ABSTRACT
2,5-diformylfuran (DFF) is a promising green platform chemical obtainable by selective oxidation of 5-(hydroxymethyl)furfural (HMF). In this work, high-purity DFF was synthesized from HMF and compared to formaldehyde (F) in terms of suitability for polymerization in aqueous solution with various phenolic model substances that may be obtainable by depolymerization of lignin. Resorcinol, catechol, and phloroglucinol formed stable hydrogels with DFF, which were converted to alcogels and subsequently dried by extraction with supercritical carbon dioxide. Thermogravimetric analysis and scanning electron microscopy of the resulting aerogels showed no significant differences between DFF and F gels. The high porosity of the aerogels was confirmed by liquid nitrogen BET surface measurement; the specific surface areas measured varied significantly, ranging from 20 to 700 m2 g−1.
Graphical Abstract

Introduction
Due to the ongoing depletion of fossil fuel reserves and the need to reduce the emission of greenhouse gases, conversion of renewable and abundant biomass to fuels and value-added compounds has been of increasing interest. Lignocellulose is a composite consisting of lignin, hemicellulose, and cellulose that makes up more than 90% of all plant biomass. Consequently, much effort has been invested in developing processes for producing platform chemicals from this renewable feedstock. Application of these renewable precursors in various industrial fields, chiefly in polymer chemistry, has attracted considerable attention from the chemical community [Citation1–4].
One recent focus has been the valorization of lignin by depolymerization, where catalyzed cleavage of primarily C-O bonds occurs at temperatures between 150°C and 300°C. This process produces a bio-oil that contains various monomeric and oligomeric phenolic compounds which could be used – after separation – as fine-chemical feedstock from renewable resources [Citation5,Citation6]. The majority of monomers in this bio-oil have been found to be phenol, methylphenol, ethylphenol, catechol, cresols, resorcinol, guaiacols, phloroglucinol, and vanillin derivatives. [Citation6–8]
Aerogels are highly cross-linked three-dimensional organic networks that extend in an air matrix and thus have many interesting properties, such as a high specific surface area (200–1000 m2 g−1), low density (0.03–0.10 g cm−3), high pore volume (0.25–1.25 cm3 g−1) and low thermal conductivity[Citation9]. Kistler [Citation10] described the behavior of inorganic aerogels – derived from silica gels obtained by supercritical extraction_ – as early as in the 1930s. Almost 60 years later, Pekala et al. [Citation11] synthesized the first organic aerogels by condensation of resorcinol with formaldehyde in water in a sol-gel process, subsequent solvent exchange replacing water with acetone and then supercritical drying with carbon dioxide, and showed that organic and inorganic aerogels have similar properties. They can be used in a wide variety of applications, for instance, as catalyst support [Citation12], thermal and sonic insulators [Citation13], superabsorbents [Citation14] and supercapacitors[Citation15]. To date, organic aerogels based on resorcinol and formaldehyde in aqueous solution with various basic and acidic activators have been the primary target of investigations, with other combinations of phenolic or aldehydic molecules accounting for only a small proportion of studies [Citation16–18].
Many classes of synthetic organic aerogels, such as those based on polyimide, polyurea, or polyamide, are neither biodegradable nor based on renewable feedstock, which makes them unsuitable for environmentally friendly applications [Citation19,Citation20]. Therefore, the synthesis of biodegradable aerogels based on renewable feedstock has recently attracted growing attention. For instance, various degradation products of cellulose and hemicellulose produced by hydrothermal preparation, such as furfural and 5 hydroxymethylfurfural (HMF), have been investigated in combination with phenolic compounds for the synthesis of bio-based aerogels. Lee et al. [Citation21] prepared organic aerogels based on HMF and phloroglucinol in ethanol using concentrated nitric acid as a catalyst. However, nitric acid is neither an environmentally friendly acid nor safe, and its application should therefore be avoided if possible[Citation22].
