 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Polyhydroxyalkanoates (PHA) are bacterial polyesters that have been described as one of the most promising sustainable alternatives to petroleum-based plastics. A major challenge for PHA to become market-competitive, is to decrease the associated production costs. According to several studies, about 20–50% of the total production costs is attributed to the raw materials and therefore, the use of different types of organic residues have been evaluated as substrates for the microorganisms. In this study, the strain Bacillus cereus ATCC 14579 was cultivated in shake flasks using a defined medium and pretreated grape peel as sole carbon source. The pretreatment of the grape peels was performed by a hydrolysis step with diluted sulfuric acid yielding up to 52.9% (w/w) of total sugars from the dry residue. PHB extraction using dry and wet biomass was performed and evaluated showing up to 3.7-higher yields when using the latter. PHB accumulation of 18.79% (w/w), corresponding to 0.53 gPHB/L, was achieved using the grape peel hydrolyzate at 72 h of cultivation. Eventually, the possibility of producing PHB by the use of non-treated lignocellulosic biomass (LCB) with high contents of fermentable sugars is presented as an interesting alternative to be optimized and projected for sustainable bioplastic production processes.
GRAPHICAL ABSTRACT
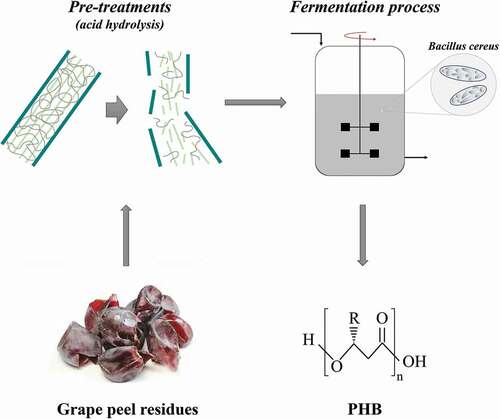
Introduction
Accumulation of organic waste is a serious and growing problem, directly impacting the release of CO2, which is one of the main causes for the greenhouse effect [Citation1]. One of the many different types of organic waste are fruit residues, which occur mainly in the form of pulp and peel. Annually, 500 million tons of fruits and vegetables are discarded worldwide [Citation2] and thus are a major challenge for local waste management. Nowadays, researchers come up with numerous ideas on how to repurpose fruit residues, including the production of biogas [Citation3–5], dietary fibers [Citation6], pharmaceuticals [Citation7], bioethanol [Citation8–10] and other fermentation products. In this way, waste streams can be redirected into new production processes to close the loops on valuable organic resources, the major principle of a circular bioeconomy. The idea of integrated bio-refineries for the synthesis of biopolymers has attracted the attention of industry and academia likewise: using organic waste materials as a sustainable carbon and energy source for microorganisms able to produce the biomolecules of interest [Citation11].
One of the microorganisms with a great potential to synthesize bioproducts from various organic sources is Bacillus cereus. The Gram-positive bacterium is capable of producing biopolymers such as polyhydroxybutyrate (PHB) [Citation12–14], a polyester belonging to the family of polyhydroxyalkanoates (PHA). This biopolymer is stored inside the microorganism’s cytoplasm in form of granules under specified culture conditions, which are characterized by a non-carbonated nutrient limitation and an excess of the carbon source. PHB is a linear molecule and insoluble in water. It is derived from alkanoic acids (specifically hydroxybutanoic acid) and contains a hydroxyl group that is added to a carbonyl group [Citation15]. Its molecular weight ranges from 2 × 105 to 3 × 106 Da. However, composition and weight depend on the microorganism and the carbon source used during cultivation [Citation16]. Due to its competitive advantage over petroleum-based materials, being a biodegradable and biocompatible polymer, PHB has been used for manufacturing plastics with applications in different fields such as biomedicine, pharmacy and packaging [Citation17–21]. Some properties of PHB like its high thermoplasticity (melting point at 180°C) and its efficiency against moisture, make this biopolymer especially suitable for food packaging applications [Citation22].
A market study performed by European Bioplastics predicts an economic growth of bioplastics from 2.1 million tonnes in 2019 to 2.4 million tonnes in 2024. However, the production scale of bioplastics is still low, representing only 1% of the total volume of produced fossil-based plastics [Citation23]. The high manufacturing cost of bioplastics, including PHA, is mainly because of the required feedstocks that make up for 20% to 50% of the total production costs [Citation24–27]. The search for novel and cost-effective substrates to yield higher process efficiency is much needed for large-scale industrial PHB production.
