Abstract
Background
Tuberculosis (TB) poses a significant risk to people with HIV (PWH), with heightened incidence and prevalence rates, especially in countries with a high TB burden. This study assesses the prevalence and incidence rates of TB among PWH during the COVID-19 pandemic, and on treatment outcomes in TB-HIV co-infections.
Methods
A retrospective study was conducted at Suddhavej Hospital, Faculty of Medicine, Mahasarakham University, Maha Sarakham, Thailand, from January 2020 to September 2023, involving newly diagnosed adult PWH. Data were collected on TB prevalence and incidence rates, with TB cases categorized as definite or possible. The primary outcomes were TB prevalence and incidence rates per 100,000 person-years of follow-up.
Results
Among 171 newly diagnosed PWH, the prevalence of TB was 5.85%, with an incidence rate of 4,568.71 per 100,000 person-years. All but one TB cases were diagnosed before antiretroviral therapy (ART) initiation. There was no incident TB during the follow-up period during ART. Nearly half of the TB cases required therapeutic trials without microbiological confirmation.
Conclusions
The study revealed a high prevalence and incidence rate of TB among PWH during the COVID-19 pandemic, comparable to pre-pandemic rates in Thailand. The findings highlight the necessity of comprehensive TB screening prior to ART initiation and the cautious implementation of universal TB preventive therapy. The use of molecular diagnostics, in addition to symptom screening, can enhance TB diagnosis among PWH, though accessibility remains an issue in many regions.
Introduction
Tuberculosis (TB) is a disease caused by Mycobacterium tuberculosis. It is mostly contracted through inhalation of infectious bacilli, leading to infection of the alveolar macrophages which then spread throughout the body [Citation1]. With a functional immune system, the bacilli can either be eliminated, remain dormant in monocytes or macrophages, or are controlled within an asymptomatic granuloma, which may reactivate following immunosuppression [Citation1]. The annual risk of people living with HIV (PWH) developing TB after M. tuberculosis infection is 5-10%. However, this risk can be over 20-fold higher compared to the general population in people with advanced HIV disease prior to antiretroviral therapy (ART) [Citation2,Citation3]. PWH are also at a heightened risk of morbidity as TB can stimulate HIV-1 replication, accelerating the progression of advanced HIV and increasing the risks for other opportunistic infections [Citation3,Citation4] as well as overall mortality [Citation5]. In the era of ART, the mortality rate of PWH with TB has declined, but the risk of TB development, prevalence, and incidence remains at least 4-fold higher than in the general population [Citation3], especially in countries with a high prevalence of TB [Citation5–13]. This might even be an underestimate, as post-mortem examinations among PWH have revealed many undiagnosed TB cases [Citation14]. In Thailand, before the COVID-19 pandemic, large multicentric retrospective cohort studies showed an incident TB rate among PWH of 466-750 per 100,000 person-years of follow-up (PYFU), compared to 150 per 100,000 person-years in 2019 for the general population [Citation5,Citation9,Citation10]. These high numbers persist despite the initiation of universal ART and more accessible care, thanks to the universal coverage policy endorsed by the Thai government.
During the COVID-19 pandemic, there was a decline in the prevalence and incidence of many respiratory viruses, including influenza, respiratory syncytial virus, and other coronaviruses [Citation15,Citation16]. Measures such as community lockdowns, universal masking, and strict hand hygiene compliance have been cited as reasons for this reduction [Citation5,Citation15,Citation16]. The World Health Organization (WHO) reported that strict lockdowns resulted in a 50% reduction in TB transmission, with mask-wearing also playing a role, although its exact impact remains unknown [Citation5]. Moreover, reports highlighted a drop in TB case notifications in 30 high TB-burden countries between 2019 and 2021 [Citation5]. Thailand saw a near 20% decrease in TB case notifications in 2021 compared to 2019 [Citation5]. Paradoxically, during the same period, there was an increase in TB-related deaths among HIV-negative individuals, reaching an estimated 1.4 million – an increase to 2017 levels after years of decline [Citation5]. Most of these increases were reported in countries severely affected by COVID-19, such as India, Indonesia, Myanmar, and the Philippines [Citation5,Citation17]. The surmise is that limited access to care as well as healthcare resource constraints during the pandemic might have disrupted and delayed TB patient care, leading to increased mortality despite fewer case notifications [Citation5]. Another adverse effect is ongoing TB transmission in communities due to undiagnosed cases, evident from the rise in case notifications after 2021 in most high TB-prevalence countries as they started to recover from the pandemic [Citation5].
