ABSTRACT
Background
Radiological response assessment is becoming challenging with novel immune-based combinations for metastatic renal cell carcinoma (mRCC). RECIST criteria appear not exhaustively adequate to capture the kinetics of treatment response, which is better reflected by tumor growth rate (TGR). We explored TGR changes during first-line treatments and its association with clinical outcomes in mRCC.
Research design and methods
We retrospectively evaluated TGR in untreated patients undergoing pembrolizumab/axitinib (P/A) or tyrosine-kinase inhibitors (TKI). TGR was calculated at the first (TGR1, after 3 months) and the second (TGR2, after 6 months) evaluation, thus assessing the TGR2-TGR1 difference.
Results
Thirty-three patients were included (P/A n = 15, TKIs n = 18). Volumes firstly decreased more rapidly with TKIs, and then more slowly. Volumes initially remained stable with P/A, quickly decreasing until the second evaluation. TGR1 was related to progression-free survival (PFS; p = 0.023) and overall survival (p = 0.046) with P/A. TGR2 was correlated with PFS in all patients (p = 0.025). Patients with higher velocity volume reduction appeared to have improved survival benefits than patients with lower velocity considering both treatments, but especially with P/A.
Conclusion
Combining immunotherapy with TKIs has an important role in enhancing the rapidity of tumor shrinkage. A rapid disease volume reduction correlates with better OS and PFS.
1. Introduction
At the current day, renal cell carcinoma (RCC) represents the 14th most common cause of cancer in the general population, with an estimated worldwide incidence of 15 cases per 100.000 population [Citation1]. Even though most RCCs are localized at the time of diagnosis, about 30% of patients are diagnosed at an advanced stage of disease. The 5-year survival rates of patients with metastatic or not metastatic kidney cancer are 10% and 85%, respectively [Citation2].
In the last few years, the therapeutic landscape of metastatic RCC (mRCC) has been revolutionized by the advent of first-line immune-based combinations, using an immune checkpoint inhibitor (ICI) coadministered with another ICI (such as nivolumab plus ipilimumab) or a vascular endothelial growth factor (VEGF) receptor inhibitor (TKI) (including pembrolizumab plus axitinib or lenvatinib, nivolumab plus cabozantinib, avelumab plus axitinib) [Citation3]. On the other hand, TKI monotherapy still represents a valuable first-line therapy for those mRCC patients in whom ICI-based combinations could be not indicated (i.e. patients with a medical history of autoimmune disease) or also patients categorized as ‘favorable-risk’ (according to the International Metastatic RCC Database Consortium – IMDC – criteria) [Citation4–6]. However, all the immune-based combinations led to significant survival benefits when compared to sunitinib, becoming the novel standards of care in mRCC. Nonetheless, a head-to-head comparison between these first line strategies is missing, and validated biomarkers, which may help physicians in selecting those patients who might benefit the most from one combination rather than others, are lacking and urgently needed in this setting [Citation7]. Growing evidence is progressively suggesting a potential role of several markers as predictors of response to available ICI-based treatments or TKIs in RCC (including genomic, epigenetic, radiomic features, and so on) [Citation7–10], but their routinary use in the clinical practice is still so far to be carried out.
Although the IMDC risk criteria still play a decisive role, the therapeutic decision-making process could also rely on themes that emerged across various studies. For example, a rapid and meaningful disease control obtained with an ICI/TKI combination is required in patients with a high-burden disease. Pembrolizumab plus axitinib or lenvatinib and nivolumab plus cabozantinib showed higher objective response (ORR) and disease control rates (DCR) than the nivolumab/ipilimumab combination, with the highest complete response rates ever seen so far in mRCC first line trials [Citation3,Citation4]. Otherwise, for asymptomatic patients in which a fast tumor shrinkage is not needed, the ICI/ICI combination may be favored, due to its higher durability of response as well as the longer treatment-free interval [Citation3,Citation4]. In 2022, Navani et al. unveiled that ICI/TKI regimens appeared to be independently associated with an increased likelihood of obtaining response when compared with ICI-doublet therapy [Citation11]. Of note, VEGF inhibitors seemed to stabilize disease in a clinically relevant manner, producing benefit even without a documented imaging response [Citation11]. Further efforts are necessary to better clarify how to translate the different types of response with available first line therapies for mRCC to improve and personalize treatment-selection.
