1. Introduction
Oncology, the medical field specializing in the study, diagnosis, and treatment of cancer, has made significant advancements over the years [Citation1]. While the advent of chemotherapy, targeted therapies, and immunotherapy has expanded treatment options, the field still grapples with numerous challenges. One such obstacle is Cancer Stem Cells (CSCs), which often evade traditional therapies and contribute to cancer recurrence [Citation2]. These CSCs further complicate treatment landscapes through resistance mechanisms mediated by ATP-Binding Cassette (ABC) transporter proteins. The adaptability of cancer cells and their intricate interactions with their surrounding microenvironments add another layer of complexity to therapeutic outcomes [Citation2]. Moreover, the absence of early symptoms in certain cancers, such as esophageal, prostate, and pancreatic types, hampers timely intervention [Citation2]. The lack of reliable biomarkers not only impedes early detection but also complicates the assessment of treatment efficacy, necessitating multifaceted approaches for successful management [Citation3].
Precision medicine emerges as a groundbreaking solution in this complex landscape. It customizes medical treatments to each patient’s unique genetic makeup, environmental factors, and lifestyle choices, aiming for optimal outcomes [Citation4]. In oncology, precision medicine serves as a pivotal innovation, offering targeted solutions to persistent challenges like CSCs and drug resistance [Citation4]. By tailoring treatments to the specific genetic and molecular profiles of each patient’s cancer, precision medicine aims to maximize effectiveness while minimizing side effects [Citation4]. This individualized strategy proves particularly useful in navigating the complexities of cancer cell interactions with their microenvironment, thereby enhancing therapeutic results. Furthermore, precision medicine has the potential to revolutionize diagnostic procedures, especially for cancers that often go unnoticed in their early stages [Citation5]. The use of targeted biomarkers could facilitate not only early detection but also a more nuanced evaluation of treatment effectiveness. Given cancer’s propensity to spread, requiring a multi-targeted approach, precision medicine stands as an invaluable asset in cancer therapy [Citation5].
This editorial aims to explore the current and future potential of precision medicine in oncology. It offers a comprehensive perspective on how this innovative approach could transform cancer treatment, making it both more effective and safer.
1.1. Discussion
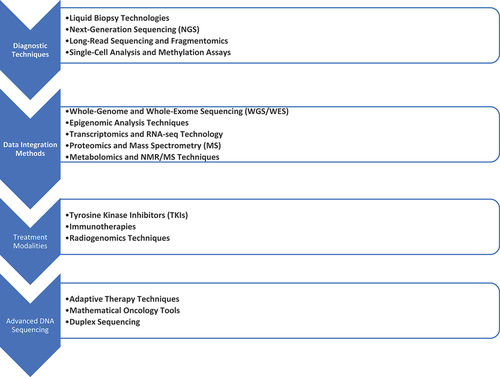
Approaches to precision medicine in cancer therapy can generally be categorized into diagnostic techniques, data integration methods, and treatment modalities (). Diagnostic tools such as Liquid Biopsy and genomic sequencing identify cancer-specific markers and mutations. Meanwhile, Multi-omics Analysis and Artificial Intelligence (AI) synthesize diverse biological and clinical data to provide a comprehensive view of the cancer landscape. Treatment modalities include targeted therapies, immunotherapies, and radiogenomics, which tailor radiation and drug treatments according to the individual characteristics of each tumor.
1.1.1. Diagnostic techniques
Liquid biopsies have rapidly emerged as a cutting-edge, noninvasive technique for biomarker detection, offering the promise of early diagnosis and continuous monitoring [Citation6]. Biopsy-guided sampling technologies such as Next-Generation Sequencing (NGS), coupled with advances in high-throughput sequencing, have revolutionized biomarker discovery. NGS platforms are particularly effective at analyzing nucleic acid materials from bodily fluids, providing a comprehensive assessment of potential biomarkers [Citation6]. Emerging techniques like long-read sequencing and fragmentomics offer deeper insights into the genetic makeup of cancer cells, while whole-genome amplification methods for single-cell analysis and whole-genome methylation assays enhance our understanding of diseases [Citation6,Citation7]. Bioinformatics tools play a crucial role in this ecosystem, assisting with data processing for NGS as well as developing software applications for liquid biopsy biomarker detection [Citation7].