This study aims to evaluate the prevalence and incidence rates of TB among PWH at our center during the COVID-19 pandemic, and examine the outcomes of treatment concerning TB-HIV co-infection during this period.
Materials and methods
Study design, setting, and data collection
This is a retrospective study conducted at Suddhavej Hospital, Faculty of Medicine, Mahasarakham University, Maha Sarakham, Thailand, from 1 January 2020 to 1 September 2023. The hospital functions as a secondary-care facility with a 140-bed capacity, catering to a nearby community of at least 60,000, which includes all campuses of Mahasarakham University, Maha Sarakham, Thailand. Patients were included if they were adults aged 18 years and above with a positive 4th-generation human immunodeficiency virus antigen/antibody test (HIV Ag/Ab) during the study period. Exclusion criteria encompassed 1) previously diagnosed PWH referred to our hospital, 2) newly diagnosed PWH referred out before completing one year of follow-up, 3) newly diagnosed PWH who were lost to follow-up for more than a year and completed less than one year of follow-up, 4) deaths not attributable to TB during the follow-up, and 5) cases with inadequate data for review. We employed an ECLIA for HIV Ag/Ab testing. All individuals testing positive for HIV Ag/Ab underwent confirmation for HIV infection as per the Thailand National Guidelines on HIV/AIDS Treatment and Prevention 2021/2022 [Citation18]. The computer program was used to determine the date of initial ART based on individual hospital numbers. The follow-up duration was calculated from the day of ART initiation to the last day of follow-up.
All newly diagnosed PWH underwent chest radiography screening. Only those clinically suspected of having pulmonary TB underwent tests for sputum acid-fast bacilli nucleic acid (using multiplex real-time polymerase chain reaction (PCR), specifically the Anyplex™ II MTB/MDR Detection (Seegene, Korea) or the Xpert® MTB/RIF assay (Cepheid, Sunnyvale, CA), based on availability), and mycobacterial culture sent to the central lab of Department of Disease Control, Ministry of Public Health. No TB preventive therapy was deployed in our center during the study period. All cases in the clinic were followed up at least once in a 4-week period after ART initiation and 3-6 months thereafter. Symptoms of TB were automatically screened as part of the isolation precaution protocol for all cases at our hospital. Additional tests for TB will be done at the physician’s discretion.
To identify TB cases among the newly diagnosed PWH, after applying the inclusion and exclusion criteria we 1) matched all cases with the International Classification of Diseases, Tenth Revision (ICD-10) codes for TB diagnosis and related conditions, 2) matched all cases with any anti-TB drugs listed in our hospital system received during the study period, and 3) reviewed for TB in cases that matched either 1) or 2). Since our center was not certified to handle multidrug-resistant TB (MDR-TB), we excluded the MDR regimen from the matching criteria.
We categorized TB into two groups: definite and possible. Definite TB cases had to test positive for M. tuberculosis complex from at least one method, either through molecular diagnostics (Seegene® or Xpert® MTB/RIF) or mycobacterial culture from a clinical specimen. Possible TB cases were those with symptoms or signs compatible with TB and who had undergone a complete anti-TB therapy course with noted clinical response either by the attending physician or radiography. All data were sourced from our computer-based program (HosXP) and medical records.
Outcome
The primary outcome of the study was the prevalence of TB at baseline (before and at ART initiation), the prevalence of TB after ART initiation, and the TB incidence rate, which were expressed as percentages and a rate per 100,000 PYFU, respectively. Prevalence of TB is calculated by the number of TB cases per all included cases, while TB incidence rate is calculated as the number of TB cases divided by the number of participants multiplied by the time of follow-up in years (expressed as 100,000 PYFU). The prevalence of TB is calculated for 2 periods: at baseline and following the start of ART treatment.