Another meaningful challenge is to define the most adequate way to assess and define response to ICIs. Currently, the updated version of Response Evaluation Criteria in Solid Tumors (RECIST) represents the standardized method to define objective response to classical antitumoral agents, in clinical trials [Citation12]. The aim of RECIST criteria is to evaluate the change in tumor burden during an oncological treatment in terms of objective response for specific target malignant lesions, considering the minimum size of measurable lesions, sums of target lesions’ diameters, number of lesions (up to 10, with a maximum of five lesions per organ site), along with the appearance of new disease localizations [Citation12]. Although a significant association between the achievements of objective imaging response (i.e. complete or partial response) and long-term survival has been reported among mRCC patients receiving an immunotherapeutic agent [Citation13], ICIs were shown to lead to unconventional radiological patterns of response compared to chemotherapies or other anticancer treatments, due to their specific mechanism of action. Among these unusual patterns, durable or dissociated responses, along with the so-called pseudoprogression (PSPD) and hyperprogression (HPD), have been described so far [Citation14]. The PSPD is reported in about 7–8% of mRCC patients treated with ICI-based therapies, reflecting an increase in the size of lesions, or the occurrence of new lesions, followed by a radiological response. This pattern is typically shown within the first two cycles of immunotherapy [Citation15]. On the other hand, the less common HPD represents the unexpected acceleration of tumor growth after ICIs’ initiation [Citation16]. Furthermore, stable disease (SD) according to RECIST criteria was described as the best response in about 30% of oncological patients treated with ICIs, but this definition was shown to be quite uninformative, involving a wide range of outcomes from no benefit to long-term benefit across and within tumor types [Citation17].
RECIST criteria may therefore not be appropriately adequate to assess the response to immunotherapy in cancer patients, thus promoting the development of novel radiological criteria of response (including immune-RECIST (iRECIST) and immune-related response criteria (irRC)) [Citation18,Citation19]. In this regard, the so-called tumor growth rate (TGR) represents a useful tool which could help physicians in better defining responses to immune-based combinations. TGR is described as the ratio between the slope of tumor growth before the treatment start and the slope of tumor growth during treatments, and the ratio between the nadir and disease progression [Citation20]. Consequently, TGR provides a dynamic and quantitative evaluation of tumor kinetics, measuring tumor volume changes between imaging assessments, per unit time [Citation21].
So far, several studies have suggested that TGR was related to tumor response or survival outcomes for many malignancies treated with different agents [Citation22–25]. Focusing on RCC, TGR was already shown to have a prognostic value in patients treated with first line TKIs (sunitinib or sorafenib), allowing an early and specific identification of signs of the antitumor activity of these drugs [Citation26–28]. More recently, a retrospective study showed that TGR-derived parameters could promote a better evaluation of mRCC response to immunotherapy in addition to RECIST criteria, also pointing out a potential association of these parameters with clinical outcomes. As a matter of fact, the deceleration in TGR, which was shown in mRCC patients with progressive disease during second- or later-line nivolumab, led to improved survival outcomes similar to that highlighted in those patients experiencing a tumor regression [Citation29]. Notably, carrying the treatment beyond progression may be an option in these selected patients, especially in those who witnessed a clinical benefit [Citation29].
In this study, we evaluate TGR in mRCC patients treated with immune-based combinations (in particular pembrolizumab plus axitinib) and in mRCC patients treated with TKIs, focusing on how TGR could change during these different therapeutic strategies. Furthermore, we assess the potential association of different TGR patterns with survival outcomes, thus clarifying their potential prognostic and predictive role in the first line treatment selection.
2. Patients and methods
2.1. Study population
Data were retrospectively collected from all the consecutive eligible patients with mRCC which have been treated with an ICI/TKI combination or a single-agent TKI as a first line therapy at Sant’Orsola-Malpighi Hospital from 1 January 2015 to 31 December 2022. To be eligible, patients had to be characterized by the following criteria of inclusion:
Confirmed histological diagnosis of RCC (both clear cell and non-clear cell histologies).
Stage IV disease.
Eighteen years or older.
Available computed tomography (CT) scans for radiological evaluation (within 30 days from treatment start, after 3 months and after 6 months).
Treatment-naive patients, who could not have received a previous line of systemic therapy.
Other meaningful data were histological subtype, gender, presence of liver metastases, presence of bone metastases, presence of central nervous system (CNS) metastases, presence of pancreatic metastases, IMDC risk group at the diagnosis.