Liquid biopsies and NGS present revolutionary approaches for early cancer detection; Recent advancements in liquid biopsies have shown that circulating tumor DNA analysis (ctDNA) can identify specific mutations, allowing for the development of targeted therapies tailored to each patient’s unique genomic profile. Additionally, emerging techniques such as long-read sequencing are being employed to detect structural variations in the genome, furthering the search for biomarkers. Concurrently, bioinformatics tools have undergone significant evolution, enabling the integration of diverse data types, including genomics, proteomics, and clinical information. These developments are instrumental in facilitating personalized cancer treatments, uniquely designed according to each patient’s genetic makeup.
However, they also have limitations in terms of sensitivity, specificity, and cost-effectiveness [Citation8]. These limitations are evidenced by discrepancies between tumor tissue mutation detection rates and those detected from cfDNA. Comparative analysis indicates that liquid biopsies offer noninvasive but limited biomarker profiling capabilities compared to NGS; emerging techniques like long-read sequencing provide nuanced insights but remain experimental [Citation8]. Future research should focus on enhancing the sensitivity and specificity of existing cancer diagnostic methods, identifying cost-effective alternatives, integrating multi-omics data for a more comprehensive understanding, and validating emerging techniques to establish their efficacy in cancer detection.
1.1.2. Data integration methods
Genomic technologies play a pivotal role in bulk omics, using Whole-Genome Sequencing (WGS) and Whole-Exome Sequencing (WES) to decode DNA sequences and their modifications [Citation9]. AI accelerates the translation of this genomic data into clinical applications. Epigenomics complements this information by employing techniques like DNA methylation analysis and Chromatin Immunoprecipitation Sequencing (ChIP-seq) to study chemical modifications affecting gene expression, which could serve as potential targets in cancer pathogenesis [Citation9].
Transcriptomics enhances our understanding by using RNA-seq technology to analyze RNA and interpret gene expression patterns, making this technique essential for cancer diagnosis and treatment optimization. Other approaches, such as proteomics, which uses Mass Spectrometry (MS) to study protein functions, and metabolomics, which employs both Nuclear Magnetic Resonance (NMR) and MS to study metabolic pathways, have also made significant contributions [Citation10].
Single-cell omics technologies go a step further by profiling individual cells, providing a more in-depth picture than bulk omics can offer. Technologies like Genome and Transcriptome Sequencing (G&T-seq) and Simultaneous Isolation of DNA and RNA (SIDR) enable simultaneous genome and transcriptome profiling at the single-cell level, offering high-resolution analysis that enriches our understanding of cancer heterogeneity and contributes to early detection and drug target discovery [Citation10].
Beyond traditional omics techniques, the field is rapidly expanding into medical imaging techniques like pathomics and radiomics, which utilize large-scale digital archives for cancer diagnosis. Radiogenomics integrates medical imaging with genomics to deepen our understanding of disease mechanisms and biomarker discovery.
Data analysis in AI has advanced from shallow to deep learning architectures, adept at handling the complexity of multi-omics data. Machine Learning (ML) methods can generally be categorized into supervised and unsupervised learning, each with its own set of challenges and opportunities in terms of data integration and interpretation [Citation11].
However, these technologies do have limitations. A major challenge is data scarcity, which impacts the successful training of AI models and meaningful interpretation of omics data. Another obstacle is the high heterogeneity in both cancer biology and omics data, complicating the development of universal models. This heterogeneity also makes the integration of multi-omics data challenging, especially when merging it with clinical information like patient history or treatment outcomes.
Collaborative efforts are essential for curating large, high-quality datasets that represent diverse populations. Open-access databases could facilitate this, allowing researchers worldwide to contribute to and access data for more comprehensive studies. Further research is needed to develop algorithms capable of handling the heterogeneity in omics data, encompassing genomics, proteomics, and patient clinical records to provide a holistic view of cancer.
Beyond traditional omics techniques, medical imaging approaches such as pathomics and radiomics are rapidly advancing in the field. Radiogenomics, which combines medical imaging with genomics, offers greater insights into disease mechanisms and biomarker discovery. AI has progressed from shallow learning architectures to more complex neural network architectures, adept at handling multi-omics data. ML methods are broadly categorized into two types: supervised and unsupervised learning, each presenting unique challenges and opportunities for data integration and interpretation.
However, these technologies are not without their limitations. A significant challenge is the scarcity of data, which hampers the successful training of AI models and the meaningful interpretation of omics data. The heterogeneity within cancer biology and omics data poses another difficulty, making the development of universal models challenging. Furthermore, integrating these technologies with clinical information, such as patient history or treatment outcomes, introduces additional complications.
