugna et al., Citation2009; Derso & Waller, Citation2003). The fungus attacks all stages of the crop from flower to ripe fruits and occasionally leaves, but the maximum crop loss occurs following the infection of green berries (Batista et al., Citation2017; Derso, Citation1997). Yield loss due to CBD is estimated to range from 24% to 30%, but it may reach up to 100% in high rainfall, high humidity and high altitude areas (Derso, Citation1997; Derso & Waller, Citation2003; Garedew et al., Citation2017). Very recently, Alemu et al. (Citation2016) have reported that the present status of the CBD in Ethiopia is remarkably on an increasing trend to the extent that the disease can negatively influence the domestic and foreign coffee markets.
Coffee berry disease can be controlled by the use of resistant coffee varieties, spraying fungicides or through improved cultural practices. Resistant varieties are by far profitable over fungicides because it guarantees stable cash income for the farmer subsistence and above all the growing resistant cultivars may lead to environmentally friendly organic coffee production (Zeru et al., Citation2008). Furthermore, breeding for resistant varieties to coffee berry disease may provide a safe, effective and sustainable long-term management of the CBD (Van der Vossen et al., Citation2015). Consequently, efforts have been devoted for the past four decades to the improvement of the genetic base of resistance through selection. However, this has faced the problem of possible pathogen variation. A good understanding of the biology of CBD pathogens could lead to the development of varieties with sufficient disease resistance (Van der Vossen et al., Citation2015).
Many research has been conducted across the globe indicating the variation among coffee berry disease pathogen through morphological, cultural and pathogenic characteristics, vegetative compatibility groups, DNA sequencing and isoenzymatic methodologies and found promising results (Bridge et al., Citation2008; Loureiro et al., Citation2011; Luzolo et al., Citation2010; Omondi et al., Citation2000;Pires et al., 2016; Vieira et al., 2018; Vieira et al., Citation2019). In Ethiopia, studies conducted some twenty years back indicated the presence of variations among and within the Colletotrichum populations isolated from diseased coffee berries based on cultural, morphological and pathogenic characteristics (Biratu, Citation1995). Derso and Waller (Citation2003) also found variation within and among Colletotrichum species from CBD infected berries sampled from Fiseha Genet, Yirgachefe, Gore, Gera and Limu districts of Ethiopia. However, the study was confined to small coffee-growing areas which are not sufficient to make meaningful conclusion about the existing variability of coffee associated fungal flora in the country. Thus, it is imperative to look at a profile of several isolates from different coffee production systems existing in the country. In addition, as variations among and within the pathogen populations might be influenced by the current climate change scenario together with the change in coffee production systems in the country (Batista et al., Citation2017; Belachew & Teferi, Citation2015; Labouisse et al., Citation2008).
Climate change has a significant effect on disease epidemics and possibly influences the reproduction of pathogen populations (Belachew & Teferi, Citation2015; Van der Vossen et al., Citation2015). Climate change influence the rate of overwintering and over summering of sexual propagules which in turn influence the evolutionary potential of pathogen population (Garrett et al., Citation2006). Chakraborty et al. (Citation2000) also suggested that the evolution of pathogen populations may accelerate from enhanced UV-B radiation and/or increased fecundity in elevated CO2. Thus, it is crucial to examine the variability of populations of Colletotrichum species from major coffee-producing areas over time using their cultural, virulence and molecular parameters (Derso & Waller, Citation2003). Knowledge on variation among Colletotrichum populations gives information on geographical patterns, possible specialization for a specific crop and the fungus potential to change (development of new variant), and might therefore, provide an input in understanding coffee berry disease management programs (Nguyen et al., Citation2009; Prihastuti et al., Citation2009). This study reports characterization of isolates of Colletotrichum species retrieved from diseased coffee berries collected from major coffee-producing regions of Ethiopia based on cultural, morphological and pathological characteristics.
2. Materials and methods
2.1. Description of the study area
The study was conducted in laboratory and growth room at Jimma Agricultural Research Center, Ethiopian Institute of Agricultural Research (EIAR) from July 2014 to June 2015.
2.2. Collection of samples
Specimens of diseased coffee berries were collected from major coffee-producing areas of southern, southwestern and eastern parts of the country. A total of 24 districts was visited and 120 coffee specimens were collected from randomly selected coffee farms (Table ). A total of fifteen to twenty green coffee berries from each farm with active CBD lesions was collected during the fruiting stage of coffee bushes. Samples were picked using disinfected forceps, packed in perforated sterile plastic bags and transported to Plant Pathology Laboratory of Jimma Agricultural Research Center and maintained at 4 ºC for further studies.
Table 1. Description of sample collection areas
2.3. Isolation and identification of the coffee berry disease pathogen
The pathogens were isolated from infected coffee berries showing active anthracnose lesions following standard method (Biratu, Citation1995). Five to ten berries from each coffee farm were washed in 5% sodium hypochlorite solution for 2 minutes and rinsed at least 5 times (2 minutes each) with sterilized distilled water. The disinfected berries were placed on sterilized moist tissue paper in sterile plastic boxes with lid to induce conidial production. The boxes were maintained at room temperature until conidial mass and/or fungal growths are observed. Conidial producing berries were aseptically transferred to 100 ml capacity beaker containing sterilized distilled water. Conidia were harvested by gentle shaking manually. Serial dilutions (10−1–10−5) were made and 0.2 ml of each suspension was streaked on Potato Dextrose Agar (PDA) amended with 100 ppm streptomycin sulfate (Gibbs, Citation1969). The inoculated plates were incubated for 5–7 days at 25 ± 2 ºC. Colletotrichum spp. were identified based on morphological, cultural and pathological characteristics (Biratu, Citation1995; Hindorf, Citation1970; Waller et al., Citation1993). The pure cultures of each isolate were stored at 4°C on PDA slants during the study.