Patients without available CT scans at each time point were excluded from the study. The end of the follow-up period was 15 March 2023.
This study was approved by local IRB (Comitato Etico Indipendente, IRCCS Azienda Ospedaliero-Universitaria di Bologna, protocol code: PRIORI, 321/2019/Sper/AOUBo) and was conducted in accordance with the principles of the Declaration of Helsinki (6th revision, 2008). Informed consent was obtained from all individual participants included in the study.
2.2. Tumor volume and tumor growth rate (TGR) calculation
Target lesions were identified on CT scans using RECIST 1.1 criteria. Consequently, tumor volumes were calculated by using the Philips IntelliSpace Portal v. 8.0 software (Philips Medical System, Eindhoven, The Netherlands). For each patient, CT assessment was performed at the baseline, 3 months after first line treatment started and then 6 months after treatment started, respectively. On the basis of the malignant cells’ tumor growth pattern, the disease volume after the start of first line therapy at time t (expressed in days at cancer assessment) has been defined using the formula V(t) = V0e(TG1t), in which V0 represents the volume at the baseline, V(t) is the volume at time t, and TG1 is the tumor growth after the start of immune-based combination or TKI. An exponential approximation of the tumor growth at the ICI/TKI or TKI therapy start (Vi) has been calculated. Consequently, we calculated the increase of tumor volume per day at the first (TGR1) and at the second (TGR2) CT evaluation with the following formulas: TGR1 = 100(eTG1–1), TGR2 = 100(eTG2–1). The difference in TGR between the two CT assessments from treatment’s start was also determined (TGR2-TGR1).
2.3. Statistical analysis
Data were presented as means, standard deviations, and frequencies. Volume data were log-normalized with the natural logarithmic function. Survival data were computed with the Kaplan–Meier estimator and presented as medians and standard errors. The Fisher’s exact test, the Pearson’s chi-squared test, the Mann–Whitney U test, the Log-rank test were used. The median follow-up was obtained by the reverse Kaplan–Meier method. The association between TGR and survival data was evaluated with univariate Cox proportional hazards regression model. All the tests were 2-tailed and a p-value of <0.05 was considered statistically significant. The statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) program version 28.0 (IBM, Armonk, NY, U.S.A.).
3. Results
Thirty-three consecutive patients were included in our study (). Of these, 15 patients were treated with first line pembrolizumab plus axitinib (P/A) combination, while 18, representing the control group, were treated with a single-agent TKI (2 patients with cabozantinib and 16 with sunitinib). We found no differences in baseline characteristics among the two groups (). Tumor histology was predominantly clear cell (ccRCC; 90.9%) vs non-clear cell (nccRCC; 9.1%). Sarcomatoid or rhabdoid dedifferentiated areas were discovered in 30.3% of patients in the overall population (26.7% of patients treated with immune-based combination, 33.3% of patients treated with TKIs).
Figure 1. Patients included in our study. Abbreviations: mRCC, metastatic renal cell carcinoma; P/A, pembrolizumab/axitinib; TKI, tyrosine kinase inhibitor; CT, computed tomography.
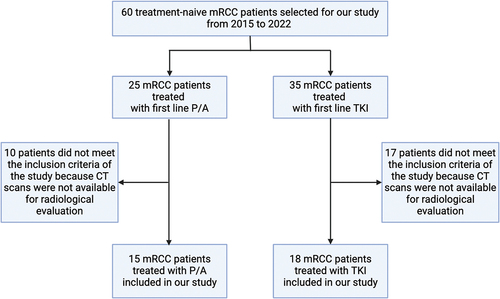
Table 1. Baseline patients’ features in both experimental (immune-based combination) and control (TKI) groups as well as in overall population, along with p-value for association between categorical or continuous variables and the two groups.
As for IMDC risk classes, 54.5% of patients were intermediate-risk at the diagnosis, while 39.4% of patients were favorable-risk and 6.1% poor-risk. Furthermore, elected patients were mostly male (69.7%) vs female (32.3%). Lung was the most common site of metastases in mRCC patients included in both groups (53.3% of patients treated with P/A, 55.6% of patients treated with TKIs).
As for objective responses, a partial response (PR) was described as the best response in 55.6% of patients treated with TKIs, whereas stable disease (SD) and progressive disease (PD) were reported in 33.3% and 11.1%, respectively. Regarding the P/A group, 53.3% of patients witnessed PR as the best response, 26.7% of patients achieved a SD and 20% a PD.