Collaborative efforts are crucial for generating large, high-quality datasets that accurately represent diverse populations. The creation of open-access databases would facilitate this by enabling researchers worldwide to contribute to and access data for more comprehensive studies. Research into algorithms capable of managing the heterogeneity in omics data, which includes genomics, proteomics, and patient clinical records, is also vital to this endeavor.
1.1.3. Treatment modalities
Targeted therapies like tyrosine kinase inhibitors (TKIs) are effective but often encounter resistance, particularly from lung cancer cells treated with EGFR inhibitors [Citation12]. Several studies explore combinations that include immunotherapies and multiple TKIs to target various melanoma biomarkers; some have received FDA approval while others are still in the experimental phase [Citation13]. Similarly, anaplastic lymphoma kinase (ALK), associated with various cancers, presents resistance challenges; a recent study suggests monoclonal antibody screening or additional combination therapies as potential solutions [Citation12].
Immunotherapies such as pembrolizumab and nivolumab have revolutionized cancer treatment but can induce adverse side effects, including autoimmune reactions [Citation14]. Clinical trials like CheckMate 067 aim to enhance efficacy while minimizing side effects by combining different immunotherapies [Citation15]. The use of immunosuppressant drugs is also being explored, although this approach raises concerns about increased infection risks.
Future research may focus on personalized treatment plans based on genetic markers and specific tumor characteristics. ML could enable real-time monitoring of treatment responses, while novel drug delivery systems like nanoparticles may improve both the safety and efficacy of therapies.
Radiogenomics integrates radiomics and genomics to deepen our understanding of breast cancer (BC), aiming to correlate imaging features with genomic markers such as mRNA profiles [Citation16]. This interdisciplinary field has seen significant growth over the past two decades, particularly in BC research. Innovative approaches like ‘radiomiRnomics,’ which merges radiomics with epigenomic miRNA profiles, could help in identifying correlations between imaging features and molecular subtypes, recurrence risks, and cellular dynamics within tumors [Citation17].
However, radiogenomics faces several barriers, such as high costs and data complexity issues, lack of comprehensive databases combining both imaging and genomic information, insufficient biopsy samples used for genomic analysis may not fully represent tumor complexity and lack of standardization in imaging/biochemical techniques hampers its creation as reliable radiogenomic biomarkers.
Radiogenomics, integrating radiomics and genomics, significantly enhances our understanding of breast cancer (BC). This approach aims to establish correlations between imaging features and genomic markers, such as mRNA profiles, offering deeper insights into BC research. Over the past two decades, radiogenomics has experienced substantial growth, especially among researchers focusing on breast cancer. One of the innovative techniques in this field is ‘radiomiRnomics,’ which merges radiomics with epigenomic miRNA profiles. This method could aid researchers in uncovering connections between imaging characteristics and various aspects of breast cancer, including molecular subtypes, recurrence risks, and the dynamics of cells within tumors.
However, radiogenomics faces several challenges. These include the high costs and complexity of handling data, the absence of comprehensive databases that amalgamate imaging and genomic information, and the inadequacy of biopsy samples for genomic analysis that accurately reflect the complexity of tumors. Additionally, the lack of standardization in imaging and biochemical techniques poses a significant obstacle to the development of radiogenomic biomarkers. These limitations necessitate a concerted effort to overcome them, ensuring the further advancement of radiogenomics in breast cancer research.
1.1.4. Advanced DNA sequencing and the complexity of cancer evolution
Precision medicine has significantly advanced cancer treatment, yet it continues to grapple with the complexities of subclonal heterogeneity and the evolutionary dynamics within tumors. Targeted therapies have notably improved outcomes, particularly in metastatic breast cancer, but treatment resistance remains a formidable challenge, often due to the emergence of resistant clones.
In response to these challenges, evolutionarily guided precision medicine has emerged, offering more personalized treatment plans and therapies, such as adaptive therapy. This approach was exemplified in Gatenby et al.‘s 2009 study published in Cancer Research, which introduced adaptive therapy as part of a treatment regimen. This method adapts to changes within the tumor microenvironment and responds to therapy-induced alterations [Citation18]. The study used mathematical models to show how resistant cancer phenotypes, initially at a disadvantage, can proliferate once their sensitive counterparts are eliminated by high-dose chemotherapy. Adaptive therapy aims to maintain stable tumor populations by allowing some chemosensitive cells to survive while suppressing emerging resistant subpopulations, thus prolonging survival compared to traditional high-dose therapies.