Sample size calculation
Based on a previous study [Citation19] and a web-based calculator [Citation20], our required sample size was determined to be 98. This accounts for a 5% prevalence of TB among PWH in Thailand [Citation10], 76% sensitivity and 100% specificity of the molecular test (Xpert® MTB/RIF) [Citation21], and a precision value of 0.05, providing a 95% confidence interval.
Statistical analysis
Categorical data are presented as frequencies and percentages, and were analyzed using the chi-square test, or Fisher’s exact test when >20% of expected cell counts were <5. Continuous data are represented as medians and interquartile ranges (IQR), and were analyzed using the Mann-Whitney test. Statistical evaluations were performed with SPSS 23 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA). We used a Bayesian one-sample test to determine the confidence intervals (95% CI) for prevalence. A P-value of ≤0.05 (two-sided) was considered significant.
Ethical approval
The Mahasarakham University’s ethics committee granted an exemption for informed consent for research involving human subjects (No.434-469/66).
Results
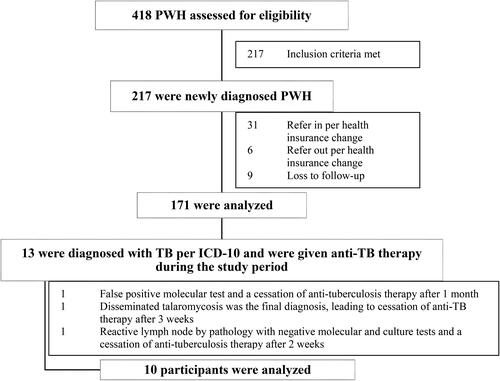
During the study period, there were 217 PWH. Of these, 171 were newly diagnosed PWH who met the inclusion criteria and were thus analyzed further (). The median age was 23 years (IQR, 22-26), with 96.5% being male. The median follow-up duration was 1.28 years (IQR, 0.63-2.25). None had a history of TB. Baseline characteristics can be found in .
Table 1. Baseline characteristics of the studied population.
Prevalence of TB and TB incidence rate
The prevalence of TB at baseline was 5.85% (95% CI 2.27-9.42%). The incidence rate was 4,568.71 (95% CI 2,372.83-7,952.48) per 100,000 person-years. There were 10 TB cases, of which nine were diagnosed before ART initiation (one case was administered with anti-TB therapy for potential TB lymphadenitis at the time of ART initiation). No cases of TB were observed after ART initiation, corresponding to a 0% prevalence of TB following the start of ART treatment.
Characteristics of TB
Definite TB accounted for 40% (4/10) and possible TB for 60% (6/10). Pulmonary TB constituted 60% (6/10), with three cases (3/6 of pulmonary TB) having probable concurrent TB lymphadenitis identified by computed tomography. The other four cases (4/10) were possible extra-pulmonary TB, including two lymphadenitis, one spondylodiscitis, and one meningitis. None had a previous history of TB. Of the definite TB cases, there was one instance each of 1) smear-negative pulmonary and pleural TB, 2) TB spondylodiscitis, 3) smear-positive pulmonary TB, and 4) smear-negative pulmonary TB with lymphadenitis. Every case tested positive either with Anyplex™ II MTB/MDR Detection or Xpert® MTB/RIF, with the spondylodiscitis case also confirmed by positive culture. No cases showed detectable resistance to isoniazid or rifampicin.