2,5-diformylfuran (DFF) is a promising platform chemical obtained by oxidation of HMF, but – due to its more reactive aldehyde group – selective oxidation of the alcohol moiety of HMF can be challenging[Citation23]. Various heterogeneous catalyst systems with very high yields and selectivities [Citation23–26] have been investigated for aerobic oxidation of HMF, but no viable large-scale processes have been developed to date. DFF has been used as a monomer in numerous bio-based polymers, such as polymeric Schiff bases [Citation27,Citation28] and furan-urea resins [Citation29]. Unlike HMF, which slowly decomposes at room temperature and has a melting point of 32°C, DFF, with a melting point of 110°C [Citation30], is stable at room temperature and is, therefore, easier to handle in industrial applications[Citation31].
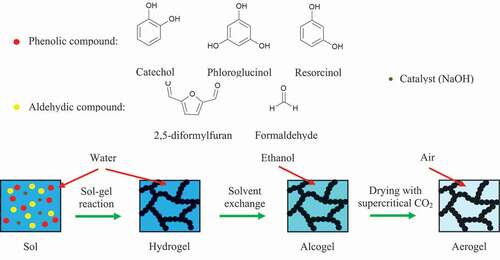
In this study, DFF was synthesized from HMF (see for an overview) and its purity determined by melting point analysis and [Citation1]H-NMR spectroscopy. In preliminary experiments, various phenolic model compounds that may be obtainable from bio-oil produced by depolymerization of lignin were used with DFF in a sol-gel reaction in order to prepare fully bio-based organic aerogels. Only catechol, resorcinol, and phloroglucinol formed stable gels with DFF, which may be attributable to the higher reactivity and smaller relative size of these phenolic compounds compared to that of other, more sterically hindered products of lignin depolymerization. To investigate the impact of using different aldehydes in the sol-gel reaction, aerogels were prepared not only with DFF but also with formaldehyde (F) and the phenolic model compounds. After solvent exchange and supercritical drying with carbon dioxide, morphology, specific surface area, and thermal decomposition of the aerogels were analyzed by scanning electrode microscopy (SEM), Brunauer-Emett-Teller analysis (BET), and thermogravimetric analysis (TGA), respectively.
Methods and materials
Materials
HMF was purchased from AVA Biochem. 2,5-diformylfuran (97%), resorcinol (99%) phloroglucinol (≥99%), catechol (≥99%), and deuterated trichloromethane (99,96%) were obtained from Sigma Aldrich. Ethyl acetate (≥99,8%), sodium nitrite (≥99%), n-heptane (≥99%), trichloromethane (≥99%), phosphoric acid (85%), and aqueous formaldehyde solution (37%) were purchased from Carl Roth. Ethanol (≥99,9%) was purchased from AustrAlco. All reagents were used as received without any further purification.
Method for synthesis and characterization of DFF
The method for synthesizing DFF from HMF was developed by adapting and scaling up the method described by Smirnova et al. [Citation30] where sodium nitrite in a solution of concentrated phosphoric acid is used for selective oxidation of HMF to DFF. This method of synthesizing DFF was chosen despite the large amount of inorganic waste it produces, because no environmentally friendly gram-scale methods have been developed to date, and the reagents were affordable and readily available. In a 1 L three-necked round-bottom flask equipped with a condenser, 10 g HMF (79.3 mmol) was dissolved in 125 mL concentrated phosphoric acid. Subsequently, 13.7 g sodium nitrite was added to the solution under vigorous stirring, and the solution was stirred at room temperature for one hour. After the specified time, the reaction solution was diluted with 400 mL water and extracted 5 times with 20 mL trichloromethane. The combined organic extracts were washed with water until the washings were neutral, dried with anhydrous sodium sulfate, and evaporated. The yellow residue was recrystallized from 1:1 (v:v) n-heptane:trichloromethane and dried over silica in vacuo for 24 h. Purity of DFF was confirmed by determining the melting point and by 1H-NMR measurement in deuterated trichloromethane using a 300 MHz Bruker NMR spectrometer.