Further supporting this concept, Beckman, Schemmann, and Yeang (2012) developed a mathematical model that integrates genetic evolutionary dynamics and single-cell heterogeneity into an effective personalized medicine strategy. Their model suggests that nonstandard personalized medicine approaches, considering these complexities, could yield superior patient outcomes compared to current methods [Citation19].
Mathematical oncology also attests to this advanced approach, using multimodal data for proactive treatment strategies. As outlined in the 2019 Roadmap for Mathematical Oncology (MAPO), this field is crucial in tailoring medicine through mathematics, modeling, and simulation based on patient-specific data. It aims to overcome therapy resistance, implement domain-specific standards for sharing model predictions, and enhance the reproducibility of models [Citation20].
Duplex Sequencing, introduced by Schmitt MW in a 2012 PNAS publication [Citation21], represents a significant advancement in DNA sequencing technology, with profound implications for cancer research and precision medicine. This innovative method uniquely tags and sequences both strands of a DNA duplex, resulting in an exceptionally low technical error rate, estimated at less than one artefactual mutation per billion nucleotides sequenced. The precision of Duplex Sequencing is particularly invaluable in cancer research, where detecting even the rarest subclones can significantly impact treatment decisions and patient prognosis. Traditional sequencing methods often struggle with high error rates, which can obscure the identification of low-frequency mutations crucial for understanding cancer evolution and therapeutic response. However, the enhanced accuracy of Duplex Sequencing provides researchers with a reliable tool to discern the true genetic landscape of tumors.
Loeb et al. addressed colorectal cancers (CRCs) [Citation22]. They highlighted the presence of both clonal and subclonal mutations in CRCs. Clonal mutations, which are positively selected and present in most cells, drive malignant progression. Subclonal mutations, on the other hand, are randomly dispersed throughout the genome and contribute significantly to tumor heterogeneity. These subclonal mutations can expand, repopulate the tumor, and lead to rapid emergence of resistance. They employed duplex sequencing methodology to quantify these mutations with high accuracy, revealing an unexpectedly high effective mutation rate. This finding suggests that no DNA locus is wild type in every malignant cell within a tumor at the time of diagnosis.
Robert A. Beckman and Lawrence A. Loeb highlighted the significance of intratumoral genetic heterogeneity in cancer evolution and therapy [Citation23]. It was found that driver mutations are mostly clonal and present in primary tumors and their metastases, indicating that a single biopsy can capture the majority of these mutations. Advanced Duplex Sequencing revealed an unexpectedly high effective mutation rate in colorectal cancer, suggesting the presence of all possible mutations, including resistance mutations, in at least one cell at diagnosis. Both studies emphasized the importance of using non-cross resistant therapies and frequent adaptation of treatment to address the complex mutational landscape of cancers, including the potential threat of hypermutator subclones that rapidly acquire multiple resistance mutations.
1.2. Conclusion
Precision medicine plays a central role in oncology, providing targeted solutions to complex challenges like drug resistance. By employing cutting-edge diagnostic techniques, data analytics and treatment modalities aimed at revolutionizing cancer care – making it more tailored, effective and safer – precision medicine stands to revolutionize cancer treatment paradigm. Future research should aim at overcoming existing limitations such as data scarcity or heterogeneity while exploring innovative approaches such as ML or novel drug delivery systems.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
AZA did the study design development, data extraction, manuscript drafting and reviewing
Additional information
Funding
References
