 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The properties of metal(loid)s in the environment are mainly determined by their chemical forms. The chemical forms of metal(loid)s affect their possible chemical and biochemical reactions, toxicity, mobility, and bioavailability in the environment. Sequential chemical extraction (SCE) has been successfully used to clarify the chemical forms of soil metal(loid)s. However, quite a few SCE procedures have been applied for speciation analysis of plant samples; there is no systematic discussion in such a field. The current review deals with the SCE of plant metal(loid)s, and compares the extraction procedures and the extractants in different SCE methods. It has been found that some chemical forms are unreliable for plant SCE analyses. The forms can be the phosphates or oxalates of some specific metals in plants, such as gadolinium or chromium, which cannot be fully extracted by the designated extractants, 2% acetic acid, or 0.6 M/L HCl. Therefore, SCE methods for the non-bivalent metal(loid)s have been emphasized in this work. Moreover, the application status, development trends, limitations, and future directions for SCE methods of plant metal(loid)s have been discussed.
Graphical abstract
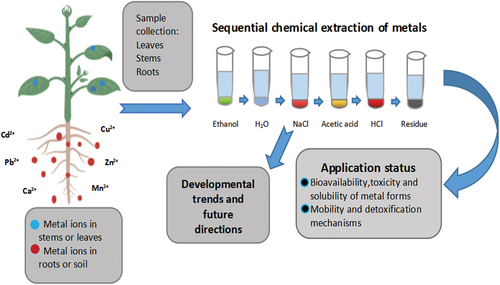
1. Introduction
Over abundance of toxic metal(loid)s in the environment causes metal pollution, which adversely affects the quality of production and life. The environmental behaviour of these metal(loid)s is highly dependent on the chemical forms in which they exist. The binding between metal(loid)s and solid components of the environmental matrix greatly influences the mobility, bioavailability, and toxicity of metal(loid)s. Consequently, there is considerable interest in understanding the natural chemical forms of metals and element-solid phase associations in polluted systems, particularly in plants. The polluted biomass is then treated and disposed of based on its chemical characteristics in order to enable large-scale phytoremediation and limit metal contamination in ecosystems [Citation1,Citation2]. Speciation analysis provides information about the identification of forms of metals based on their molecule or compound structure, electronic or oxidation state, and composition of isotopes. The forms of metals continuously migrate into nature and transform into different forms. It creates complexity in natural systems, and the precise speciation of elements becomes difficult [Citation1]. Passive or active uptake and accumulation of metal(loid)s in plants is a matter of concern because metals are non-biodegradable [Citation3,Citation4] and their speciation analysis is a critical area of research that bridges the gap between environmental science, ecology, and human health. The use of analysis methodologies can be difficult since the toxic or beneficial nature of metals is not always indicated by their organic or inorganic form [Citation5,Citation6] or the oxidative species [Citation7]. Speciation analysis of metal(loid)s can be achieved by direct determination (spectral analyses) by use of analytical instrumental techniques to such as extended X-ray absorption fine structure (EXAFS) spectroscopy, synchrotron-based X-ray radiation fluorescence (SXRF) [Citation8,Citation9], particle-induced X-ray emission (PIXE) [Citation9], X-ray absorption near edge structure (XANES) [Citation10], synchrotron-based X-ray absorption spectroscopy (XAS), nuclear magnetic resonance (NMR) spectroscopy [Citation11–13]. However, these techniques provided researchers with powerful tools for identifying and quantifying specific metal species [Citation11–13], but not widely employed, and their low detection range limits their use to highly polluted samples [Citation2,Citation14].
Thus, the limiting factors of direct analysis techniques draw research interest towards indirect determination methods of metal speciation in plants. One method that has gained prominence in this endeavour is sequential chemical extraction (SCE), which has emerged as a valuable tool for speciation analysis, allowing researchers to fractionate metal(loid)s species from different plant compartments and shed light on their mobility and bioavailability. Sequential chemical extraction (SCE) employed a fractional scheme, in which a systematic series of reagents were introduced to selectively extract various metal species from complex plant matrices. Then, the separation technique is used to separate forms of metals, and ultimately, quantitative analysis is performed for the total metal content [Citation1]. Sequential chemical extraction was considered as an example of operational analysis [Citation2] that dates back to 1991, when Ure [Citation15] defines chemical speciation as ‘the active process of identification and quantification of different defined species forms or phases in which an element occurs in a material’ or the description of the amounts and kinds of species, forms, or phases present in the material’. He categorizes speciation into three classes: a) Classic speciation: speciation refers to specific chemical compounds or oxidation states of elements, e.g. cerussite (PbCO3) vs. pyromorphite [Pb5(PO4)3Cl]; CrIII vs. CrVI [Citation2]. b) Functional speciation: speciation refers to the identified role or actions of an element. c) Operational speciation: speciation refers to the condition in which the reagent employed to extract the sample defines the species.