Lignocellulosic biomass (LCB) such as agricultural, industrial and forest waste represents a potential low-cost and renewable carbon source [Citation28]. To generally make sufficient amounts of fermentable sugars available, LCB must be subjected to pretreatments, which have been classified into physical, chemical, and biological procedures and/or a hydrolysis step [Citation29,Citation30]. Some specific types of fruit residues already contain high concentrations of free sugars, such as glucose and fructose, that are directly available as carbon source in the fermentation process. Additional pretreatments of fruit residues to hydrolyze the cellulose and hemicellulose polymers can then even enhance the amount of available fermentable sugars, which makes fruit residues a promising alternative carbon source for an efficient PHB production process [Citation31]. Previous studies report the utilization of various fruit residues, such as banana peel, mango peel, orange peel or pineapple peel, for the production of PHB with different strains of the genus Bacillus and obtained promising results [Citation32–39].
The present study is focused on the feasibility of PHB production by fermentation of B. cereus using grape residues as sole carbon source. The established protocol involves a widely available and low-cost feedstock with high sugar content leading to the development of a bioprocess that is in accordance with the principles of a circular bioeconomy.
Materials and methods
Hydrolyzate preparation
Prior to hydrolysis, grape peels were dried at 90°C for 48 h and stored at room temperature. Hydrolyzates were prepared using 5% w/v of dried grape peels in dH2O. Three variables were considered for optimization of the acid hydrolysis: temperature (RT, 80, 100, 120°C), acid concentration (0, 0.5, 1, 2, 5, 10 % v/v) and incubation time (0, 60, 120 min). To study the effect of the different treatments, a completely randomized experimental design was used. After hydrolysis, the samples were cooled, filtered, and neutralized to pH 5 to 7 using 10 M NaOH as needed. Finally, the obtained hydrolyzates were stored at −20°C for further analysis. Each procedure was performed in triplicates.
Cultivation conditions
The microorganism used in this study was B. cereus ATCC 14579, which was cryopreserved at −20°C using glycerol 50% v v−1. The inoculum was prepared in shake flasks with 50 mL of Luria-Bertani (LB) medium and incubated at 30°C and 150 rpm for 24 h. The main culture was performed in 500-mL shake flasks containing 100 mL medium at 30°C, 150 rpm for 48 and 72 h. One liter of mineral salt medium (MSM) was prepared as follows: 90 mL of stock 1 (Na2HPO4 x 7H2O 4.0 g, KH2PO4 2.0 g, NH4Cl 1.0 g y NaCl 0.1 g), 1 mL of stock 2 (MgCl2 100 g l), 1 mL of stock 3 (CaCl2 20 g l−1), 1 mL of stock 4 (FeCl2 5 g l−1), 100 µl of stock 5 (ZnCl2 1 g, MnCl2 0.2 g, H3BO3 3.0 g, CoCl2 1.0 g, CuCl2 0.1 g, NiCl2 0.1 g y NaMoO4 x 2H2O 0.3 g) and 907 mL of stock 6 corresponding to 10 g l−1 of the carbon source in combination with dH2O depending on the concentration of the hydrolyzate. Each stock was sterilized separately. Samples were taken every 4 h. Cell density was measured by spectrophotometry at 600 nm (OD600) and the calculation of its concentration was determined by cell dry weight. Cultivations were performed in triplicates.
Quantification of reducing sugars and ammonia
The concentration of reducing sugars obtained after hydrolysis and during the cultivations was determined using the DNS method [Citation40]. For this, samples were diluted conveniently and analyzed using a spectrophotometer (Shimadzu, UVmini-1240) at 540 nm. Similarly, ammonia present in the cultivation medium was quantified using the phenol-hypochlorite method [Citation41] at 635 nm.
Recovery and quantification of PHB
For the extraction of PHB, biomass was recovered by centrifugation at 9000 × g for 20 min. PHB extraction was performed using wet and dry biomass. For better comparison, each extraction was done using the biomass from 40 mL of cultivation broth in triplicates. Biomass was resuspended in 2 mL of sodium hypochlorite (10% v/v) and 2 mL of chloroform. The mixture was vigorously mixed by vortexing and incubated at room temperature for 24 h. The lower phase (chloroform phase) was recovered, the polymer was precipitated with 10 mL of isopropanol and recovered by filtration [Citation42].
