Abstract
Marchiafava-Bignami disease (MBD) is an uncommon neurological disorder characterized by corpus callosum demyelination and necrosis, primarily associated with alcohol use disorder and vitamin deficiencies, notably thiamine. We present the case of a 27-year-old female who was diagnosed by MRI after developing stroke-like symptoms, and was found to have undetectable serum thiamine concentrations, likely due to alcohol use disorder and noncompliance with prescribed vitamin supplements after Roux-en-Y gastric bypass surgery. She was prescribed thiamine supplementation, and upon follow-up one month later, she had no residual neurological deficits. MBD is a rare cause of stroke-like symptoms, and thorough medical and surgical history is needed in patients who may not be in the typical age range for development of the disease.
Introduction
Marchiafava-Bignami disease (MBD) is a rare neurological disorder characterized by demyelination and necrosis of the corpus callosum. The etiology is unclear but is attributed to direct alcohol-induced toxicity as well as deficiencies in B-complex vitamins [Citation1], including thiamine. The clinical presentation of MBD is variable and nonspecific, and can include dementia, altered mental status, spasticity, dysarthria, ataxia, gait abnormalities, and seizures [Citation1]. We describe an unusually young patient [Citation2] who developed MBD due to a combination of alcohol use disorder (AUD) and noncompliance with prescribed vitamin supplements after Roux-en-Y gastric bypass surgery [Citation3], likely causing an undetectable serum thiamine concentration.
Case presentation
A 27-year-old female with a history of AUD and Roux-en-Y in 2018 presented to the emergency department (ED) complaining of stroke-like symptoms. She had a 13-h episode of chest pain, right facial droop, right arm weakness, and difficulty speaking that lasted until just prior to ED evaluation. Computed tomography angiography (CTA) of her head and neck were normal. The patient was admitted to the hospital and was empirically started on vitamin supplementation for her AUD, including thiamine 100 mg IV daily, on the first day of hospitalization. The primary team consulted neurology, who recommended further stroke workup including magnetic resonance imaging (MRI). The patient’s MRI showed abnormal T2 hyperintensity and diffusion restriction involving the splenium of the corpus callosum and bilateral frontoparietal lobes, consistent with toxic leukoencephalopathy ( and ). The patient had a similar episode of chest pain, right-sided weakness, and facial droop lasting 8 h following her MRI on hospital day two. Medical toxicology was consulted for her abnormal MRI and findings of toxic leukoencephalopathy. During our interview with the patient, she reported daily alcohol use as well as noncompliance with her vitamin supplementation that was recommended after her Roux-en-Y surgery. She did not report exposures to other causes of toxic leukoencephalopathy, including recreational drug use (heroin inhalation, toluene use), pesticide exposure (including sodium monofluoroacetate), and exposure to various industrial and environmental hazards (carbon dioxide, paradichlorobenzene). While she did drink homemade “hootch” over a month month earlier, her symptoms had no temporal relationship to this. We recommended serum thiamine, folate, and B12 testing due to suspicion of MBD. The patient had normal serum folate and B12 concentrations; however, her serum thiamine concentration was undetectable (). She had no other stroke-like symptoms during her three day hospitalization, and she was discharged to a rehabilitation facility with prescriptions for thiamine 100 mg daily, repeat MRI, and neurology follow-up. Upon follow-up the subsequent month, she had no residual deficits. She did not complete her 6-month follow-up for repeat MRI.
Figure 1. T2W FLAIR, DWI, and ADC sequences, respectively. Abnormal T2 hyperintensity and diffusion restriction involving the bilateral cerebral white matter, including the corona radiata and the centrum semiovale of the frontoparietal lobes.
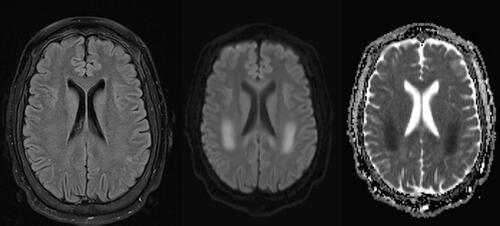
Figure 2. T2W FLAIR, DWI, and ADC sequences, respectively. Abnormal T2 hyperintensity and diffusion restriction involving the splenium of the corpus callosum.
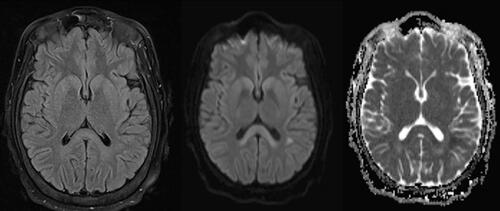
Table 1. Serum thiamine, folate, and B12 concentrations.
Discussion
MBD is most reported in patients with AUD that puts them at risk for vitamin deficiencies, with a mean age of onset in the late 40s [Citation4, Citation5]. There are only a few hundred published cases of MBD [Citation1, Citation6] and only seven cases reported to the literature of patients in their 20s with suspected MBD based on radiographic findings [Citation2, Citation7–12]. Three of these patients had risk factors other than alcohol use [Citation7–9]. To our knowledge, this patient is the youngest patient reported in the literature to have MBD with a confirmed undetectable serum thiamine concentration.
Strengths to our approach to the case included a thorough history, as she did not offer that she had a Roux-en-Y surgery in the past, or that she was noncompliant with her vitamin supplementation, which was crucial to the diagnosis of MBD in this case. Early diagnosis and available send-out testing allowed for prompt treatment and resolution of recurrent episodes. Given the rarity of the disease, standardized treatment have not been established. Our final conclusions are limited given the lack of repeat MRI at the 6-month follow-up.