According to IUPAC report [Citation16] on analysis of trace elements, ‘despite some drawbacks, the sequential extraction method can provide a valuable tool to distinguish among trace elements fractions of different solubility related to mineralogical phases. The understanding of the speciation of trace elements in solid samples is still rather unsatisfactory because the appropriate techniques are only operationally defined’. Thus, this statement indicates complications in the use of the sequential extraction method, such as it has certain limitations, including pH dependency of method and lack of selectivity for particular species [Citation17].
For the analysis of water and soil sediments, two standard SCE procedures have been set up: a five-step Tessier method [Citation18] and improved three-step European Community Bureau of Reference (BCR) method [Citation19,Citation20], respectively. Due to some commonality of speciation analysis in plants and soils, the principle and goal of the SCE method for plants can be glimpsed by comparing the Tessier method and the European Community Bureau of Reference (BCR) method. Thus, some adaptations have been proposed for various matrices, including plants, i.e. lichens [Citation21]. However, so far, many methods have been reported, but there is no standard SCE method for plant samples. The reason is the difference in the chemical composition of the soil and plant samples [Citation22]. Metals in plants are present in complicated forms, and complexity increases with plant diversity and the geological distribution of metals. Therefore, it is not easy to establish a unified process for determining metals in plants [Citation23,Citation24]. So, for the determination of metal forms in plants, the current more commonly used method is the six-step sequential chemical extraction method, proposed by Xu J. et al. [Citation25] and others with reference to the seventies and eighties Yasuda O. et al. [Citation26] and Koji A. et al. [Citation27]. The plant six-step SCE method was initially employed to determine the chemical forms of metals, including cadmium, lead, copper, and some other bivalent metals, in plants. By this method, inorganic Cd including nitrate, chloride, and amino-phenol cadmium (F1) is extracted with 80% ethanol, soluble Cd-organic acid complexes and Cd(H2PO4)2 (F2) are extracted with deionized water, Cd integrated with pectates and proteins (F3) were extracted with 1 M sodium chloride solution, insoluble CdHPO4 and Cd3(PO4)2 and other Cd phosphate complexes (F4) are extracted with 2% acetic acid (HAc), cadmium oxalate (F5) is extracted with 0.6 M HCl, and Cd in residues (F6) is extracted with HNO3-HClO4 (3:1, v/v). Since the properties, valances, and ion radii of the metals in phosphates are different from each other, the solubility of metal phosphates is also different. Therefore, this plant SCE method that is fit for bivalent metal extraction perhaps might not fit for all kinds of plant metal(loid)s. The criteria for determining the form of metal oxalate in plants are similar [Citation25]. In SCE operation, each chemical form of metal (loid)s is actually related to extractant agents, and the rate of extraction depends on the strength of the metal-solid association [Citation2].
At present, the SCE method has been widely used for the extraction of various metals from a variety of plants. Most of the selected plant samples are roots, stems, leaves, buds, or roughly divided into aboveground and underground parts. However, depending on the research requirements of plants, sequential chemical extraction needs to be flawless [Citation23]. The speciation analysis uncovered the uptake and excessive accumulation of metals in plants, hence encouraging the development of phytoremediation technology [Citation1]. It is evident from the literature that for desirable results, the SCE method has undergone many modifications throughout the past years, such as the use of fewer steps to lessen the operation time [Citation28], use of less harsh reagents and the addition of extractants rather than replacing them in each step, with tiny aliquots left aside for analysis [Citation17,Citation21]. Except for the worldwide usage of the SCE method, some shortcomings exist: (a) redistribution of metalloids among phases during extraction; (b) non-selectivity for target phases; (c) incomplete extraction; and (d) precipitation of new phases during extraction [Citation2,Citation19,Citation25], (e) poor reproducibility of research results; (f) irregular operation procedures, etc. [Citation1]. In view of the above shortcomings, the SCE approach for plants requires an established standard method to unravel the complexities of metal interactions within plant ecosystems and the development of phytoremediation techniques.
This review aimed to systematically summarize the methodologies of sequential chemical extraction in plants for different metals (bivalent or non-bivalent), according to their solubility products. At the same time, it highlights the potential of sequential extraction to revolutionize our understanding of metal(loid)s speciation in plants. In addition to the development trend, application status and limitations are also analysed. By navigating challenges regarding the SCE method, recent progress in plant-metal systems and future prospects have been discussed.