Chromatographic analysis of PHB
Qualitative analysis of the extracted PHB was done using gas chromatography-mass spectrometry (GC-MS). Prior to the analysis, extracts were subjected to methanolysis using 2 mL chloroform and 2 mL methanol/sulfuric acid (85:15 v/v). Samples were heated at 100°C for 2 h and, after cooling, 2 mL distilled water and 2 mL chloroform were added. The lower organic phase was recovered and filtered using 0.22 µm PTFE filters. The resulting methyl ester monomers were injected (injection volume: 1 µL) into the GC-MS (Shimadzu QP2010) using a Rtx-5 ms column and helium as the mobile phase with a flow rate of 1 mL min−1. Injector temperature was set at 200°C and the temperature conditions during analysis were the following: 50°C for 3 min, followed by an increase to 325°C within 15 min, holding this final temperature for 5 min.
Calculations
The specific cell growth rate (µ), the substrate yield on biomass (YX/S) and PHB (YPHB/S), specific substrate consumption rate (qS), volumetric productivity (QPHB), and specific production rate (qPHB) were calculated as follows:
where X(t) is the cell concentration at time t (g L−1), Xf is the final cell concentration (g L−1), X0 is the initial cell concentration (g L−1), S0 is the initial substrate concentration (g L−1), Sf is the final substrate concentration (g L−1), and PHBf is the final PHB concentration (g L−1).
Results and discussion
Over the last decades, multiple Bacillus species were studied regarding the synthesis of PHA with synthetic culture media and using low-cost substrates derived from waste streams or agro-industrial residues [Citation27,Citation35,Citation43–47]. The capability of Bacillus species to produce PHA utilizing cost-effective, highly available and unrelated carbon sources has attracted the attention of researchers in order to decrease the PHA associated production costs and promote a sustainable circular bioeconomy at the same time. Although strains of B. cereus have been studied for the production of PHB, the utilization of fruit residues as sole carbon source was not investigated up to now.
Pretreatment of grape peel residues to obtain reducing sugars
Acid hydrolysis of grape peel residues was performed in order to analyze the amount of sugars coming from this pre-treatment reaction. The reducing sugar concentration after acid hydrolysis is presented in . In general, hydrolyzates showed higher sugar concentrations when the hydrolysis time was increased from 60 to 120 min. The maximum concentration of reducing sugars (26.46 ± 2.83 g L−1) was achieved at a temperature of 100°C using 0.5% v/v of H2SO4 and a hydrolysis time of 120 minutes. At acid concentrations from 0 to 2 % v/v, the sugar contents increase with higher temperatures. However, at acid concentrations of 5 and 10% v/v, this tendency cannot be observed and using 10% v/v of H2SO4 and 120 min hydrolysis time, a clear decrease of the sugar concentrations was obtained with higher temperatures. A decrease of sugars such as glucose, galactose, arabinose, xylose and mannose during acid hydrolysis at high temperatures and high acid concentrations has been reported in previous studies [Citation48–52]. At these conditions, sugar derivative products such as organic acids increase, which causes lower sugar yields [Citation53]. From these results, we can conclude that grape peels already contain high amounts of simple sugars prior to the acid hydrolysis step. According to Sousa et al. [Citation54], the content of sugars for direct fermentation of a mixture of pulp, peel and seed was quantified to be 29.2% w/w, with glucose and fructose being the main sugars when the grapes were mature at the time of analysis. Varandas et al. [Citation55], however, reported a glucose content of only 14.2 g per kg of grape peel. Analyzing the dried grape peel mass used in our study, a total sugar content of 31.4% w/w was measured without any pretreatments, while the maximum content of sugars obtained after hydrolysis was increased to 52.9% w/w. Even though the specific sugars present in the grape peel were not identified, the increased concentration of sugars in the samples after hydrolysis clearly indicates the breakdown of the lignocellulosic structure. The breakdown of cellulose yields glucose molecules, while hemicellulose provides a mixture of sugars such as glucose, arabinose, mannose, galactose and xylose [Citation30].
Cultivation of B. cereus using grape peel as substrate
Based on the hydrolysis results, the hydrolyzate obtained at 100°C with 0.5% v/v of sulfuric acid after an incubation for 120 min was selected as sole carbon source for the following cultivation experiments (cultivation 1). Additionally, two other carbon sources were used as controls: i) the non-hydrolyzed sample (cultivation 2) and ii) pure glucose (data not shown). Considering the different sugar concentrations of the samples, the cultivation medium was designed to contain 10 g/L of the carbon source.