Synthesis of aerogels
All hydrogels were prepared by the sol-gel condensation reaction between a phenolic compound (resorcinol, phloroglucinol, or catechol) and an aldehydic compound (F or DFF) with sodium hydroxide as a base catalyst. This procedure has previously been described for resorcinol-formaldehyde (RF) gels in numerous publications and can be applied to various precursor compounds [Citation11,Citation32,Citation33]. Both the aldehydic and phenolic compounds were dissolved at the specified molar ratio in deionized water, the pH was set to 10–11 with 20% sodium hydroxide solution, and the mixture was then diluted with deionized water to achieve a total solid content of 15 wt%. The reaction mixtures were sealed in glass vials with screw caps and cured in a heating cabinet in a 1-day/1-day/3-day cycle at room temperature, 323 K and 363 K, respectively. The molar ratios of phenolic to aldehydic compounds investigated were 1:1, 1:2, and 1:3 for F gels, and – due to solubility issues at higher DFF concentrations – only 1:1 for DFF-gels. After the specified time, if gelation had occurred, the pore liquid was replaced with ethanol. Subsequently, the alcogels were placed in a heated high-pressure stainless-steel extraction vessel, where carbon dioxide above the critical point (Tc = 304 K; Pc = 7.4 MPa) was passed through the samples for two hours to remove any solvent within the pores.
Aerogel characterization
The specific surface areas of the aerogels were determined by nitrogen gas adsorption at 77.35 K by means of an Autosorb-iQ automatic volumetric sorption analyzer from Anton Paar GmbH. Measurements were performed with approximately 50 mg sample material; to remove adsorbed water, the samples were degassed for 60 min under high vacuum at 383 K prior to measurement. The Brunauer-Emett-Teller (BET) method was used to calculate the specific surface area of the aerogels.
Thermogravimetric analysis of the aerogels was performed on a TGA 4000 thermogravimetric analyzer from PerkinElmer. Approximately 10 mg of sample material were heated from 40 to 950°C at a heating rate of 20°C min−1 under a nitrogen flow of 20 mL min−1.
The morphology of the aerogels was investigated with a Phenom Pro X scanning electron microscope (SEM). The aerogels were gold-sputtered prior to analysis to achieve the desired electrical conductivity, which was accomplished with an SC7620 Mini Sputter Coater from Quorum Technologies.
Results and discussion
DFF synthesis
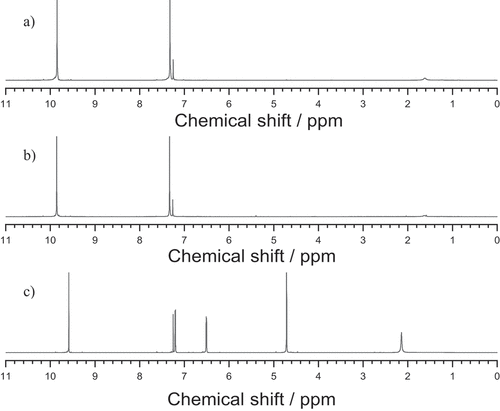
After recrystallization and drying of the product, beige-white 2,5-diformylfuran crystals were obtained. In the process, 38.2 mmol of the product were obtained using 79.2 mmol HMF, which corresponds to a yield of 48%. The product had a melting range of 109.5–110.4°C, which agrees very well with that of 108–110°C described in the literature [Citation30]. shows the 1H-NMR spectra in deuterated chloroform of a) synthesized DFF, b) DFF obtained from Sigma Aldrich and c) the HMF used in the synthesis. The product spectrum of synthesized DFF is in good agreement with that of DFF obtained from Sigma Aldrich, and no peaks of residual HMF were observed. Chemical shifts in ppm a): 7.32 s (2 H, = CH); 9.85 s (2 H, CHO); b): 7.33 s (2 H, = CH); 9.86 s (2 H, CHO); c): 4.71 s (2 H, CH2O); 6.51 d (1H, = CH); 7.20 d (1H, = CH); 9.59 s (1H, CHO).
Gel preparation
lists the combinations of phenolic and aldehydic reagents investigated in this study and indicates whether gelation occurred. After the specified reaction time, gelation of DFF at a 1:1 aldehyde:phenol (A:P) ratio was observed for phloroglucinol, resorcinol, and catechol. However, with F only catechol led to gelation. Increasing the molar A:P ratio to 2:1 or 3:1 resulted in gelation of F with all phenolic monomers investigated. This is in accordance with the literature [Citation32–36], where F has been used mainly at an A:P ratio of 2:1. In preliminary experiments, the reaction mixture of HMF with resorcinol was examined under the same basic conditions at a ratio of 1:1, but no gelation occurred. Therefore, the use of HMF as a possible aldehyde monomer under these conditions was rejected. Phloroglucinol, resorcinol, and catechol hydrogels were prepared with DFF and F at 1:1 and 2:1 A:P ratios, respectively, and – after gelation – dried by supercritical extraction. Generally, hydrogels prepared with F required shorter gelation times at room temperature than the corresponding DFF gels, which typically showed gelation after 24 h at 363 K. This may be due to the higher chemical stability of DFF or the lower A:P ratio compared to that in the F hydrogels. The resulting opaque aerogels were dark red to black and exhibited shrinkage ranging from 10% to 30%.