2.4. Cultural and morphological characterization of isolates of Colletotrichum species
2.4.1. Cultural characteristics
A total of 35 isolates of Colletotrichum species from diseased coffee berries was selected from large collection (Over 211 Colletotrichum isolates) representing 24 districts in nine major coffee-growing administrative zones and assessed for their colony color, colony size, mycelia growth type (vigor), apical mycelia growth (elevation) and tendency to form sectoring and conidia masses (acervuli). Sample isolates from each district representing the distinct group of Colletotrichum sp. were selected based on the preliminary observation on cultural and morphological characteristics.
2.4.1.1. Colony growth size
Cultures of Colletotrichum isolates were inoculated on Potato Dextrose Agar (PDA), Malt Extract Agar (MEA) and Sabouraud Dextrose Agar (SDA) media with 5 mm diameter mycelial plugs excised from the actively growing margin of a 7-day-old PDA culture with three replicates. Cultures were incubated in the darkness at 25°C. The radial colony growth rate (mm/day) of each isolate was estimated from a measurement of colony diameter (size) taken at perpendicular plane on the reverse side of the plate three times at 7, 10 and 15 days after inoculation (Biratu, Citation1995).
2.4.1.2. Colony color, density, apical growth, fruiting structure and saltation
The growing mycelia color on obverse side was described in reference to standard RGB color charts (Anonymous, Citation2013). The colony density of each Colletotrichum isolates was scored as sparse, slightly dense and dense with visual observation while growing them on PDA, MEA and SDA media at 25°C (Biratu, Citation1995). The varying degree of colony elevation of each isolate from the agar surface was determined as flat, slightly raised and raised (Zeru, Citation2006). The presence and absence of fungus fruiting bodies such as acervuli were observed and relative tendency of each isolate to form sectors (saltation or dissociation) was also determined.
2.4.2 Morphological characteristics
2.4.2.1 Sporulation capacity
Representative isolates of Colletotrichum spp were cultured on PDA, MEA and SDA media and incubated at 25 ºC in darkness. After ten days the colony of each isolate was washed by flooding with 10 ml sterile-distilled water and rubbed gently from the agar surface with sterile scalpel. The spore suspension was then transferred into 50 ml sterile beaker and thoroughly stirred for 10 to15 minutes with magnetic stirrer to extract the spores from the interwoven mycelia. Thereafter, the suspension was filtered into another sterile beaker through double-layer cheese clothes. The number of conidia per milliliter was counted and determined using hemocytometer (Biratu, Citation1995).
2.4.2.2 Conidial size and shape
Variation in conidia size among Colletotrichum isolate was determined by measuring the length and width of 30 randomly selected conidia following the method employed by Peres et al. (Citation2002). The frequency of various conidial shapes for each isolate was determined and grouped into five most frequent conidia shapes as described by Hindorf (Citation1973) and Biratu (Citation1995).
2.5. Pathogenicity test
2.5.1. Pathogenicity test on detached coffee berries
The detached berry test was conducted in laboratory following the procedure employed by Van der Graaff (Citation1981). Fully expanded green coffee berries from a standard CBD susceptible cultivar 370 were collected. Conidial suspension of each Colletotrichum species was prepared by washing 10 days old PDA cultures with sterile-distilled water. Spores were suspended by scraping the colony surface with sterile loop. The suspension was then transferred into a sterile test tube and the spore concentration was determined using a hemocytometer. Apparently, healthy coffee berries were surface sterilized by dipping in 1% sodium hypochlorite solution for 2 minutes before they were repeatedly rinsed thoroughly in sterile-distilled water and air dried. The surfaces of berries were air dried. The berries were placed in sterile Petri dishes with moistened Whitman filter paper and inoculated with a drop of conidia suspension of respective isolates of Colletotrichum spp. having a concentration of 2 × 106 conidia/ml. The Petri dishes with the inoculated berries were then covered to maintain high level of humidity and incubated at room temperature. A total of 10 coffee berries was used to test each isolate. After 14 days, the reaction of each of the isolate was recorded as positive (+) showing the sign of lesions and negative (–) showing no reaction.
2.5.2. Seedling inoculation test
2.5.2.1 Raising coffee seedlings
Coffee seedlings were raised in growth-room from freshly picked seeds of the standard CBD susceptible cultivar 370. Ripened cherries were picked from mother trees in the field and dried under shade after removing the pulp by hand. After removing the parchment, the seeds were soaked in sterilized distilled water and kept for 48 hours. Thereafter, seeds were sown (40 seed per box) in heat sterilized and moistened sandy soil in disinfected plastic boxes each with 2295 cm3 capacity arranged on benches and covered with chip wood in growth-room.
2.5.2.2. Inoculum preparation
Inoculum of each Colletotrichum spp. was prepared by sub-culturing from the stock culture on potato dextrose agar (PDA) medium and incubated at 25ºC. Conidial suspension was prepared by washing 10 days old PDA cultures with sterile-distilled water. Spores were suspended by scraping the colony surface with sterile loop. The suspension was then transferred into a sterile test tube and the spore concentration was determined using a hemocytometer and adjusted to 2 × 106 conidia/ml.