Growth curves of B. cereus using hydrolyzed and non-hydrolyzed grape peels are shown in . Cultivations 1 and 2 exhibited a similar behavior growing to a maximum cell density of 7.43 and 7.30 (OD600), respectively. Specific growth rates of 0.16 h−1 and 0.12 h−1 were calculated for B. cereus growing with the non-hydrolyzed and hydrolyzed substrate, respectively. Detailed cultivation parameters are shown in .
Table 1. Kinetic and stoichiometric parameters of cultivations of B. cereus using grape peel residues as sole carbon source
Figure 2. Shake flask cultivation of B. cereus using grape peel with (Δ) and without (□) hydrolysis pretreatment. Curves represent mean values from triplicates. Standard deviation is indicated

Analyzing the late stationary phase (after 54 h), a decrease in the cell density can be observed when using the grape peel hydrolyzate. This might be explained by the presence of aliphatic acids, such as levulinic acid, acetic acid and formic acid, which affect cell growth and viability and can be formed during the hydrolysis step. Thermochemical degradation of polysaccharides in the presence of acids causes the formation of formic and levulinic acid, while the formation of acetic acid occurs mainly due to the cleaving of acetyl groups present in hemicellulose [Citation30]. Moreover, furfural, which is a secondary product of xylose, has been identified a as recurrent inhibitor in lignocellulosic hydrolyzates [Citation56]. We assume that the lower specific growth rate and the decrease of the cell density by the end of cultivation 1 can be a consequence of by-products formed during the preceding hydrolysis step.
Both cultivations showed differences in the substrate yield on biomass (YX/S) when comparing the harvesting times. The decrease in YX/S from 48 h to 72 h was mainly due to the decrease of the sugar concentration, while the biomass stayed relatively constant during the stationary phase. It is important to point out that both cultivations were not limited by the carbon source, which is an important condition in order to promote PHB accumulation. The medium was designed to limit cell growth by the nitrogen source, which was confirmed by measuring the ammonium concentrations in the supernatants. After 30–33 h of cultivation, ammonium (present as NH4Cl) was completely consumed by the cells, which corresponds to the moment when B. cereus stopped growing and entered to the stationary phase ().
PHB recovery and quantification
One of the advantages of using Bacillus species for the production of PHB is the absence of an external lipopolysaccharide layer making the extraction process simpler than for other bacterial strains [Citation57]. Interestingly, there are not any reports comparing PHB extraction from dry or wet biomass. Therefore, the accumulation of PHB was analyzed at the early (48 h) and late stationary phase (72 h) using wet and dry biomass. For all evaluated conditions, a clear increase of the PHB accumulation was observed in the late stationary phase, reaching a maximum PHB accumulation of 16.33 % w/w and 18.79 % w/w for cultivations 1 and 2, respectively (). Furthermore, by using wet biomass it was possible to obtain greater amounts of PHB per biomass and therefore higher percentages of PHB accumulation compared to extractions performed with dry biomass. Consequently, YPHB/S, QPHB and qPHB were higher at 72 h using wet biomass, except for the sample from cultivation 1 at 72 h when PHB production analyzed using dry and wet biomass was not significantly different (). Analyzing the PHB production parameters obtained in B. cereus cultures 1 and 2, we can indicate that the use of an acid hydrolysis would not be necessary. This is of great importance, since we would be avoiding a pre-treatment to the process and consequently it implies lower costs, less energy consumption and shorter associated times. According to our results a theoretical yield of 25 g of PHB can be produced from 1 kg of grape peel without pre-treatments.
Table 2. PHB production parameters for cultivations of B. cereus using different extraction methods. Cultivation 1: non-hydrolyzed substrate; cultivation 2: hydrolyzed substrate; D: dry biomass; W: wet biomass
Figure 3. PHB accumulation (% w/w) in cultures of B. cereus growing with non-hydrolyzed substrate (cultivation 1) and hydrolyzed substrate (cultivation 2). Extractions were performed using dry (D) and wet (W) biomass

The 3-hydroxybutyrate methyl ester molecules obtained as a result of the methanolyzed PHB was detected by GC-MS, confirming the presence of the polymer in the extracted samples (). A predominant peak corresponding to the 3HB methyl ester was noted at 9.87 min, and its mass spectrum was obtained by full scan mode and analyzed though the NIST database. The fragmentation patterns () of the 3-HB methyl ester coming from the full scan analysis were in accordance with previous results, where the hydroxyl end is represented by the peak at m/z 103 in the mass spectrum [Citation58].