Table 1. Phenolic compounds screened in this study for gelation with DFF and F
Characteristic surface area
The initial pH before curing, composition, A:P ratio, and specific BET surface area of each F and DFF aerogel are listed in . Aerogels denoted with A and B contain F and DFF as aldehydic compounds, respectively. The results of the BET surface determination indicate that aerogels with high porosity were produced, which may be attributable to gaps between chains of connected particles. The values obtained for the specific BET surfaces correspond relatively well to those of RF gels described in the literature, which range from 200–700 m2 g−1. However, due to the large differences between individual measured values, no conclusions can be drawn concerning the replacement of F by DFF in these aerogels.
Table 2. Specific BET surface areas of the aerogels investigated
Gel morphology
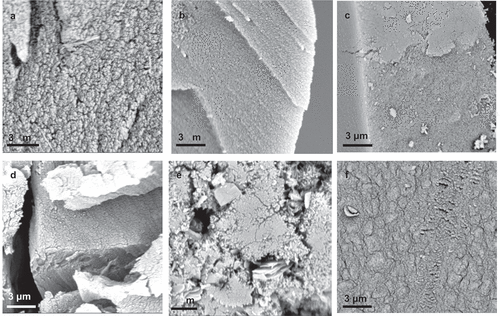
SEM images of gold-sputtered aerogels of phloroglucinol, resorcinol, and catechol with DFF (1:1) and F (1:2) as aldehydic components are shown in . The gels were generally relatively similar in morphology, containing uniform frameworks with an open-cell structure and evenly distributed porosity. Note that no significant differences between F and DFF aerogels were observed.
Thermal decomposition
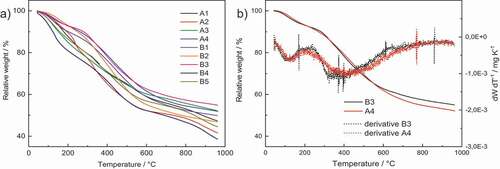
The aerogels were analyzed thermogravimetrically to characterize their behavior during a potential pyrolysis step that leads to carbon aerogels (see ). a shows that, in the range between 100°C and 140°C, the size of the batch has a particular influence on the weight loss, as the 10 g batches lost about twice the total weight (10%) of the 1 g batches (5%). This discrepancy between batch sizes may be attributable to incomplete solvent removal from the pores of the larger-sized batches. The greatest mass loss was observed in a range from 250°C to 550°C, which – according to the literature [Citation37] – can be attributed to the loss of oxygen in the form of carbon dioxide. b shows a direct comparison of the curves for 1 g batches of 1:1 (A:P) catechol-F (A4) and catechol-DFF (B3) aerogels: no significant differences can be observed when F is replaced with DFF as the aldehydic component.
Conclusion
This work focused primarily on using phenolic model compounds that may be obtainable by depolymerization of lignin to produce bio-based aerogels. Further, 2,5-diformylfuran was synthesized from 5-(hydroxymethyl)furfural and investigated as an aldehydic feedstock alternative to formaldehyde in the sol-gel reaction under basic aqueous conditions. Using DFF resulted in no significant physical or morphological differences of the aerogels, as the SBET values varied considerably, ranging from 13.8 to 719.4 m2 g−1. The thermogravimetric analysis curves of DFF and F aerogels were largely similar. Note, however, that – unlike F – DFF is not classified as a toxic substance and can be used to produce phenolic hydrogels at an A:P ratio of 1:1, whereas other aldehydes have been used at a ratio of at least 1.5:121 or higher. One drawback of replacing F with DFF is reduced reactivity and thus longer gelation times at higher temperatures. However the higher stability may also constitute an advantage since, in contrast to F and HMF, DFF can be stored as a solid at room temperature with no decomposition or side reactions. In conclusion, DFF is an interesting alternative to F in the production of bio-based organic aerogels. Additional research must be conducted to investigate the effects of varying the process variables, for instance, A:P ratio, pH, and solvent concentration. Further development of bio-based aerogels synthesized from diformylfuran will require upscaling of DFF production using heterogeneous catalysts.