2.5.2.3. Inoculation of coffee hypocotyls
Two days prior to inoculation, seedlings reached a stage just before unfolding of the cotyledons (i.e. six week after sowing). To maintain 100% relative humidity for the seedlings, the boxes were closed for 48 hours with plastic sheets. Then, the coffee hypocotyls were inoculated with conidial suspension by stem brushing procedure with fine camel hairbrush as described by Van der Graaff (Citation1981). The second re-inoculation was conducted after 48 hours following the same procedures. In order to create more humid condition for infection, all the treated hypocotyls were immediately covered with transplant plastic sheet and maintained in a cool place with a mean temperature of 20 ± 2 ºC in a growth room. The reaction of each hypocotyls was assessed in a week interval using the symptom classifications of Van der Graaff (Citation1981) with 0–4 scale; where 0 = no symptom; 1 = from very tiny to 1 or 2 narrow brown lesion up to 0.5 mm wide; 2 = more than 2 brown lesions or brown coalescing lesions exceed 0.5 mm, black dots if present are rare; 3 = wide brown lesions with numerous black dots and/or black lesion may completely surrounded the stem but the top remains alive and 4 = black lesion girdling the stem and top killed. A disease index (DI) for each assessment was expressed as a percentage of the maximum possible infection using the following formula:
where v = number of hypocotyls in class 0, w = number of hypocotyls in class 1, x = number of hypocotyls in class 2, y = number of hypocotyls in class 3, z = number of hypocotyls in class 4.
2.6 Statistical analysis
Data were subjected to analysis of variance (ANOVA) using SAS V 9.2 software package (SAS, Citation2008). Mean comparisons were made using Duncan’s Multiple Range Test (DMRT) at 5% probability level. Cluster analysis was used to determine the relationship of isolates of Colletotrichum species based on cultural, morphological and pathological characteristics using the Unweight Paired-Group Method with Arithmetic Mean method (Mahmoud et al., Citation2007).
3. Results
3.1. Cultural and morphological characteristics of Colletotrichum species
3.1.1. Cultural appearance
Cultural characteristics of Colletotrichum species isolated from diseased coffee berries varied on Potato Dextrose agar (PDA), Malt Extract Agar (MEA) and Sabouraud Dextrose Agar (SDA). Based on the colony color, the isolates were grouped into nine different classes. Most of the isolates exhibited light grey, grey and dark grey colony color on the upper side and the reverse side of the plates revealed a range of colors including light grey, light greenish grey, greenish grey, grey, dim gray, dark grey and dark Olivia green (Table ).
Table 2. Proportion of variation in colony color among isolates of Colletotrichum species from diseased coffee berries from Ethiopia
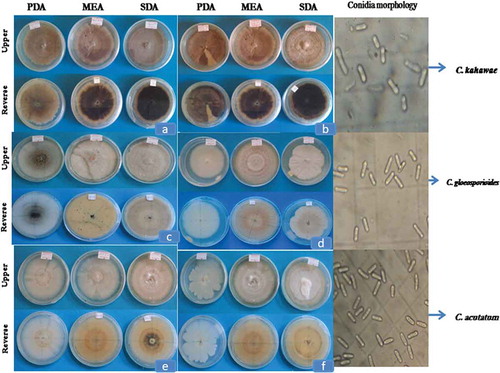
Isolates CBD014, CBD027 and CBD182 displayed a sole colony color with the upper side ranged from white to light grey with orange mass of conidia covered with gelatinous matrix. Similarly, the reverse side of the plate showed a colony with unevenly white to grey color with orange conidia mass. CBD005, CBD042, CBD099, CBD154 and CBD186 exhibited distinct colony characteristics that significantly varied from the other isolates. The colonies had creamy white to white with abundant orange with very small black dots aggregated in the center of the colony. On the reverse side of the colony, white with abundant pink and very small black dots aggregated in the center of the colony were observed. Isolate CBD119 and CBD163 which displayed white on the obverse side of the colony, but lacking black dots aggregated in the center of the colony. Some isolates showed light yellow colony on the reverse side of both MEA and SDA media (Figure ).
Figure 1. Colony characteristics of Colletotrichum species isolated from diseased coffee berry where (a) CBD007, (b) CBD33, (c) CBD014, (d) CBD119, (e) CBD005 and (f) CBD099 (PDA, Potato Dextrose agar; MEA, Malt Extract Agar; SDA, Sabouraud Dextrose Agar)
The isolates also were categorized into 3 classes on the basis of aerial mycelia growth (vigor): dense, irregular (scarce) and very scarce colony types (Table ). Among the total isolates 60.0%, 31.4% and 8.6% on PDA, 65.7%, 25.7% and 8.6% of isolates on MEA and 85.7%, 14.3% and 0.0% isolates on SDA indicated dense, irregular (scarce) and very scarce types of aerial mycelial growth, respectively. A total of 48% of the isolates showed consistent dense aerial mycelia growth on all types of culture media. On the other hand, 42.9%, 45.7% and 20% of the isolates form sectoring on PDA, MEA and SDA media, respectively, while 25.7%, 22.9% and 14.3% of isolates form acervuli (Table ).