At baseline, no difference in terms of total tumor volume was reported between patients in both P/A and TKI groups (). Post-baseline tumor volumes in patients treated with the ICI/TKI combination were significantly higher than post-baseline volumes detected in patients from the TKI group, at the first (p = 0.047) as well as at the second (p = 0.046) radiological evaluation ().
Consequently, TGR was calculated for each post-baseline CT assessment (TGR1 and TGR2 respectively). On the basis of TGR changes during first line therapies, three different patterns of TGR were identified:
patients presenting a disease progression with no deceleration of cancer growth (13.3% of patients in P/A group; 11.1% of patients in TKI group).
patients with progressive disease but characterized by a deceleration of TGR (13.3% for P/A group; 11.1% for TKI group).
patients responding or witnessing a SD to P/A (46.7%) or single-agent TKI (66.7%).
A meaningful difference between patients treated with immune-based combination and patients treated with TKI was shown calculating TGR2-TGR1 (). As a matter of fact, TGR increased by 0.012 in the TKI group, while decreased by 0.005 in the P/A group (p = 0.026). In more detail, tumor volumes decreased faster at the first CT evaluation (TGR1–0.010), and then more slowly at the second evaluation (TGR2–0.001) in the control group. Conversely, in patients treated with P/A combination, tumor volumes remained almost stable at the first CT evaluation (TGR1–0.001), then decreased quickly at the second assessment (TGR2–0.008).
We also evaluated the association between survival outcomes and TGR () and we found a significant correlation between TGR1 and PFS (p = 0.012), between TGR2 and PFS (p = 0.025) and between TGR1 and OS (p = 0.002). The association between TGR1 and both PFS and OS was also confirmed in the ICI/TKI group (p = 0.023 and p = 0.046, respectively; ). The median follow-up was 35.4 months. In order to graphically represent the results of the univariate survival analysis of Cox, both patients treated with P/A combination or TKI were further divided in two groups according to the median cutoff of TGR = −0.0038, showing a different trend of tumor volume changing:
Figure 2. PFS in patients in the control group, treated with a single-agent TKI. Patients were further divided in two groups: group 1 with high velocity volume reduction (HvVR) and group 2 with lower velocity volume reduction or increase (LvVR).
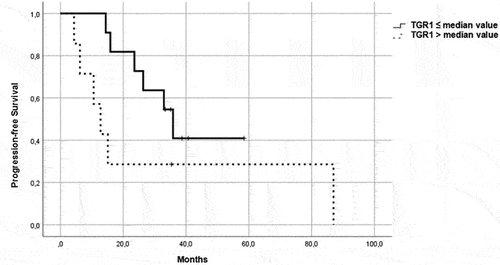
Figure 3. PFS in patients in the immunotherapy group, treated with pembrolizumab plus axitinib. Patients were further divided in two groups: group 1 with high velocity volume reduction (HvVR) and group 2 with lower velocity volume reduction or increase (LvVR).
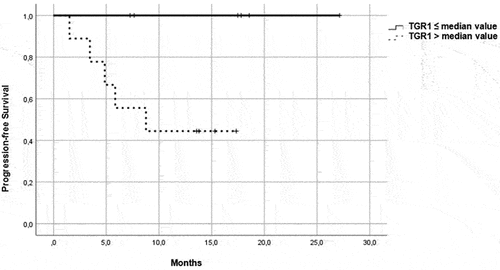
Figure 4. OS in patients in the control group, treated with a single-agent TKI. Patients were further divided in two groups: group 1 with high velocity volume reduction (HvVR) and group 2 with lower velocity volume reduction or increase (LvVR).
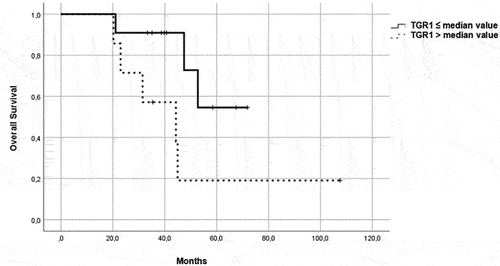
Figure 5. OS in patients in the immunotherapy group, treated with pembrolizumab plus axitinib. Patients were further divided in two groups: group 1 with high velocity volume reduction (HvVR) and group 2 with lower velocity volume reduction or increase (LvVR).