Figure 4. GC-MS analysis of extracted PHB from cultures of B. cereus growing on grape residues. A) Chromatogram in selected ion mode (SIM). B) Comparison of the peak (Rt: 9.87 min) with mass spectra MS library

The utilization of different fruit residues as sole carbon source in cultures of Bacillus species has been reported. To date however, grape residues have not been evaluated. Getachew and Woldesenbet [Citation35] isolated a strain of Bacillus sp., which was able to synthesize PHB using banana residues that were pretreated by hydrolysis with zinc chloride. They reported a PHB accumulation of 27% w/w, corresponding to 2.1 gPHB L−1. Although the percentage of polymer accumulation is similar with our result, the higher PHB concentration reported in this study compared to our results (0.53 g L−1) is due to the higher biomass concentration. This was achieved by the incorporation of 40 g L−1 of the hydrolyzate, which is 4-times higher than our initial condition. In another study, mango peel was used in shake flasks experiments with B. thuringiensis IAM 12077. A cell concentration of 7.86 g L−1 and a PHB concentration of 4.03 g L−1 was achieved using a nutrient broth medium supplemented with yeast extract and peptone [Citation36]. Analysis of the fermentation kinetics, yielded a YPHB/S of 0.1 g g−1 and a QPHB of 0.168 g L−1 h−[Citation1]. In comparison with our results, a lower a YPHB/S (0.05 g g−1) and a significant lower QPHB (6.85 mg L−1 h−1) was obtained, however, the use of yeast extract and peptone might have influenced on the parameter calculation finding several carbon sources that can contribute to the synthesis of PHB. Sukan et al. (2014) investigated a modified strain of B. subtilis for the synthesis of PHB using orange peel in a 0.5 L fermenter and obtained a maximum concentration of 1.24 gPHB L−1 corresponding to a PHB accumulation of 40% w/w [Citation38]. Pineapple residues in the form of juice have been used as the carbon source in a defined medium for the synthesis of PHB by Bacillus sp. SV13 [Citation39]. In this study, a 5-L bioreactor was operated at 37°C and 250 rpm, while varying the pH and aeration conditions. The highest PHB accumulation (40.54% w/w) was achieved when the pH was not controlled and aeration was set at 2.5 vvm. Although B. cereus strain has not been studied with fruit residues as a carbon source, some studies with other substrates have been reported. Al Bhagowati et al. [Citation59] characterized the production of PHB in cultures of B. cereus SE-1 using a complex medium with beef extract and peptone reaching PHB accumulation of 22.1 and 40.0% w/w after 48 and 72 h of cultivation, respectively. In another study, strain B. cereus SPV was cultivated in 20-L fermenters using glucose as sole carbon source and a fed-batch cultivation strategy [Citation60]. The results pointed out that PHB production depended on the harvesting time and cultivation strategy reaching PHB accumulations between 16 and 38% w/w. In general, previous studies have used complex medium components and not fruit waste as sole carbon source. This affects cultivation yields and productivities making a proper comparison hard to achieve. It is proposed that in order to achieve sustainable PHB production through the use of waste and to encourage competitiveness in the cost of the polymer production, the use of complex nutrients should be avoided.
Conclusion
Renewable feedstocks in the form of by-products bring with them a number of challenging aspects to be considered, in order to be applied as suitable substrates PHA production. The high amount of fermentable sugars available in grape peels offers the opportunity of avoiding complex and costly pretreatments, which makes them a valuable and highly promising carbon source for PHA synthesis. For the first time, B. cereus has been studied regarding its growth and PHB production using grape residues as sole carbon source yielding promising results that can be further optimized and scaled-up to develop a sustainable and low-cost production process.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
R. Andler
Dr. Rodrigo Andler is a Biochemical Engineer with a Master in Biochemical Engineering Sciences and a PhD in Biotechnology from the University of Münster, Germany. Currently, Dr. Andler is an Assistant Professor at Universidad Católica del Maule, Chile and his research interests are in the synthesis of biopolymers and biodegradation processes.