Table 3. Comparisons among Colletotrichum isolate on colony density, sector and acervuli
Mycelial growth rate varied significantly (p < 0.05) among Colletotrichum spp. across the different culture media (Table ). Mycelial growth ranged between 5.19 and 8.42 mm/day, 3.26 and 8.99 mm/day and, 4.20 and 8.95 mm/day on PDA, MEA and SDA media, respectively. The overall average radial mycelial growth on the three media ranged between 3.97 and 8.69 mm (Table ).
Table 4. Radial mycelia growth of Colletotrichum species on PDA, MEA and SDA
Isolates CBD014 and CBD182 exclusively showed the fastest growth (8.7 and 8.5 mm/day, respectively) across the three different media compared to the other isolates. Isolates CBD027 and CBD119 also showed fast growth with significant (p < 0.05) difference from other isolates. However, isolate CBD110 showed slow growth rate (3.97 mm/day) than the other isolates. The results also indicated that culture media has a pronounced effect on mycelial growth. Overall, relatively faster growth was observed on SDA than PDA and MEA media (Table ).
3.1.2. Morphological characteristics
3.1.2.1. Sporulation capacity
Conidial production that was taken from ten days old cultures showed highly significant (p < 0.05) differences among isolates (Table ). Isolates of Colletotrichum species produced an average number of conidia that ranged between 2.4 × 105(CBD051) to 1.3 × 107(CBD042), 2.9 × 105 (CBD135) to 1.07 × 106 (CBD014 and CBD042) and 1.9 × 105 (CBD027) to 7.5 × 106 conidia/ml (CBD154) on PDA, MEA and SDA media, respectively. Isolate CBD42 uniquely produced mean higher conidia (8.1 × 106 conidia/ml) on PDA, MEA and SDA medium followed by isolates CBD014, CBD154 and CBD099 with a mean conidia production of 7.0 × 106, 4.8 × 106 and 4.0 × 106 per milliliter, respectively. However, isolate CBD135, produced lower (6.4 × 105 conidia/ml) conidia than the other isolates of Colletotrichum. The overall mean average conidia production of Colletotrichum species on PDA, MEA and SDA was 2.06 × 106 conidia/ml. The fungal isolates produced more abundant conidia on PDA than MEA and SDA (Table ).
Table 5. Variation among isolates of Colletotrichum species in sporulation capacity
3.1.2.2. Conidia size
Conidia harvested from PDA culture manifested irregular sizes (Table ). However, the length and width variations had a distinct persistent pattern in a species. The mean conidia length and width varied between 12.33–17.71 μm, and 3.57–5.10 μm, respectively. Isolates CBD164 and CBD166 had longer conidia of 17.71 and 17.38 μm, respectively. On the other hand, isolate CBD186 had the smallest conidia size (12.33 μm). Similarly, isolates CBD 154, CBD042, CBD099 and CBD005 had also the smallest conidia size. The isolates significantly (p < 0.05) varied in width of their conidia (Table ). Isolate CBD005, CBD182 and CBD186 had thin conidia with values of 3.57, 3.61and 3.63 μm, respectively, but with conidia width of CBD042 which was much thicker than any other species. Overall, average conidia length and width of all Colletotrichum isolates were 14.85 μm and 4.42 μm, respectively.
Table 6. Variation of conidia size of Colletotrichum isolates on potato dextrose agar medium
3.1.2.3. Shape of conidia
The dominant form of conidia was cylindrical and round at both ends (63–90%) followed by cylindrical acute at one and round at the other ends (3-27%). Isolate CBD005, CBD042, CBD099, CBD154 and CBD186 were dominated (33–47%) by cylindrical acute at one and round at the other ends followed by oblong to elliptical shape. Clavate-round at both ends that started attenuating from one-fourth of its length and reniform or kidneys shaped were the other conidia types observed.
3.2. Pathogenicity test
Among the 35 isolates of Colletotrichum spp, all the grey, light grey and dark grey mycelia forms were pathogenic to both detached berries and hypocotyls of the susceptible coffee cultivar 370 except isolate CBD014, CBD027 and CBD182 (Figure ). On the other hand, coffee hypocotyls which were inoculated with CBD005, CBD042, CBD099, CBD119, CBD154, CBD163, and CBD186 remained healthy similar to the control (Table ). Those hypocotyls inoculated with pathogenic species (C. kahawae) developed disease symptoms within five to seven days after inoculation on detached berries and hypocotyls. On hypocotyls test, initially the hypocotyls had small brown spots which latter turned into grayish brown and coalesced to form dark brown lesion which caused the upper part of the seedling to wilt and die (Figure ). Re-isolation of the inoculated culture from the diseased coffee berries and hypocotyls showed the typical colony of C. kahawae.
Figure 2. Pathogenicity of isolates of Colletotrichum species on detached berry test on coffee cultivar 370 and (a-c) Disease symptom development on green berries inoculated with isolates of C. kahawae (CBD068) (d) Isolates of C. gloeosporioides (CBD182), (e) Isolates of C. acutatum (CBD154) and (f) Control

Figure 3. Pathogenicity of isolates of Colletotrichum species on seedling inoculation test on 370 coffee cultivar (a-c) Isolates of C. kahawae; (d) Isolates of C. gloeosporioides; (e) Isolates of C. acutatum and (f) Control

Table 7. Pathogenic variability among isolates of Colletotrichum species
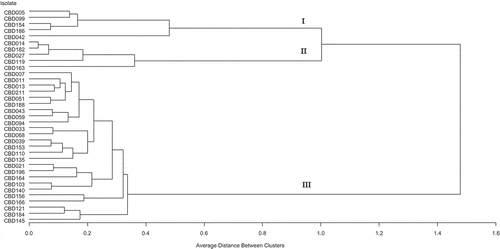
3.3. Cluster analysis
Cluster analysis on cultural, morphological and pathological characteristics grouped the isolates into three categories (Figure ). The first group included 5 isolates (CBD005, CBD042, CBD154, CBD186 and CBD142); the second group also included 5 isolate (CBD014, CBD182, CBD027, CBD119 and CBD163) isolates and the third-largest group comprising all the 25 pathogenic Colletotrichum species (Figure ).