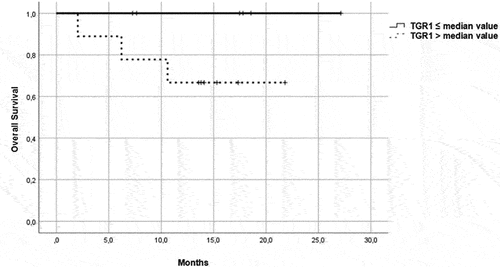
Group 1 characterized by a TGR ≤median (patients who witnessed a faster decrease in tumor volume), called ‘high velocity volume reduction’ (HvVR);
group 2 characterized by a TGR > median (patients who observed a slower volume decrease or a slower volume increase), called ‘lower velocity volume reduction’ (LvVR).
Table 2. Association between survival outcomes and TGR at each CT assessment from first-line therapy’s start in patients treated with immune-based combination, single-agent TKI and in the overall population.
Focusing on patients treated with ICI/TKI combination, Kaplan–Meier curves showed that HvVR patients were significantly characterized by better PFS () and OS () than LvVR patients. A benefit in terms of PFS () and OS () was reported also in HvVR patients treated with a single-agent TKI, although less evident than in patients treated with P/A.
4. Discussion
We examined the role of TGR in the context of the first line of treatment for mRCC to investigate the correlation with clinical outcomes. The results of our study revealed that immune-based combinations lead to a decreased TGR compared to TKI monotherapy. Immunotherapy is considered to help to gain disease control long term, acting especially in terms of overall survival and maintenance of a prolonged response, even though it seems to be less beneficial in terms of PFS. Nonetheless, our study showed that the activity of TKI in terms of decreasing the rate of tumor growth is enhanced by the combination with ICIs. Considering the plethora of available first line treatments for mRCC and given the absence of predictive factors of response to guide the therapeutic decision [Citation7], one of the main factors to consider in the clinical scenario is the burden of disease [Citation3,Citation4]. TKIs are a treatment cornerstone in patients in need for a shrinkage of disease with a high burden of metastatic lesions considering the elevated ORRs observed with these therapies. In contrast, ICIs are associated with lower rate of response and ability to contain a high burden of disease. Nonetheless, our study showed that ICIs seem to have a relevant role also in decreasing the growth rate of the tumor lesions. Another meaningful aspect to take into account is also represented by the different safety profiles of these drugs, which are tightly related to their specific mechanism of action, and which may help physicians in the treatment decision-making process [Citation30–32].
Our study presents several limitations, including its retrospective design as well as the small sample size. Moreover, patients’ baseline characteristics (as outlined above in ) should be considered in interpreting the results of our analysis. As a consequence, more robust data obtained with prospective designed studies are surely necessary to confirm our analysis. Lastly, we did not perform a centralized review of radiological imaging, but the use of the exponential approximation of the tumor growth (Vi) is one strength of our work because it allows to calculate the increase of tumor lesions between radiological assessment and the ICI/TKI or TKI therapy start.
The role of TGR has been investigated in different types of cancers, including RCC in different settings and lung cancer [Citation23,Citation25–29]. TGR may have an additional role to RECIST criteria, commonly used to assess treatment response [Citation29–32]. Nonetheless, RECIST criteria present several limitations, considering that they use a unidimensional method of assessing lesions’ changes, not taking into consideration the kinetics of tumor response to treatments. In terms of response to ICIs, several new patterns of response have been recognized, including hyperprogression and pseudoprogression, that are a result of the mechanism of action of immunotherapy that interacts with tumor lesions through the enhanced response of the immune system [Citation14]. These types of responses may be not captured by the assessment through RECIST criteria, while they may be integrated when TGR is evaluated, thus reflecting the real response to an ICI-based treatment.