V. Pino
Valentina Pino is a student of Biotechnology Engineering at Universidad Católica del Maule, Chile.
F. Moya
Felipe Moya is a Biotechnology Engineer from Universidad Católica del Maule, Chile.
E. Soto
Esteban Soto is a student of Biotechnology Engineering at Universidad Católica del Maule, Chile.
C. Valdés
Cristian Valdés is a Biochemist with a Master in Biochemistry and a PhD in Applied Sciences from the University of Talca, Chile. Currently, Dr. Valdés is doing a postdoctoral fellowship in polymers synthesis and degradation systems at Universidad Católica del Maule, Chile.
C. Andreeßen
Christina Andreeßen is a Microbiologist with a PhD in Biotechnology from the University of Münster, Germany. After a postdoctoral fellowship at MIT, Boston (MA), she now works as a project manager at DECHEMA in Germany.
References
- Yoshida H, Gable JJ, Park JK. Evaluation of organic waste diversion alternatives for greenhouse gas reduction. Resour. Conserv. Recycl. 2012;60:1–9.
- Banerjee J, Singh R, Vijayaraghavan R, MacFarlane D, Patti AF, Arora A. Bioactives from fruit processing wastes: green approaches to valuable chemicals. Food Chem. 2017;225:10–22.
- Achinas S, Achinas V, Euverink GJW, et al. Overview of biogas production from biowaste. Engineering. 2017;3(3):299–307.
- Pavi S, Kramer LE, Gomes LP, et al. Biogas production from co-digestion of organic fraction of municipal solid waste and fruit and vegetable waste. Bioresour Technol. 2017;228:362–367.
- Sitorus B, Sukandar, Panjaitan SD. Biogas recovery from anaerobic digestion process of mixed fruit -vegetable wastes. Energy Procedia. 2013;32:176–182.
- Sharma K, Mahato N, Cho MH, et al. Converting citrus wastes into value-added products: economic and environmently friendly approaches. Nutrition. 2017;34:29–46.
- Sagar NA, Pareek S, Sharma S, et al. Fruit and vegetable waste: bioactive compounds, their extraction, and possible utilization. Compr Rev Food Sci Food Saf. 2018. DOI:https://doi.org/10.1111/1541-4337.12330
- Sarkar N, Ghosh SK, Bannerjee S, et al. Bioethanol production from agricultural wastes: an overview. Renewable Energy. 2012;37(1):19–27.
- Choi IS, Lee YG, Khanal SK, et al. A low-energy, cost-effective approach to fruit and citrus peel waste processing for bioethanol production. Appl Energy. 2015;157:953–973.
- Guerrero AB, Ballesteros I, Ballesteros M. The potential of agricultural banana waste for bioethanol production. Fuel. 2018;213:176–185.
- Dietrich K, Dumont MJ, Del Rio LF, et al. Sustainable PHA production in integrated lignocellulose biorefineries. N Biotechnol. 2019;49:161–168.
- Valappil SP, Peiris D, Langley GJ, Herniman JM, Boccaccini AR, Bucke C, Roy I. Polyhydroxyalkanoate (PHA) biosynthesis from structurally unrelated carbon sources by a newly characterized Bacillus spp. J. Biotechnol. 2007;127(3):475–487.
- ŁAbuzek S, Radecka I. Biosynthesis of PHB tercopolymer by Bacillus cereus UW85. J. Appl. Microbiol. 2001;90(3):353–357.
- Valappil SP, Misra SK, Boccaccini AR, Keshavarz T, Bucke C, Roy I. Large-scale production and efficient recovery of PHB with desirable material properties, from the newly characterised Bacillus cereus SPV. J Dairy Sci. 2013;132:251–258.
- Lee B, Pometto AL, Fratzke A, Bailey TB. Biodegradation of degradable plastic polyethylene by phanerochaete and streptomyces species. Appl. Environ. Microbiol. 1991;57:678–685.
- Sudesh K, Abe H, Doi Y. Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Polym Sci. 2000. DOI:https://doi.org/10.1016/S0079-6700(00)00035-6
- Zhang J, Shishatskaya EI, Volova TG, et al. Polyhydroxyalkanoates (PHA) for therapeutic applications. Mater Sci Eng C. 2018. DOI:https://doi.org/10.1016/j.msec.2017.12.035
- Wang Y, Yin J, Chen GQ. Polyhydroxyalkanoates, challenges and opportunities. Curr Opin Biotechnol. 2014;30:59–65.