Regarding possible TB, two cases had TB lymphadenitis (one exhibited granuloma from pathology but was negative for both the molecular test (Anyplex™ II MTB/MDR Detection) and mycobacterial culture; the other did not undergo a diagnostic workup and was given a therapeutic trial of anti-TB therapy). Another three were diagnosed with pulmonary TB (two with lymphadenitis). They presented a typical radiographic pattern of upper lung infiltrates but tested negative for both molecular tests (one sputum sample for Anyplex™ II MTB/MDR Detection, one for Xpert® MTB/RIF, and one for both Anyplex™ II MTB/MDR Detection and Xpert® MTB/RIF) and mycobacterial culture (tested in only one case). The final case was potential TB meningitis, presenting with subacute lymphocytic meningitis and hypoglycorrhachia (CSF tested negative for Anyplex™ II MTB/MDR Detection and mycobacterial culture), which responded to anti-TB therapy without the need for corticosteroids. All cases completed anti-TB therapy without recurrence at the last follow-up. There were no recorded instances of paradoxical immune reconstitution inflammatory syndrome (IRIS) or corticosteroid prescriptions during the follow-up.
The period between the start of anti-TB therapy and ART initiation was 19 days (IQR, 13.75-38.25). The median CD4 count was 340 cells/mm³ (IQR, 89.5-612.25) and the median percentage of individuals with TB was 21.5% (IQR, 5.4-28.9%). No significant difference was found in terms of CD4 count and percentage between PWH with TB and those without ().
Discussion
This study is the most recent epidemiological assessment of TB prevalence and incidence among PWH in Thailand during the COVID-19 pandemic. Our findings indicate a high prevalence and incidence rate, primarily in young male PWH residing in the urban community of Maha Sarakham province and the Mahasarakham University campus. Actions taken during COVID-19, such as universal masking, physical distancing, rigorous community lockdowns, and the transition to online education, were anticipated to reduce the transmission rates of respiratory pathogens, including TB [Citation5]. Nevertheless, we identified TB incidence and prevalence rates comparable to those in a major Thai cohort before the COVID-19 pandemic [Citation10]. However, in our cohort, we observed that all incident TB cases occurred either before (90%, 9/10) or at the time of ART initiation (10%, 1/10). It is noteworthy that the previous cohort aimed at the incidence after ART initiation and excluded participants with TB at baseline either before or at ART initiation [Citation10]. The previous Thai cohort found the highest TB incidence rate during the initial three months of ART [Citation10]. This contrasts with our findings of having no incident TB after ART initiation. The incident TB early after ART initiation was consistent in other nations [Citation22,Citation23]. Another Thai cohort excluding TB at baseline and incident TB during the first 3 months of ART initiation demonstrated a prevalence of around 1.5% during the excluded period [Citation9]. This suggests that the incident TB early after ART initiation is mostly part of unmasking IRIS, prompting a critical requirement for comprehensive TB screening prior to ART initiation. TB preventive therapy (TPT) has been shown to reduce mortality in countries with high TB burdens and limited TB diagnostic resources [Citation24]. However, the universal application of TPT for newly diagnosed PWH (recommended by both the WHO HIV guidelines [Citation25] and the Thai Clinical Practice Guideline on Tuberculosis Preventive Treatment 2023 [Citation26]) should be cautiously implemented, because of risks of evolving resistant TB from treating undetected TB with TPT regimens and unmasking IRIS [Citation9,Citation10,Citation22,Citation23,Citation27].
Given our study’s limited follow-up duration, we could not identify any incident TB among PWH while on ART. Two previous cohorts in Thailand reported high TB incidence rates after extended ART durations. The incident TB mostly occurred among individuals with CD4 < 50 cells/mm³ with a cumulative incidence of 20% in 7 years; in contrast another study found CD4 > 350 cells/mm³ having a cumulative incidence of 1.2% in 7 years and only 0.2% in the first year [Citation9]. This finding is concordant with other cohorts where a low CD4 count is a significant risk factor for incident TB [Citation6–13], especially when the CD4 count is <100 cells/mm³, which increases the risk fourfold [Citation10]. HIV viremia, even when on ART, also poses a risk - particularly during the early stages of ART initiation, potentially as part of unmasking IRIS [Citation10,Citation22,Citation23].