- Witt CM, Balneaves LG, Cardoso MJ, et al. A comprehensive definition for integrative oncology. J Natl Cancer Inst Monogr. 2017 Nov;2017(52). doi: 10.1093/jncimonographs/lgx012
- Phi LTH, Sari IN, Yang Y-G, et al. Cancer Stem cells (CSCs) in drug resistance and their therapeutic Implications in cancer treatment. Stem Cells Int. 2018;2018:1–16. doi: 10.1155/2018/5416923
- Tappia PS, Ramjiawan B. Biomarkers for early detection of cancer: molecular aspects. Int J Mol Sci. 2023;24(6):5272. Switzerland, Mar. doi: 10.3390/ijms24065272
- Di Nicolantonio F, Vitiello PP, Marsoni S, et al. Precision oncology in metastatic colorectal cancer — from biology to medicine. Nat Rev Clin Oncol. 2021;18(8):506–525. doi: 10.1038/s41571-021-00495-z
- Singla P, Musyuni P, Aggarwal G, et al. Precision medicine: an emerging Paradigm for improved diagnosis and safe therapy in pediatric oncology. Cureus. 2021 Jul;13(7):e16489. doi: 10.7759/cureus.16489
- Brockley LJ, Souza VGP, Forder A, et al. Sequence-based platforms for discovering biomarkers in liquid biopsy of non-small-cell lung cancer. Cancers (Basel). 2023 Apr;15(8):2275. doi: 10.3390/cancers15082275
- Huang L, Ma F, Chapman A, et al. Single-cell whole-genome amplification and sequencing: methodology and applications. Annu Rev Genomics Hum Genet. 2015;16(1):79–102. doi: 10.1146/annurev-genom-090413-025352
- Szász I, Kiss T, Mokánszki A, et al. Identification of liquid biopsy-based mutations in colorectal cancer by targeted sequencing assays. Mol Cell Probes. 2023;67:101888. doi: 10.1016/j.mcp.2022.101888
- Babu M, Snyder M. Multi-omics profiling for health. Mol & Cell Proteomics. 2023 Jun;22(6):100561. doi: 10.1016/j.mcpro.2023.100561
- Hong M, Tao S, Zhang L, et al. RNA sequencing: new technologies and applications in cancer research. J Hematol Oncol. 2020;13(1):166. doi: 10.1186/s13045-020-01005-x
- Arjmand B, Hamidpour SK, Tayanloo-Beik A, et al. Machine learning: a New prospect in multi-omics data analysis of cancer. Front Genet. 2022;13:824451. doi: 10.3389/fgene.2022.824451
- Zhao S, Li J, Xia Q, et al. New perspectives for targeting therapy in ALK-positive human cancers. Oncogene. 2023;42(24):1959–1969. doi: 10.1038/s41388-023-02712-8
- Rager T, Eckburg A, Patel M, et al. Treatment of metastatic melanoma with a combination of immunotherapies and molecularly targeted therapies. Cancers (Basel). 2022;14(15):3779. doi: 10.3390/cancers14153779
- Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–668. doi: 10.1038/s41577-020-0306-5
- Branchoux S, Sofeu CL, Gaudin A-F, et al. Time to next treatment or death as a candidate surrogate endpoint for overall survival in advanced melanoma patients treated with immune checkpoint inhibitors: an insight from the phase III CheckMate 067 trial. ESMO Open. 2022 Feb;7(1):100340. doi: 10.1016/j.esmoop.2021.100340
- Gallivanone F, Bertoli G, Porro D. Radiogenomics, breast cancer diagnosis and characterization: Current status and Future directions. Methods Protoc. 2022 Oct;5(5). doi: 10.3390/mps5050078.
- Gallivanone F, Cava C, Corsi F, et al. In Silico Approach for the Definition of radiomiRNomic Signatures for Breast Cancer Differential Diagnosis. Int J Mol Sci. 2019 Nov;20(23). doi: 10.3390/ijms20235825.
- Gatenby RA, Silva AS, Gillies RJ, et al. Adaptive therapy. Cancer Res. 2009 Jun;69(11):4894–4903. doi: 10.1158/0008-5472.CAN-08-3658
- Beckman RA, Schemmann GS, Yeang C-H. Impact of genetic dynamics and single-cell heterogeneity on development of nonstandard personalized medicine strategies for cancer. Proc Natl Acad Sci U S A. 2012 Sep;109(36):14586–14591. doi: 10.1073/pnas.1203559109
- Rockne RC, Hawkins-Daarud A, Swanson KR, et al. The 2019 mathematical oncology roadmap. Phys Biol. 2019 Jun;16(4):41005. doi: 10.1088/1478-3975/ab1a09
- Schmitt MW, Kennedy SR, Salk JJ, et al. Detection of ultra-rare mutations by next-generation sequencing. Proc Natl Acad Sci U S A. 2012 Sep;109(36):14508–14513. doi: 10.1073/pnas.1208715109
- Loeb LA, Kohrn BF, Loubet-Senear KJ, et al. Extensive subclonal mutational diversity in human colorectal cancer and its significance. Proc Natl Acad Sci U S A. 2019 Dec;116(52):26863–26872. doi: 10.1073/pnas.1910301116
- Beckman RA, Loeb LA. Rare mutations in cancer drug resistance and implications for therapy. Clin Pharmacol Ther. 2020;108(3):437–439. doi: 10.1002/cpt.1938