Acknowledgments
The authors would like to thank M.Sc.Gottfried Aufischer from Wood Kplus for his valuable input into this work and guidance for testing.
Disclosure statement
No potential conflict of interest was reported by the authors. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Data availability statement
Data availability statement Data will be made available upon request to the corresponding author at [email protected]; www.wood-kplus.at.
Additional information
Funding
Notes on contributors
Christoph Derflinger
Christoph Derflinger is a PhD student at the Institute for Chemical Technology of Organic Materials at the Johannes Kepler University Linz working for Wood K plus – Competence Center for Wood Composites & Wood Chemistry. He carried out the preparative work on the synthesis of the bio-based aerogels and is currently working on the development of heterogeneously catalyzed production methods for furandicarbaldehyde.
Birgit Kamm
Birgit Kamm is a Key researcher at Wood Kplus, Austría and an Honorary Professor Biorefinery Technology and Biobased Products at Brandenburg University of Technology, BTU Cottbus-Senftenberg. Her research is focused on commercially viable biobased platform chemicals and materials. She is currently working on understanding the structure–property relationships between renewably sourced biobased polymers, biobased polymer additives.
Christian Paulik
Christian Paulik is a Professor at the Johannes Kepler University Linz and Head of the Institute for Chemical Technology of Organic Materials with expertise in conventional and biobased plastics and biotechnololgy.
References
- Hu LAH, Bai X. Producing high yield of levoglucosan by pyrolyzing nonthermal plasma-pretreated cellulose. Green Chem. 2020;22:2036.
- Delidovich I, Leonhard K, Palkovits R. Cellulose and hemicellulose valorisation: an integrated challenge of catalysis and reaction engineering. Energy Environ Sci. 2014;7:2803.
- Luo Z, Qin S, Chen S, et al. Selective conversion of lignin to ethylbenzene. Green Chem. 2020;22:1842.
- Kamm B, Gruber PR, Kamm M. Ullmann’s encyclopedia of industrial chemistry. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2000. p. 1.
- Agarwal A, Rana M, Park J-H. Advancement in technologies for the depolymerization of lignin. Fuel Process Technol. 2018;181:115.
- Sun Z, Fridrich B, de Santi A, et al. Bright Side of Lignin Depolymerization: toward New Platform Chemicals. Chem Rev. 2018;118(2):614.
- Wang H, Tucker M, Ji Y. Recent Development in Chemical Depolymerization of Lignin: A Review. J Appl Chem. 2013;2013:1.
- Wendisch VF, Kim Y, Lee J-H. Chemicals from lignin: recent depolymerization techniques and upgrading extended pathways. Cur Opin Green Sustain Chem. 2018;14:33. .
- Mirzaeian M, Hall PJ. The control of porosity at nano scale in resorcinol formaldehyde carbon aerogels. J Mater Sci. 2009;44(10):2705.
- Kistler SS. Coherent Expanded-Aerogels. J Phys Chem. 1932;36:52.
- Pekala RW. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J Mater Sci. 1989;24:3221.
- Lee Y, Choi J-W, Suh DJ, et al. Ketonization of hexanoic acid to diesel-blendable 6-undecanone on the stable zirconia aerogel catalyst. Appl Catal A Gen. 2015;506:288.
- Buratti C, Moretti E, Belloni E, et al. Development of Innovative Aerogel Based Plasters: preliminary Thermal and Acoustic Performance Evaluation. Sustainability. 2014;6:5839.
- Gui X, Wei J, Wang K, et al. Carbon Nanotube Sponges. Adv Mater. 2010;22:617.
- Dong X-C, Xu H, Wang X-W, et al. 3D Graphene–Cobalt Oxide Electrode for High-Performance Supercapacitor and Enzymeless Glucose Detection. ACS Nano. 2012;6:3206.