With respect to TB manifestation, all cases presented clinical symptoms consistent with TB. Nearly half (40%) underwent therapeutic trials without microbiological confirmation. This observation aligns with a prior cohort where 47% of PWH with TB required therapeutic trials [Citation9]. Such scenarios are significant in resource-limited countries where investigative capabilities are constrained. Beyond the immediate availability of a multidisciplinary team to secure adequate clinical specimens, meta-analyses indicate that adding molecular diagnostics to the initial WHO-recommended four-symptom screen (W4SS) enhances the sensitivity of TB diagnosis among PWH, increasing to 73% with W4SS plus Xpert® MTB/RIF Ultra compared to 57% with W4SS alone [Citation28]. Regrettably, these tools are not universally accessible, especially in many secondary or primary care hospitals. A Thai cost-effectiveness analysis suggests an increased in quality-adjusted life year (QALY) when adding Xpert® MTB/RIF to the standard sputum microscopy and culture, yielding an increase of up to 3,940 baht per QALY for pulmonary TB [Citation29]. Although the National Tuberculosis Control Programme Guideline of Thailand 2021 advocates for universal molecular testing for those suspected of having pulmonary TB [Citation30], the distribution and implementation of readily-available molecular diagnostics remain limited in certain regions of Thailand and across many Southeast Asian countries [Citation31].
Our study presents some limitations. Being retrospective in nature, certain data points and confounding factors might not have been fully addressed. The interval between HIV acquisition and the subsequent HIV diagnosis and ART initiation is the period with the highest risk for TB acquisition and ensuing disease manifestation. We were unable to acquire data concerning the duration between HIV infection and the commencement of ART. This might lead to an overestimation of the TB incidence rate due to the shorter follow-up duration considered in the calculations.
Conclusion
During the COVID-19 pandemic, there was a high prevalence and incidence rate of PWH with TB, comparable to those seen in pre-COVID-19 Thai cohorts. In most situations, TB can be diagnosed or, if inconclusive, a therapeutic trial can be initiated before starting ART to prevent the emergence of unmasking IRIS. The implementation of TPT should be carefully considered, ensuring TB exclusion using W4SS complemented with molecular diagnostics.
Acknowledgments
We extend our gratitude to the molecular laboratory technicians for their unwavering dedication and hard work throughout the COVID-19 pandemic.
Disclosure statement
The authors have no conflicts of interest to disclose.
Additional information
Funding
References
- Pai M, Behr MA, Dowdy D, et al. Tuberculosis. Nat Rev Dis Primers. 2016;2:16076.
- Glynn JR. Resurgence of tuberculosis and the impact of HIV infection. Br Med Bull. 1998;54(3):579–593.
- Bell LCK, Noursadeghi M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat Rev Microbiol. 2018; 16(2):80–90.
- Mlcochova P, Sutherland KA, Watters SA, et al. A G1-like state allows HIV-1 to bypass SAMHD1 restriction in macrophages. Embo J. 2017;36(5):604–616. 1
- Global tuberculosis report 2022. Geneva: World Health organization; 2022. [Internet]. [Cited 2023 Oct 15]. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports.
- Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359(9323):2059–2064.
- Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19(18):2109–2116.
- Kabali C, Mtei L, Brooks DR, et al. Increased mortality associated with treated active tuberculosis in HIV-infected adults in Tanzania. Tuberculosis (Edinb). 2013;93(4):461–466.
- Gatechompol S, Sophonphan J, Ubolyam S, et al. Incidence and factors associated with active tuberculosis among people living with HIV after long-term antiretroviral therapy in Thailand: a competing risk model. BMC Infect Dis. 2022;22(1):346.
- Suwanpimolkul G, Gatechompol S, Kawkitinarong K, et al. Incidence of active tuberculosis among people living with HIV receiving long-term antiretroviral therapy in high TB/HIV burden settings in Thailand: implication for tuberculosis preventive therapy. J Int AIDS Soc. 2022;25(4):e25900.
- Dravid A, Natarajan K, Medisetty M, et al. Incidence of tuberculosis among HIV infected individuals on long term antiretroviral therapy in private healthcare sector in pune, Western India. BMC Infect Dis. 2019;19(1):714.