4. Discussion
The cultural characteristics were mostly in harmony with the description of Colletotrichum species reported by many scholars (Biratu, Citation1995; Hindorf, Citation1970; Waller et al., Citation1993). Most of the Colletotrichum isolates displayed light grey, grey and dark grey mycelia color, which is a typical colony characteristic of the C. kahawae with easily distinguishable features from the other isolates that had pale grey to white colonies or pinkish adpressed colonies in the case of C. acutatum (Waller et al., Citation1993). The grey mycelia form of C. gloeosporioides produced orange mass of conidia (acervuli) as observed on CBD014, CBD027 and CBD182 that assisted to distinguish it from the pathogenic species (Waller et al., Citation2007). Over 45% of the isolates made sectoring or saltations, which differed in color from the mother isolates. Sectors in all groups were lighter in color and the isolates varied in their tendency to form saltant on different culture media. The reason for saltations has not been explained fully yet. According to Biratu and Hulluka (Citation1989), if culture variants arising by saltation from single spore culture are more often than not irreversible and heritable, then mutation in a general sense would explain their origin. When change in the culture medium can greatly affect the rate as revealed in the present study, then mutation theory is acceptable. A more likely explanation may be heterokaryosis and parasexualism (Biratu & Hulluka, Citation1989). Differences in aerial mycelia growth and formation of acervuli reported previously reports (Biratu, Citation1995; Emana, Citation2015; Zeru, Citation2006) were also observed in the present study.
Mycelial growth rate varied greatly among isolates of Colletotrichum spp. Isolates of C. gloeosporioides showed fast mean daily radial growth (6.80 to 8.69 mm/day) on PDA, MEA and SDA compared to C. acutatum (4.39 to 7.15 mm/day) and C. kahawae (3.97 to 6.7 mm/day). The growth of C. kahawae was significantly slower than the other Colletotrichum species. This is in agreement with the description of previous scholars (Biratu, Citation1995; Varzea et al., Citation2002; Waller et al., Citation2007). Recently, Weir et al. (Citation2012) also indicate the slow growth rate is among the unique feature used to differentiate C. kahawae subsp. kahawae form the other Colletotrichum spp. Hindorf (Citation1970) and Waller et al. (Citation1993) recorded a relatively low growth rate (1.9–4 mm/day) for C. kahawae. However, Biratu and Hulluka (Citation1989) and Zeru (Citation2006) recorded 4.1–6.7 mm/day growth rate on PDA. Biratu (Citation1995) indicated that the growth rate of C. kahawae and other Colletotrichum species was highly modified by pH, temperature, and repeated sub-culturing. The author also found that repeated sub-culturing could increase the growth rate of C. kahawae by over 20%. Differences in growth rate among the isolates of different species have been considered as a reliable criterion for species delineation of C. kahawae from C. acutatum and C. gloeosporioides (Talhinhas et al., Citation2005; Vinnere et al., Citation2002; Waller et al., Citation2007). The study also revealed that culture media had a pronounced effect on the colony growth. C. kahawae and C. acutatum showed faster growth on SDA, while the isolates of C. gloeosporioides showed faster growth on PDA.
Considerable variation was observed among the isolates of Colletotrichum species in their conidia production. Isolates of C. gloeosporioides and C. acutatum exhibited higher mean conidia production than C. kahawae. The study also showed that culture media has significantly affected conidia production capacity of the fungi. Isolates of C. kahawae exhibited relatively lower conidia production than the two species on PDA and MEA. However, higher conidia of C. kahawae were harvested from SDA ranging between 9.3 × 105 to 2.7 × 106 conidia/ml. Biratu (Citation1995) and Zeru (Citation2006) recorded 1.2–5.2 × 105 conidia/ml and 2.6 × 105–2.5 × 106 conidia/ml production, respectively, for C. kahawae on PDA. Biratu (Citation1995) also indicated that, conidia production could vary depending on media/substrate quality, pH value, temperature, light/dark, culture age and other factors.
The morphological characteristics of the isolates of Colletotrichum sp. were observed on PDA. Conidial length and width varied significantly among the isolates as also noted in other studies (Biratu, Citation1995; Hindorf, Citation1973; Waller et al., Citation1993). Although almost all isolates showed cylindrical with one or both ends obtuse, which is a typical characteristic of conidia of C. kahawae and C. gloeosporioides to distinguish them from C. acutatum, a species with oblong to elliptical shape as observed in the present study from isolates of CBD005, CBD042, CBD099, CBD154 and CBD186 (Waller et al., Citation2007). Despite this fact, conidia shape types and sizes have very limited use for distinguishing and comparing the isolates of Colletotrichum. Overlapping shape and sizes of different Colletotrichum spp. were also reported by McDonald (Citation1926) and Nutman and Roberts (Citation1960).