The exact pathophysiology of MBD is unknown and is likely to be multifactorial. Ethanol can cause direct central nervous system (CNS) toxicity through several pathways, including NADPH oxidase mediated free radical production [Citation13]. In addition, acetaldehyde production from ethanol metabolism induces the xanthine oxidase pathway, also contributing to formation of reactive oxygen species (ROS) [Citation14]. Induction of brain catalase and CYP2E1 through chronic consumption of alcohol may further increase the production of acetaldehyde locally in the CNS, leading to more ROS generation and direct cellular damage [Citation15].
Thiamine deficiency may contribute to the demyelination seen in MBD through several mechanisms. First, thiamine deficiency interrupts pyruvate dehydrogenase (PDH) activity, which leads to decreased ATP production and downstream failure in myelin synthesis [Citation15]. Transketolase is also a thiamine-dependent enzyme that can disrupt myelin synthesis via decreased NADPH synthesis [Citation16]. Dysfunction in PDH and transketolase also contribute to decreased protection against ROS which may contribute to the pathophysiology of the disease.
Diagnosis is difficult, particularly in younger patients, given the lack of standardized management guidelines. Most case reports show favorable response to treatment modalities used in Wernicke-Korsakoff syndrome, like high-dose thiamine and alcohol cessation [Citation4].
Conclusion
Though typically a disease in older patients, MBD can develop in young patients with significant risk factors Our patient developed MBD at an unusually young age likely due to continued alcohol use disorder and nonadherence with prescribed vitamin supplements after Roux-en-Y gastric bypass surgery, resulting in severe thiamine depletion.
Disclosure statement
The authors have no conflicting interests to disclose.
Data availability statement
No additional data for this case report are available for sharing in order to protect patient privacy.
Additional information
Funding
References
- Tian TY, Pescador Ruschel MA, Park S, et al. Marchiafava-Bignami disease. In: StatPearls [internet]. Treasure Island (FL): StatPearls Publishing; 2023. [Accessed November 29, 2023].
- Tosunoğlu B, Çokal BG, Güneş HN, et al. Marchiafava-Bignami disease caused by chronic alcohol use in a young patient: case report. Asian J Med Case Rep. 2022;4(1):1–4.
- Bahardoust M, Eghbali F, Shahmiri SS, et al. B1 vitamin deficiency after bariatric surgery, prevalence, and symptoms: a systematic review and meta-analysis. Obes Surg. 2022;32(9):3104–3112. doi:10.1007/s11695-022-06178-7.
- Hillbom M, Saloheimo P, Fujioka S, et al. Diagnosis and management of Marchiafava-Bignami disease: a review of CT/MRI confirmed cases. J Neurol Neurosurg Psychiatry. 2014;85(2):168–173. doi:10.1136/jnnp-2013-305979.
- Garcia-Santibanez R. Marchiafava-Bignami disease presenting as acute dysarthria and ataxia. Alcohol Alcohol. 2015;50(2):256–257. doi:10.1093/alcalc/agu093.
- Helenius J, Tatlisumak T, Soinne L, et al. Marchiafava-Bignami disease: two cases with favourable outcome. Eur J Neurol. 2001;8(3):269–272. doi:10.1046/j.1468-1331.2001.00212.x.
- Rusche-Skolarus LE, Lucey BP, Vo KD, et al. Transient encephalopathy in a postoperative non-alcoholic female with Marchiafava-Bignami disease. Clin Neurol Neurosurg. 2007;109(8):713–715. doi:10.1016/j.clineuro.2007.05.005.
- Sera S, Ichiba T. Marchiafava-Bignami disease in a patient with no alcohol abuse. Neurol India. 2019;67(4):1169. doi:10.4103/0028-3886.266247.
- Maki N, Hokoishi K, Komori K, et al. [A case of Marchiafava-Bignami disease caused by anorexia nervosa]. No to Shinkei. 2001;53(7):669–671. [Article in Japanese].
- Lucato LT, Freua F, Kok F. Chronic stage of Marchiafava-Bignami disease. Arq Neuropsiquiatr. 2015;73(10):890–890. doi:10.1590/0004-282X20150103.
- Machado A, Soares-Fernandes J, Ribeiro M, et al. Alcohol abuse and acute behavioural disturbances in a 24-year-old patient. Diagnosis: marchiafava-Bignami disease (MBD). J Clin Neurosci. 2009;16(6):811, 859. doi:10.1016/j.jocn.2008.08.040.
- Logak M, Fève A, Samson Y, et al. [Contribution of position emission tomography in a case of marchiafava bignami disease: morel’s laminar sclerosis?]. Rev Neurol (Paris). 1996;152(1):47–50. [Article in French].
- Haorah J, Ramirez SH, Floreani N, et al. Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic Biol Med. 2008;45(11):1542–1550. doi:10.1016/j.freeradbiomed.2008.08.030.
- Oei HH, Zoganas HC, McCord JM, et al. Role of acetaldehyde and xanthine oxidase in ethanol-induced oxidative stress. Res Commun Chem Pathol Pharmacol. 1986;51(2):195–203.
- Fernandes LMP, Bezerra FR, Monteiro MC, et al. Thiamine deficiency, oxidative metabolic pathways and ethanol-induced neurotoxicity: how poor nutrition contributes to the alcoholic syndrome, as Marchiafava-Bignami disease. Eur J Clin Nutr. 2017;71(5):580–586. doi:10.1038/ejcn.2016.267.
- Singleton CK, Martin PR. Molecular mechanisms of thiamine utilization. Curr Mol Med. 2001;1(2):197–207. doi:10.2174/1566524013363870.