- Girardi E, Sabin CA, d‘Arminio Monforte A, et al. Antiretroviral therapy cohort collaboration. Incidence of tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis. 2005;41(12):1772–1782.
- Liu E, Makubi A, Drain P, et al. Tuberculosis incidence rate and risk factors among HIV-infected adults with access to antiretroviral therapy. AIDS. 2015;29(11):1391–1399.
- Gupta RK, Lucas SB, Fielding KL, et al. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS. 2015;29(15):1987–2002.
- Principi N, Autore G, Ramundo G, et al. Epidemiology of respiratory infections during the COVID-19 pandemic. Viruses. 2023;15(5):1160.
- Chow EJ, Uyeki TM, Chu HY. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat Rev Microbiol. 2023;21(3):195–210.
- Emery JC, Richards AS, Dale KD, et al. Self-clearance of Mycobacterium tuberculosis infection: implications for lifetime risk and population at-risk of tuberculosis disease. Proc Biol Sci. 2021;288(1943):20201635.
- แนวทางการตรวจรักษาและป้องกันการติดเชื้อเอชไอวี ประเทศไทย ปี 2564/2565. (Thailand National Guidelines on HIV/AIDS Treatment and Prevention 2021/2022) [Internet]. [Cited 2023 October 20]. Available from: https://www.thaiaidssociety.org/thailand-hiv-aids-guideline/.
- Humphry RW, Cameron A, Gunn GJ. A practical approach to calculate sample size for herd prevalence surveys. Prev Vet Med. 2004;65(3-4):173–188.
- Sample size to estimate a true prevalence with an imperfect test. [Internet]. [Cited 2023 October 1]. Available from: https://epitools.ausvet.com.au/prevalencess
- Saeed M, Hussain S, Riaz S, et al. GeneXpert technology for the diagnosis of HIV-associated tuberculosis: is scale-up worth it? Open Life Sci. 2020;15(1):458–465.
- Hermans SM, Kiragga AN, Schaefer P, et al. Incident tuberculosis during antiretroviral therapy contributes to suboptimal immune reconstitution in a large urban HIV clinic in Sub-Saharan Africa. PLoS One. 2010;5(5):e10527.
- Haddow LJ, Moosa MY, Mosam A, et al. Incidence, clinical spectrum, risk factors and impact of HIV-associated immune reconstitution inflammatory syndrome in South Africa. PLoS One. 2012;7(11):e40623.
- Danel C, Moh R, Gabillard D, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–822.
- Consolidated guidelines on HIV prevention, testing. treatment, service delivery and monitoring: recommendations for a public health approach. Geneva: World Health Organization; 2021 [Internet]. [Cited 2023 October 15]. Available from: https://www.who.int/publications/i/item/9789240031593.
- Clinical Practice Guideline Tuberculosis Preventive Treatment. 2023Internet]. [Cited 2023 October 15]. Available from: http://www.tbthailand.org/download/Manual/แนวทางเวชปฏิบัติ%20วัณโรคระยะแฝง%. 202566%20CPG-TPT%202023.pdf.
- Nyangu S, Kagujje M, Mwaba I, et al. Breakthrough TB among people living with HIV on TB preventive therapy. Public Health Action. 2022;12(4):153–158.
- Dhana A, Hamada Y, Kengne AP, et al. Tuberculosis screening among ambulatory people living with HIV: a systematic review and individual participant data meta-analysis. Lancet Infect Dis. 2022;22(4):507–518.
- Chitpim N, Jittikoon J, Udomsinprasert W, et al. Cost-Utility analysis of molecular testing for tuberculosis diagnosis in suspected pulmonary tuberculosis in Thailand. Clinicoecon Outcomes Res. 2022;14:61–73.
- National Tuberculosis Control Programme Guideline of Thailand. 2021 [Internet]. [Cited 2023 October 15]. Available from: https://ddc.moph.go.th/uploads/publish/1253220220330064337.pdf.
- Bhatia V, Srivastava R, Reddy KS, et al. Ending TB in Southeast Asia: current resources are not enough. BMJ Glob Health. 2020;5(3):e002073.