Pathogenicity test with the isolates of C. kahawae confirmed a positive reaction on both detached coffee berries and hypocotyls. Waller et al. (Citation1993) have demonstrated that pathogenicity towards developing coffee berries and seedling hypocotyls is the most distinctive characteristic of the C. kahawae, which separates it on a functional basis from all other Colletotrichum species. Similar results were also reported by several investigators (Biratu, Citation1995; Biratu & Hulluka, Citation1989; Gibbs, Citation1969; Hindorf, Citation1970; Varzea et al., Citation2002) who have emphasized pathogenicity as a unique feature of C. kahawae. Although the C. kahawae appears to be closely related to the grey C. gloeosporioides and possibly evolved from it fairly recently Waller et al. (Citation1993) it is sufficiently distinct in its pathogenicity, colony morphology and cultural characteristics to warrant recognition as a distinct species as described in the present study.
In agreement with the present study characteristics, previous studies (Biratu, Citation1995; Biratu & Hulluka, Citation1989; Derso & Waller, Citation2003) have also reported that the Colletotrichum populations on Arabica coffee in Ethiopia consist of C. kahawae, C. gloeosporioides and C. acutatum. Colletotrichum kahawae was found to be distinct from other Colletotrichum species by several cultural and morphological characteristics. The color, growth habit and the density of aerial mycelia, the absence of stroma of acervuli and its pathogenic ability to both detached berry and hypocotyls are some distinct characteristics used to identify C. kahawae from isolates of C .gloeosporioides and C. acutatum that most probably survive as saprophytic or sequential colonizer of dead tissues.
5. Conclusion
Our results confirmed that C. kahawae, C. gloeosporioides and C. acutatum are associated with diseased coffee berries from major coffee-growing areas of Ethiopia. The study also revealed the existence of variations in cultural, morphological and pathogenicity characteristics among and within Colletotrichum populations. The C. kahawae population is the only Colletotrichum species pathogenic to Arabic coffee by causing coffee berry disease in the country. C. gloeosporioides and C. acutatum populations exist probably as a saprophytic or sequential colonizer of dead tissues. Thus, further study on the role of saprophytes (C. gloeosporioides and C. acutatum) in CBD pathosystem should be studied. Moreover, variations in aggressiveness among the C. kahawae population need to be investigated in a more detail using various advanced supplementary techniques to reveal the diversity of the pathogen in the study areas.
Acknowledgements
The authors would like to acknowledge Jimma University and Assosa University for financial support for the research work. We are also indebted to Jimma Agricultural Research Center (JARC) for providing laboratory and greenhouse facilities.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors

Kumlachew Alemu
Dr. Kumlachew Alemu is an Assistant Professor of Plant Pathology in the Department of Plant Science, Assosa University. He is currently engaged in teaching and conducting researcher in the area of plant protection.
Girma Adugna
Dr. Girma Adugna is an Associate Professor of Plant Pathology in the Department of Horticulture and Plant Science, Jimma University. He is a senior pathologist who worked and published a lot on coffee diseases and their managements.
Fikre Lemessa
Prof. Fikre Lemessa, is a Professor of Plant Pathology, in the Department of Horticulture and Plant Science, Jimma University. He is senior pathologist who worked and published a lot on plant diseases.
Diriba Muleta
Dr. Diriba Muleta is an Associate Professor of Soil Microbiology Institute of Biotechnology, Addis Ababa University. He is senior Microbiologist who worked and published a lot on coffee microbiology.
References
- Adugna, G., Jafuka, C., Zeru, A., & Tessfaye, A. (2009). Advances in coffee diseases research in Ethiopia. Pp 275–20. In: Tadesse A(ed). Increasing crop production through improved plant protection. volume II. Proceeding of the 14th annual conference of the plant protection society of Ethiopia (PPSE). 19-22 December 2006, Addis Ababa, Ethiopia: PPSE and EIAR, Addis Ababa Ethiopia.
- Alemu, K., Adugna, G., Lemessa, F., & Muleta, D. (2016). Current status of coffee berry disease (Colletotrichum kahawae waller & bridge) in Ethiopia. Archives of Phytopathology Plant Protection, 49(17-18), 421–433. https://doi.org/10.1080/03235408.2016.1228736
- Anonymous. (2013). RGB color charts. Accessed November, 2013. http://www.multirip.com/colormanagement.html
- Batista, D., Silva, D. N., Vieira, A., Cabral, A., Pires, A. S., Loureiro, A., Guerra-Guimarães, L., Pereira, A. P., Azinheira, H., Talhinhas, P., & Silva, M. D. C. (2017). Legitimacy and implications of reducing Colletotrichum kahawae to subspecies in plant pathology. Frontiers in Plant Science, 7, 2051. https://doi.org/10.3389/fpls.2016.02051
- Belachew, K., & Teferi, D. (2015). Climatic variables and impact of coffee berry diseases (Colletotrichum kahawae) in Ethiopian coffee production. Journal of Biology, Agriculture and Healthcare, 5(7), 55–64.
- Berecha, G., Aerts, R., Vandepitte, K., Van Glabeke, S., Muys, B., Roldán‐Ruiz, I., & Honnay, O. (2014). Effects of forest management on mating patterns, pollen flow and intergenerational transfer of genetic diversity in wild arabica coffee (coffea arabica l.) from afromontane rainforests. Biological Journal of the Linnean Society, 112(1), 76–88. https://doi.org/10.1111/bij.12274
- Bhattacharya, S., & Bagyaraj, D. J. (2002). Effectiveness of arbuscular mycorrhizal fungal isolates on arabica coffee (coffea arabica L.). Biological Agriculture and Horticulture, 20(2), 125–131. https://doi.org/10.1080/01448765.2002.9754956
- Biratu, T. (1995). Studies on Colletotrichum population of Coffea arabica L. in Ethiopia and evaluation of the reactions of coffee germplasms. Ph.D. Thesis, University of Bonn.