5. Conclusions
The velocity of tumor volume reduction, regardless of RECIST criteria assessment, results to be correlated with survival outcomes of patients treated with pembrolizumab plus axitinib. The use of immunotherapy as a first line strategy in combination with a TKI appears to have an important role also in enhancing the rapidity of tumor shrinkage. Moreover, a rapid tumor volume reduction correlates with better OS and PFS. Further and larger prospective studies are warranted to validate these retrospective findings.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Ethical approval
This study was approved by local IRB (Comitato Etico Indipendente, IRCCS Azienda Ospedaliero-Universitaria di Bologna, protocol code: PRIORI, 321/2019/Sper/AOUBo) and was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Author contributions
Francesco Massari and Veronica Mollica contributed to the study conception and design. Material preparation, data collection and analysis were performed by Veronica Mollica, Matteo Rosellini, Stefano Brocchi and Andrea Marchetti. The first draft of the manuscript was written by Veronica Mollica, Matteo Rosellini, Francesco Massari and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Declaration of interest
F Massari has received research support and/or honoraria from Astellas, BMS, Janssen, Ipsen, MSD and Pfizer outside the submitted work; M Santoni has received research support and honoraria from Janssen, Bristol Myers Squibb, Ipsen, MSD, Astellas and Bayer, all unrelated to the present paper. The work reported in this publication was supported by the Italian Ministry of Health, RC-2022- 2773458. The other Authors have no relevant financial or non-financial interests to disclose.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763
- Graves A, Hessamodini H, Wong G, et al. Metastatic renal cell carcinoma: update on epidemiology, genetics, and therapeutic modalities. Immunotargets Ther. 2013. 22(2):73–90. doi: 10.2147/ITT.S31426
- Navani V, Heng DYC. Treatment selection in first-line metastatic renal cell carcinoma—the contemporary treatment paradigm in the age of combination therapy: a review. JAMA Oncol. 2022;8(2):292–299.
- Rosellini M, Marchetti A, Tassinari E, et al. Guiding treatment selection with immunotherapy compared to targeted therapy agents in patients with metastatic kidney cancer. Expert Rev Precis Med Drug Dev. 2022;7(1):131–149. doi: 10.1080/23808993.2022.2156786
- Bolek H, Yekedüz E, Ürün Y. Combination therapies in patients with favorable risk metastatic renal cell carcinoma: a systematic review and meta-analysis. Cancer Treatment Reviews. 2024;122:102667. doi: 10.1016/j.ctrv.2023.102667
- Brown JE, Royle KL, Gregory W, et al. Temporary treatment cessation versus continuation of first-line tyrosine kinase inhibitor in patients with advanced clear cell renal cell carcinoma (STAR): an open-label, non-inferiority, randomised, controlled, phase 2/3 trial. Lancet Oncol. 2023;24(3):213–227. doi: 10.1016/S1470-2045(22)00793-8
- Rosellini M, Marchetti A, Mollica V, et al. Prognostic and predictive biomarkers for immunotherapy in advanced renal cell carcinoma. Nat Rev Urol. 2023;20(3):133–157. doi: 10.1038/s41585-022-00676-0
- Boussios S, Devo P, Goodall ICA, et al. Exosomes in the diagnosis and treatment of renal cell cancer. Int J Mol Sci. 2023;24(18):14356.
- Topalian SL, Hodi FS, Brahmer JR, et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non–small cell lung cancer treated with nivolumab. JAMA Oncol. 5(10):1411–1420. 2019 doi: 10.1001/jamaoncol.2019.2187
- Dromain C, Beigelman C, Pozzessere C, et al. Imaging of tumour response to immunotherapy. Eur Radiol Exp. 2020;4(1):2. doi: 10.1186/s41747-019-0134-1
- Queirolo P, Spagnolo F. Atypical responses in patients with advanced melanoma, lung cancer, renal-cell carcinoma and other solid tumors treated with anti-PD-1 drugs: a systematic review. Cancer Treat Rev. 2017;59:71–78. doi: 10.1016/j.ctrv.2017.07.002
- Hwang I, Park I, Yoon SK, et al. Hyperprogressive disease in patients with urothelial carcinoma or renal cell carcinoma treated with PD-1/PD-L1 inhibitors. Clin Genitourin Cancer. 2020;18(2):e122–e133. doi: 10.1016/j.clgc.2019.09.009