2. Historical background and recent progress of sequential chemical extraction (SCE)
In the early stages, single-step extraction was used to estimate the abundance of different species. However, these approaches did not accurately capture the complexity of element speciation. So in the late 1970s and early 1980s, researchers designed multi-step procedures that involved subjecting samples to different reagents in a specific order, aiming to selectively dissolve different chemical species. Initially, sequential chemical extraction was employed to separate potential toxic elements (PTEs) bound to marine sediments. Since the Tessier method gained popularity, researchers have continued to transform this method for different substrates. Sequential extraction is based on the selection of suitable reagents and their ability to bind with targeted components of the sample [Citation18]. Furthermore, the European Community Bureau of Reference (BCR) perform research and established a standard method that has been widely employed to find chemical forms of metals in soil and sediments [Citation1]. According to the literature, the five-step Tessier method [Citation18] and the revised three-step BCR (European Community Bureau of Reference) [Citation19] are considered two successful standard SCE methods, employed on soil and water sediments.
Many developments have been made to improve sequential extraction procedures, but there is still a need for in-depth studies on the forms of metals in various environmental substrates [Citation1], especially in plants. The reason is the different composition of the soil and plant samples. After plant absorption, metals change into organic forms, and their physiochemical properties have changed. So, speciation analysis schemes not only adjust according to the substrates but also the physiochemical properties of the metal(loid)s to be analyzed [Citation23]. On the other hand, the wide variety of habitats and plant species in nature also makes it difficult to differentiate metals in plants [Citation24]. However, so far, there is neither a standard SCE method nor specific terminology that denotes the SCE method for plants. Scientists from Japan extracts forms of metals from plants for the first time [Citation1]. In 1970, Tian employed SCE to extract forms of calcium metal from plants, while ethanol, deionized water, NaCl, acetic acid, and hydrochloric acid were applied as extraction agents [Citation29]. Later on, the above method was followed by a number of researchers to extract metal forms from plants.
According to , in the last 18 years, the number of publications on metal speciation in plants has increased and gained attention from readers due to its importance in metal speciation. After introducing the phytoremediation technology for the removal of heavy metals from soil, the researchers paid attention to studying the chemical forms of metals in plants. The most common metals in plants are cadmium (Cd), lead (Pb), zinc (Zn), copper (Cu), calcium (Ca), and manganese (Mn). In the current studies, the six-step SCE method is most commonly used for these metals [Citation30–33], as shown in , but in order to reduce procedure time and errors, some researchers proposed methods with fewer steps. In 2012, Wu Huimei et al. proposed a two-step SCE method for the forms of Cd, Zn, Cu, Ca, and Mn in roots, stems, leaves, and fruit samples of tea and cucumbers, i.e. ethanol extraction state, hydrochloric acid extraction state, and residual state of the metals [Citation34]. Perronnet et al. used water immersion and calcium chloride to extract plant cadmium and zinc [Citation35]. Xue et al. obtained the ethanol extraction state, hydrochloric acid extraction state, and residual state of cadmium [Citation36]. Some other SCE (3–6 steps) methods are shown in . These methods are used for the speciation of metals (Cd, Pb, Zn, Cu, Ca, and Mn) in various plant species (details in ). In the present literature, chemical forms of cadmium (Cd) in plants are widely studied through 6-step SCE, and the plants for study are rice [Citation144], barley [Citation30], peppers [Citation145], tobacco [Citation97], and algae [Citation35]. In some plants, the 3-step and 4-step SCE methods are used for cadmium speciation. The plants for copper (Cu) speciation include crops [Citation101], tea [Citation34] and lettuce [Citation99]. The plants for zinc (Zn) speciation include tea [Citation34], corn [Citation146] and rice [Citation147]. Hu et al. proposed to use 80% ethanol and 2% acetic acid, deionized water, 0.6 mol/L hydrochloric acid, and 1 mol/L sodium chloride solution for the analysis of lead (Pb) [Citation148]. The plants for lead (Pb) speciation include crops [Citation25], tea [Citation34] and Bauhinia Zeeland [Citation149]. Tang et al. used deionized water, hydrochloric acid (HCl = 1:3), and concentrated nitric acid to obtain soluble free, inorganic, and organic calcium, respectively [Citation129]. For calcium speciation, the plants studied are pear [Citation150] and rice [Citation147]. For manganese speciation, the plants studied include tea [Citation34] and lettuce [Citation37]. Most plant materials used for SCE are roots, stems, leaves, or a certain part of the fruit. The number of citations for this methodology from 1970 to 2023 (Web of Science) indicates that it is well acknowledged for its accuracy. Although many improvements have been made to the experimental process, further work in this field is needed.
Figure 1. Publication in recent years on sequential chemical extraction (SCE) method for speciation analysis of metal(loid)s in plants.

Table 1. 6-step sequential chemical extraction (SCE) method for speciation analysis of metal(loid)s in plants [36–39].