- Biratu, T., & Hulluka, M. (1989). Colletotrichum species associated with coffee berry disease in hararge. Ethiopian Journal of Agricultural Science, 11, 1–6.
- Boansi, D., & Crentsil, C. (2013). Competitiveness and determinants of coffee exports, producer price and production for Ethiopia. Journal of Advanced Research in Economics and International Business1(1), 31–56.
- Bridge, P. D., Waller, J. M., Davies, D., & Buddie, A. G. (2008). Variability of Colletotrichum kahawae relation to other Colletotrichum species from tropical perennial crops and the development of diagnostic techniques. Journal of Phytopathology, 156(5), 274–280. https://doi.org/10.1111/j.1439-0434.2007.01354.x
- Chakraborty, S., Tiedemann, A. V., & Teng, P. S. (2000). Climate change: Potential impact on plant diseases. Environmental Pollution, 108(3), 317–326. https://doi.org/10.1016/S0269-7491(99)00210-9
- Derso, E. (1997). Coffee diseases and their significance in Ethiopia. The proceedings of ASIC 17(I).723–726. Nairobi, Kenya.
- Derso, E., & Waller, J. M. (2003). Variation among Colletotrichum isolates from diseased coffee berries in Ethiopia. Crop Protection, 22(3), 561–565. https://doi.org/10.1016/S0261-2194(02)00191-6
- Emana, B. T. (2015). Coffee berry disease (Colletotrichum kahawae): Status, pathogenic variability and reactions of coffee landraces in Hararghe, Eastern Ethiopia. International Journal of Plant Breeding Crop Science, 1(2), 018–027.
- Garedew, W., Lemessa, F., & Pinard, F. (2017). Assessment of berry drop due to coffee berry disease and non-CBD factors in arabica coffee under farmer’s fields of Southwestern Ethiopia. Crop Protection, 98, 276–282. https://doi.org/10.1016/j.cropro.2017.04.012
- Garrett, K. A., Dendy, S. P., Frank, E. E., Rouse, M. N., & Travers, S. E. (2006). Climate change effects on plant disease: Genomes to ecosystems. Annual Review of Phytopathology, 44(1), 489–509. https://doi.org/10.1146/annurev.phyto.44.070505.143420
- Gibbs, J. N. (1969). Inoculum sources for coffee berry disease. Ann. Appl. Biol, 64(3), 515–522. https://doi.org/10.1111/aab.1969.64.issue-3
- Hindorf, H. (1970). Colletotrichum spp. isolated from coffea arabica in Kenya. Z. Pflanzenkrh. Pflanzenschutz. 77: 328–331.
- Hindorf, H. (1973). Colletotrichum -population on coffea arabica L. in Kenya: II. Qualitative and quantitative differences in the Colletotrichum -population. Journal of Phytopathology 77(3): 216–234.
- International Coffee Organization [ICO]. (2015). World coffee market. ICO annual review Retrieved November 2016. http://www.ico.org/. International Coffee Organization, 222 Gray's Inn Road, London WC1X 8HB.
- International Monetary Fund [IMF]. (2016). Article IV consultation—press release; staff report; and statement by the executive director for the federal democratic Republic Of Ethiopia. IMF Country Report No. 16/322, October 2016. International Monetary Fund.
- Labouisse, J. P., Bellachew, B., Kotecha, S., & Bertrand, B. (2008). Current status of coffee (coffea arabica L.) genetic resources in Ethiopia: Implications for conservation. Genetic Resources and Crop Evolution, 55(7), 1079–1093. https://doi.org/10.1007/s10722-008-9361-7
- Loureiro, A., Guerra-Guimarães, L., Lidon, F. C., Bertrand, B., Silva, M. C., & Várzea, V. (2011). Isoenzymatic characterization of Colletotrichum kahawae isolates with different levels of aggressiveness. Tropical Plant Pathology, 36(5), 287–293. https://doi.org/10.1590/S1982-56762011000500003
- Luzolo, M., Talhinhas, P., Várzea, V., & Neves-Martins, J. (2010). Characterization of Colletotrichum kahawae isolates causing coffee berry disease in Angola. Journal of Phytopathology, 158(4), 310–313. https://doi.org/10.1111/jph.2010.158.issue-4
- Mahmoud, Y. A. G., Gaafar, R. M., & Mubarak, H. M. (2007). Genetic diversity among nile delta isolates of rhizoctonia solani kuhn based on pathogenicity, compatibility isozyme analysis and total protein pattern. Turk Journal of Botany, 31(1), 19–29.