- Abbas Q, Mirzaeian M, Ogwu AA. Electrochemical performance of controlled porosity resorcinol/formaldehyde based carbons as electrode materials for supercapacitor applications. Int J Hydrogen Energy. 2017;42:25588.
- Allahbakhsh A, Bahramian AR. Self-assembled and pyrolyzed carbon aerogels: an overview of their preparation mechanisms, properties and applications. Nanoscale. 2015;7:14139.
- Biener J, Stadermann M, Suss M, et al. Advanced carbon aerogels for energy applications. Energy Environ. Sci. 2011;4:656.
- Chen B, Zheng Q, Zhu J, et al. Mechanically strong fully biobased anisotropic cellulose aerogels. RSC Adv. 2016;6:96518.
- Wu D, Fu R, Sun Z, et al. Low-density organic and carbon aerogels from the sol–gel polymerization of phenol with formaldehyde. J Non-Crystalline Solids. 2005;351:915.
- Lee Y, Yoon JS, Suh DJ, et al. 5-hydroxymethylfurfural as a potential monomer for the preparation of carbon aerogel. Mater Chem Phys. 2012;136:837.
- Henderson RK, Hill AP, Redman AM, et al. Development of GSK’s acid and base selection guides. Green Chem. 2015;17:945.
- Nie J, Liu H. Efficient aerobic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran on manganese oxide catalysts. J Catal. 2014;316:57.
- Yadav GD, Sharma RV. Biomass derived chemicals: environmentally benign process for oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran by using nano-fibrous Ag-OMS-2-catalyst. Appl Catal B Environ. 2014;147:293.
- Antonyraj CA, Jeong J, Kim B, et al. Selective oxidation of HMF to DFF using Ru/γ-alumina catalyst in moderate boiling solvents toward industrial production. J Ind Eng Chem. 2013;19(3):1056.
- Ventura M, Lobefaro F, de Giglio E, et al. Selective Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Diformylfuran or 2-Formyl-5-furancarboxylic Acid in Water by using MgO⋅CeO2 Mixed Oxides as Catalysts. ChemSusChem. 2018;11(8):1305.
- Ma J, Wang M, Du Z, et al. Synthesis and properties of furan-based imine-linked porous organic frameworks. Polym Chem. 2012;3:2346.
- Xiang T, Liu X, Yi P, et al. Schiff base polymers derived from 2,5-diformylfuran. Polym Int. 2013;62:1517.
- Amarasekara AS, Green D, Williams LD. Renewable resources based polymers: synthesis and characterization of 2,5-diformylfuran–urea resin. Eur Polym J. 2009;45:595.
- Smirnova NV, Klushin VA, Bezbozhnaya TV, et al. Selective Oxidation of 5-(Hydroxymethyl)furfural to Furan-2,5-dicarbaldehyde with Sodium Nitrite in Phosphoric Acid. Russ J Org Chem. 2018;54:414.
- Rathod PV, Nale SD, Jadhav VH. Metal Free Acid Base Catalyst in the Selective Synthesis of 2,5-Diformylfuran from Hydroxymethylfurfural, Fructose, and Glucose. ACS Sustain Chem Eng. 2017;5:701.
- Saliger R, Bock V, Petricevic R, et al. Carbon aerogels from dilute catalysis of resorcinol with formaldehyde. J Non-Crystalline Solids. 1997;221:144.
- Petričević R, Reichenauer G, Bock V, et al. Structure of carbon aerogels near the gelation limit of the resorcinol–formaldehyde precursor. J Non-Crystalline Solids. 1998;225:41.
- Schaefer DW, Pekala R, Beaucage G. Origin of porosity in resorcinol-formaldehyde aerogels. J Non-Crystalline Solids. 1995;186:159.
- Bruno MM, Cotella NG, Miras MC, et al. A novel way to maintain resorcinol–formaldehyde porosity during drying: stabilization of the sol–gel nanostructure using a cationic polyelectrolyte. Colloids Surf A Physicochem Eng Asp. 2010;362:28.
- Chang Y-M, Wu C-Y, Wu P-W. Synthesis of large surface area carbon xerogels for electrochemical double layer capacitors. J Power Sources. 2013;223:147.
- Patton A, ed. Formaldehyde: synthesis, applications, and potential health effects. New York: Nova Science Publishers Inc; 2015.