- Chen GQ. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev. 2009. DOI:https://doi.org/10.1039/b812677c
- Raza ZA, Abid S, Banat IM. Polyhydroxyalkanoates: characteristics, production, recent developments and applications. Int Biodeterior Biodegrad. 2018;126:45–56.
- Brigham, Sinskey. Applications of Polyhydroxyalkanoates in the Medical Industry. Int J Biotechnol Wellness Ind. 2012. DOI:https://doi.org/10.6000/1927-3037.2012.01.01.03
- Siracusa V, Rocculi P, Romani S, et al. Biodegradable polymers for food packaging: a review. Trends Food Sci Technol. 2008;19(12):634–643.
- Bioplastic E. Bioplastics market data 2019. Global production capacities of bioplastic 2019-2024. Eur Bioplastic. 2019.
- Choi J, Lee SY. Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl Microbiol Biotechnol. 1999;51(1):13–21.
- Reis MAM, Serafim LS, Lemos PC, Ramos AM, Aguiar FR, Van Loosdrecht MCM. Production of polyhydroxyalkanoates by mixed microbial cultures. Bioprocess Biosyst. Eng. 2003;25(6):377–385. .
- Strong PJ, Laycock B, Mahamud SN, Jensen PD, Lant PA, Tyson G, Pratt S. The opportunity for high-performance biomaterials from methane. Microorganisms. 2016;4(1):11.
- Koller M, Maršálek L, de Sousa Dias MM, et al. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. N Biotechnol. 2017;37:24–38.
- Brodin M, Vallejos M, Opedal MT, et al. Lignocellulosics as sustainable resources for production of bioplastics – a review. J Clean Prod. 2017;162:646–664.
- Taherzadeh MJ, Karimi K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci. 2008;9(9):1621–1651.
- Obruca S, Benesova P, Marsalek L, et al. Use of lignocellulosic materials for PHA production. Chem Biochem Eng Q. 2015;29(2):135–144.
- Ayeni AO, Adeeyo OA, Oresegun OM, et al. Compositional analysis of lignocellulosic materials: evaluation of an economically viable method suitable for woody and non-woody biomass. Am J. Eng Res. 2015.
- Ramadas NV, Singh SK, Soccol CR, et al. Polyhydroxybutyrate production using agro-industrial residue as substrate by Bacillus sphaericus NCIM 5149. Brazilian Arch. Biol. Technol. 2009;52(1):17–23.
- Naranjo JM, Cardona CA, Higuita JC. Use of residual banana for polyhydroxybutyrate (PHB) production: case of study in an integrated biorefinery. Waste Manag. 2014;34(12):2634–2640.
- Zhang Y, Sun W, Wang H, et al. Polyhydroxybutyrate production from oil palm empty fruit bunch using bacillus megaterium R11. Bioresour Technol. 2013;147:307–314.
- Getachew A, Woldesenbet F. Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res Notes. 2016;9(1):1–9.
- Gowda V, Agrowaste-based Polyhydroxyalkanoate SS. (PHA) production using hydrolytic potential of Bacillus thuringiensis IAM 12077. Brazilian Arch. Biol. Technol. 2014;57(1):55–61.
- Rao A, Haque S, El-Enshasy HA, et al. RSM–GA based optimization of bacterial PHA production and In Silico modulation of citrate synthase for enhancing PHA production. Biomolecules. 2019;9(12):872.
- Sukan A, Roy I, Agro-Industrial Waste KT. Materials as substrates for the production of Poly(3-Hydroxybutyric acid). J. Biomater. Nanobiotechnol. 2014;5(4):229–240.
- Suwannasing W, Imai T, Kaewkannetra P. Cost-effective defined medium for the production of polyhydroxyalkanoates using agricultural raw materials. Bioresour Technol. 2015;194:67–74.
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31(3):426–428.
- Weatherburn MW. Phenol-Hypochlorite Reaction for Determination of Ammonia. Anal. Chem. 1967;39(8):971–974.
- García A, Pérez D, Castro M, et al. Production and recovery of poly-3-hydroxybutyrate [P(3HB)] of ultra-high molecular weight using fed-batch cultures of Azotobacter vinelandii OPNA strain. J. Chem. Technol. Biotechnol. 2019;94(6):1853–1860.