- Luo J, Wu S, Rizvi H, et al. Deciphering radiological stable disease to immune checkpoint inhibitors. Ann Oncol. 2022;33(8):824–835. doi: 10.1016/j.annonc.2022.04.450
- Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. doi: 10.1016/S1470-2045(17)30074-8
- Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624
- Grande E, Martínez-Sáez O, Gajate-Borau P, et al. Translating new data to the daily practice in second line treatment of renal cell carcinoma: the role of tumor growth rate. World J Clin Oncol. 2017. 8(2):100–105. doi: 10.5306/wjco.v8.i2.100
- Gomez-Roca C, Koscielny S, Ribrag V, et al. Tumour growth rates and RECIST criteria in early drug development. Eur J Cancer. 2011;47(17):2512–2516. doi: 10.1016/j.ejca.2011.06.012
- Lamarca A, Ronot M, Moalla S, et al. Tumor growth rate as a validated early radiological biomarker able to reflect treatment-induced changes in neuroendocrine tumors: the GREPONET-2 study. Clin Cancer Res. 2019;25(22):6692–6699. doi: 10.1158/1078-0432.CCR-19-0963
- He LN, Fu S, Ma H, et al. Early on-treatment tumor growth rate (EOT-TGR) determines treatment outcomes of advanced non-small-cell lung cancer patients treated with programmed cell death protein 1 axis inhibitor. ESMO Open. 2022;7(6):100630. doi: 10.1016/j.esmoop.2022.100630
- Ramtohul T, Cohen A, Rodrigues M, et al. Tumour growth rate improves tumour assessment and first-line systemic treatment decision-making for immunotherapy in patients with liver metastatic uveal melanoma. Br J Cancer. 2022;127(2):258–267. doi: 10.1038/s41416-022-01793-8
- Dall’olio FG, Parisi C, Marcolin L, et al. Monitoring tumor growth rate to predict immune checkpoint inhibitors’ treatment outcome in advanced NSCLC. Ther Adv Med Oncol. 2022;14:17588359211058391. doi: 10.1177/17588359211058391
- Ferté C, Koscielny S, Albiges L, et al. Tumor growth rate provides useful information to evaluate sorafenib and everolimus treatment in metastatic renal cell carcinoma patients: an integrated analysis of the TARGET and RECORD phase 3 trial data. Eur Urol. 2014;65(4):713–720. doi: 10.1016/j.eururo.2013.08.010
- Stein WD, Wilkerson J, Kim ST, et al. Analyzing the pivotal trial that compared sunitinib and IFN-α in renal cell carcinoma, using a method that assesses tumor regression and growth. Clin Cancer Res. 2012;18(8):2374–2381. doi: 10.1158/1078-0432.CCR-11-2275
- Iacovelli R, Massari F, Albiges L, et al. Evidence and clinical relevance of Tumor flare in patients who discontinue tyrosine kinase inhibitors for treatment of metastatic renal cell carcinoma. Eur Urol. 2015;68(1):154–160. doi: 10.1016/j.eururo.2014.10.034
- Mollica V, Brocchi S, Dall’olio FG, et al. Tumor growth rate decline despite progressive disease may predict improved nivolumab treatment outcome in mRCC: when RECIST is not enough. Cancers (Basel). 2021;13(14):3492. doi: 10.3390/cancers13143492
- Quhal F, Mori K, Remzi M, et al. Adverse events of systemic immune-based combination therapies in the first-line treatment of patients with metastatic renal cell carcinoma: systematic review and network meta-analysis. Curr Opin Urol. 2021;31(4):332–339. doi:10.1097/MOU.0000000000000889
- Ivanyi P, Wiegmann JP, Eggers H, et al. First line medical treatment of metastatic renal cell carcinoma: a podcast on choosing wisely and managing safely. Adv Ther. 2023;40(9):3620–3625. doi: 10.1007/s12325-023-02571-5
- Savaliya M, Surati D, Surati R, et al. Posterior reversible encephalopathy syndrome after pazopanib therapy. Diseases. 2023;11(2):76. doi: 10.3390/diseases11020076
- Rosellini M, Mollica V, Marchetti A, et al. Chromosome 3p gene alterations as biomarkers for immunocombinations in metastatic renal cell carcinoma: A hypothesis-generating analysis. Pathol Res Pract. 2024;254:155142. doi: 10.1016/j.prp.2024.155142
- Saliby RM, Saad E, Kashima S, Schoenfeld DABraun DA Update on Biomarkers in Renal Cell Carcinoma. Am Soc Clin Oncol Educ Book. 2024;44(2):e430734. doi: 10.1200/EDBK_430734
- Navani V, Ernst M, Wells JC, et al. Imaging Response to Contemporary Immuno-oncology Combination Therapies in Patients With Metastatic Renal Cell Carcinoma. JAMA Netw Open. 2022;5(6):e2216379.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. doi: 10.3390/ijms241814356