- McDonald, J. (1926). A preliminary account of a disease of green coffee berries in Kenya colony. Transactions of the British Mycological Society, 11(1-2), 145–154. https://doi.org/10.1016/S0007-1536(26)80033-6
- Nguyen, T. H. P., Säll, T., Bryngelsson, T., & Liljeroth, E. (2009). Variation among Colletotrichum gloeosporioides isolates from infected coffee berries at different locations in Vietnam. Plant Pathology, 58(5), 898–909. https://doi.org/10.1111/ppa.2009.58.issue-5
- Nutman, F., & Roberts, F. M. (1960). Investigations on a disease of coffea arabica caused a form of Colletotrichum coffeanum noack. II. Some factors affecting germination and infection and their relationship to disease distribution. Transactions of the British Mycological Society, 43(4), 643–659. https://doi.org/10.1016/S0007-1536(60)80055-1
- Omondi, C., Hindorf, H., Welz, H., Saucke, D., Ayiecho, P., & Mwang’ombe, A. (2000). Reaction of some coffee arabica genotypes to strains of Colletotrichum kahawae, the cause of coffee berry disease. Journal of Phytopathology, 148(1), 61–63. https://doi.org/10.1111/j.1439-0434.2000.tb04626.x
- Peres, N. A., Kuramae, E. E., Dias, M. S., & De Souza, N. L. (2002). Identification and characterization of Colletotrichum spp. affecting fruit after harvest in Brazil. Journal of Phytopathology, 150(3), 128–134. https://doi.org/10.1046/j.1439-0434.2002.00732.x
- Pires, A. S., Azinheira, H. G., Cabral, A.,Tavares, S., Tavares, D., Castor, M., Varzea, V., Silva M.C., Abranches, R., Loureiro, J., & Talhinhas, P. (2016). Cytogenomic characterization of Colletotrichum kahawae, the causal agent of coffee berry disease, reveals diversity in minichromosome profiles and genome size expansion. Plant Pathology, 65(6), 968–977. https://doi.org/10.1111/ppa.2016.65.issue-6
- Prihastuti, H., Cai, L., Chen, H., EHC, M., & Hyde, K. D. (2009). Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Diversity, 39(1), 89–109.
- SAS. (2008). SAS user’s guide (version 9.2).
- Talhinhas, P., Sreenivasaprasad, S., Neves-Martins, J., & Oliveira, H. (2005). Molecular and phenotypic analyses reveal the association of diverse Colletotrichum acutatum groups and a low level of Colletotrichum gloeosporioides with olive anthracnose. Applied Environmental Microbiology, 71(6), 2987–2998. https://doi.org/10.1128/AEM.71.6.2987-2998.2005
- Van der Graaff, N. A. (1981). Selection for Arabica coffee types resistant to CBD in Ethiopia. PhD Thesis.Wageningen, the Netherlands
- van der Vossen, H., Bertrand, B., & Charrier, A. (2015). Next generation variety development for sustainable production of arabica coffee (coffea arabica L.): A review. Euphytica, 204(2), 243–256. https://doi.org/10.1007/s10681-015-1398-z
- Varzea, V. M. P., Rodrigues, C. J., & Lewis, B. G. (2002). Distinguishing characteristics and vegetative compatibility of Colletotrichum kahawae in comparison with other related species from coffee. Plant Pathology, 51(2), 202–207. https://doi.org/10.1046/j.1365-3059.2002.00622.x
- Vieira, A., Diniz, I., Loureiro, A., Pereira, A. P., Silva, M. C., Várzea, V., & Batista, D. (2019). Aggressiveness profiling of the coffee pathogen Colletotrichum kahawae. Plant Pathology, 68(2), 358–368. https://doi.org/10.1111/ppa.2019.68.issue-2
- Vieira, A., Silva, D. N., Várzea, V., Paulo, O. S., & Batista, D. (2018). Novel insights on colonization routes and evolutionary potential of Colletotrichum kahawae, a severe pathogen of coffea arabica. Molecular Plant Pathology, 19(11), 2488–2501. https://doi.org/10.1111/mpp.2018.19.issue-11
- Vinnere, O., Fatehi, J., Wright, S. A. I., & Gerhardson, B. (2002). The causal agent of anthracnose of rhododendron in Sweden and Latvia. Mycological Research, 106 (1), 60–69. https://doi.org/10.1017/S0953756201005366
- Waller, J. M., Bigger, M., & Hillocks, R. J. (2007). Coffee pests, diseases and their management. UK CAB International.
- Waller, J. M., Bridge, P. D., Black, R., & Hakizal, G. (1993). Characterization of the coffee berry disease pathogen, Colletotrichum kahawae sp. nov. Mycological Research, 97(8), 989–994. https://doi.org/10.1016/S0953-7562(09)80867-8
- Weir, B. S., Johnston, P. R., & Damm, U. (2012). The Colletotrichum gloeosporioides species complex. Studies in Mycology, 73, 115–180. https://doi.org/10.3114/sim0011
- Worako, T. K., Van Schalkwyk, H. D., Alemu, Z. G., & Ayele, G. (2008). Producer price and price transmission in a deregulated Ethiopian coffee market. Agrekon, 47(4), 492–508. https://doi.org/10.1080/03031853.2008.9523812
- Zeru, A. (2006). Diversity of Arabica coffee populations in afromontane rainforests of Ethiopia in relation to Colletotrichum kahawae and Gibberella xylarioides M.Sc. Thesis. School of Graduate Studies, Department of Biology, Addis Ababa University. Pp. 80.
- Zeru, A., Teferi, D., Jafuka, C., Tesfaye, S., Siyoum, M., & Adugna, G. (2008). Success stories in managing coffee berry disease in Ethiopia.G. Adugna, B. Bellachew, T. Shimbir, E. Taye, & T. Kufa Eds., Coffee diversity and knowledge. Proceeding of a national workshop four decades of coffee research and development in Ethiopia (239–249). 14-17 August 2007.Ethiopian Institute of Agriculture Research (EIAR), Addis Ababa, Ethiopia.