- Khiyami MA, Al-fadual SM, Bahklia AH. Polyhydroxyalkanoates production via Bacillus (PCS) biofilm and date palm syrup. J Med Plants Res. 2011.
- Kumar P, Ray S, Patel SKS, et al. Bioconversion of crude glycerol to polyhydroxyalkanoate by Bacillus thuringiensis under non-limiting nitrogen conditions. Int. J. Biol. Macromol. 2015;78:9–16.
- Sharma P, Bajaj BK. Cost-effective substrates for production of poly-β-hydroxybutyrate by a newly isolated Bacillus cereus PS-10. J. Environ. Biol. 2015;36(6):1297–1304.
- Bhattacharya S, Dubey S, Singh P, et al. Biodegradable polymeric substances produced by a marine bacterium from a surplus stream of the biodiesel industry. Bioengineering. 2016;3(4):34.
- Mohammed S, Behera HT, Dekebo A, et al. Optimization of the culture conditions for production of Polyhydroxyalkanoate and its characterization from a new Bacillus cereus sp. BNPI-92 strain, isolated from plastic waste dumping yard. Int J Biol Macromol. 2020;156:1064–1080.
- Lenihan P, Orozco A, O’Neill E, Ahmad MNM, Rooney DW, Walker GM. Dilute acid hydrolysis of lignocellulosic biomass. Chem. Eng. J. 2010;156(2):395–403. .
- Xiang Q, Lee YY, Torget RW. Kinetics of glucose decomposition during dilute-acid hydrolysis of lignocellulosic biomass. Appl Biochem Biotechnol Part A Enzyme Eng Biotechnol. 2004;115(1–3):1127–1138.
- Lenihan P, Orozco A, O’Neill E, Ahmad MNM, Rooney DW, Mangwandi C, Walker GM. Kinetic modelling of dilute acid hydrolysis of lignocellulosic biomass. Biofuel Production-Recent Dev Prospect. 2011. DOI:https://doi.org/10.5772/17129
- Yoon SY, Han SH, Shin SJ. The effect of hemicelluloses and lignin on acid hydrolysis of cellulose. Energy. 2014;77:19–24.
- Lavarack BP, Griffin GJ, Rodman D. The acid hydrolysis of sugarcane bagasse hemicellulose to produce xylose, arabinose, glucose and other products. Biomass Bioenergy. 2002;23(5):367–380.
- Świątek K, Gaag S, Klier A, Kruse A, Sauer J, Steinbach D. Acid hydrolysis of lignocellulosic biomass: sugars and furfurals formation. Catalysts. 2020;10(4):437.
- Sousa EC, Uchôa-Thomaz AM, Carioca JO, Morais SM, Lima AD, Martins CG, Alexandrino CD, Ferreira PA, Rodrigues AL, Rodrigues SP, Silva JD, Rodrigues LL. Chemical composition and bioactive compounds of grape pomace (Vitis vinifera L.), Benitaka variety, grown in the semiarid region of Northeast Brazil. Food Sci. Technol. 2014;34(1):135–142.
- Varandas S, Teixeira MJ, Marques JC, Marques JC, Aguiar A, Alves A, Bastos MM. Glucose and fructose levels on grape skin: interference in Lobesia botrana behaviour. Anal Chim Acta. 2004;513(1):351–355.
- Heer D, Sauer U. Identification of furfural as a key toxin in lignocellulosic hydrolysates and evolution of a tolerant yeast strain. Microb. Biotechnol. 2008;1(6):497–506.
- Mohapatra S, Maity S, Dash HR, Das S, Pattnaik S, Rath CC, Samantaray Det al. Bacillus and biopolymer: prospects and challenges. Biochem Biophys Rep. 2017;12:206–213.
- Tsang TK, Roberson RW, Vermaas WFJ. Polyhydroxybutyrate particles in Synechocystis sp. PCC 6803: facts and fiction. Photosynth Res. 2013;118(1–2):37–49.
- Bhagowati P, Pradhan S, Dash HR, et al. Production, optimization and characterization of polyhydroxybutyrate, a biodegradable plastic by Bacillus spp. Biosci. Biotechnol. Biochem. 2015;79(9):1454–1463.
- Valappil SP, Misra SK, Boccaccini AR, Keshavarz T, Bucke C, Roy I. Large-scale production and efficient recovery of PHB with desirable material properties, from the newly characterised Bacillus cereus SPV. J. Biotechnol. 2007;132(3):251–258.