Table 2. Other sequential chemical extraction (SCE) methods for speciation analysis of metal(loid)s in plants.
Table 3. Literature on sequential chemical extraction (SCE) for speciation analysis of cadmium (Cd) in plants.
Table 4. Literature on sequential chemical extraction (SCE) for speciation analysis of lead (Pb), copper (Cu) and zinc (Zn) in plants.
Table 5. Literature on sequential chemical extraction (SCE) for speciation analysis of calcium (Ca) and manganese (Mn) in plants.
3. Scope and significance of sequential chemical extraction
In order to understand the significance of sequential chemical extraction, the most important thing to consider is the presence of metal(loid)s in plants. Sequential chemical extraction helps to understand the migration mechanism, toxicity, and bioavailability of chemical forms of metal(loid)s. For example, studies have found that the chemical form of cadmium in plants is related to its migration, as ethanol-extracted cadmium and water-soluble cadmium have the highest activity [Citation30]. In the genotypes that are relatively resistant to Cd, the NaCl extraction state of Cd is higher and the content of water-soluble Cd is lower. They speculate that this is a possible mechanism for the tolerance to Cd toxicity [Citation39]. Cr is transported and stored in a less toxic chemical form than pure Cr(VI), suggesting that this is a detoxification mechanism for plants. It is proposed to determine the chemical morphology and morphological regulatory processes of specific plant systems to help predict the transfer of pollutants in the agro-food chain [Citation38]. The selection of appropriate methods to analyze the occurrence of metals in plants is of great significance for studying the toxic effects, solubility, migration, transformation mechanisms, and bioavailability of the metals [Citation47]. Sequential chemical extraction is suitable for screening phytoremediation methods, monitoring pollution status and evaluating the phytoremediation effect of contaminated sites.
In view of relevant studies, the annual quantities of plant SCE studies are calculated and plotted in . It is shown that the number of relevant studies of plant SCE has increased dramatically in recent years. Plant SCE studies have become one of the research hotspots, and their importance cannot be concealed by shortcomings. The speciation analysis of metal(loid)s in plants needs more advancement due to the complex plant habitats and diverse chemical forms of metals. Therefore, sequential extraction has great significance for speciation analyses of metal(loid)s in plants and very high scope in the future.
4. Issues and limitations of sequential chemical extraction for plant samples
Although sequential extraction has been frequently used for the speciation of metals in environmental samples, it still does not have proper terminology or abbreviations. A variety of terms and abbreviations are employed for the operation, such as: sequential chemical extraction (SCE), selective sequential dissolution (SSD), selective sequential extraction (SSE), sequential extraction procedure (SEP), sequential extraction scheme (SES), sequential extraction test (SET), chemical continuous extraction (CTE), and short sequential extraction procedure (SSEP). Another issue is the methodology for the SCE operation and data interpretation. It is revealed that the conditions described by the authors are different from those specified by the BCR procedure. So, consistency is important for methodologies and extraction conditions [Citation2]. The SCE operation of many studies is different from the procedure of the popular six-step SCE method. Most scientists make amendments to the SCE method according to their experimental work, so there are a number of procedures employed by scientists. This approach hinders the presentation of a well-organized standard extraction procedure for plants. Generally, since SCE generates large sets of data, the presentation and interpretation of the obtained data in an efficient manner is not an easy task. Bar charts are widely used for data presentation, but if there are too much data in one chart, the meaning of the figure will become less clear. In order to compare the data from different works, the consistency of methodologies and extraction schemes is important. Moreover, apart from the number of applications, sequential extraction also has some limitations, such as confirmation of the combination state and concentration product of different binding states of the elements involved in the method. Therefore, two requirements need to be considered: (1) the corresponding binding state of the element is close to completely dissolved in solution, and (2) the extraction process of the previous step does not affect the extraction of the next step. Additionally, the conditions at each step need to be consistent and reduce errors. The intrinsic weaknesses are (a) redistribution of metalloids among phases during extraction, (b) non-selectivity for target phases, (c) incomplete extraction, and (d) precipitation of new phases during extraction, whereas the extrinsic weaknesses are (e) the analytical method is time-consuming, (f) usually only off-line detection is combined with the SCE method, and (g) the data from the SCE method have not been systematically studied to achieve their proper value or significance [Citation2,Citation19,Citation25]. Measures have been taken to overcome the above limitations. Adjusting the pH value is an important method since the use of extractants and the studied samples themselves change the pH values of the system and change the selectivity of the SCE [Citation14,Citation51]. The SCE involves two types of uncertainties: those from the operators and those from the technologies. The former one includes sample collection, sample pre-treatment, and the dilution of the extractants [Citation23,Citation50], and the latter one includes the detection limits of the instruments for metal analyses. As the number of extraction steps increases, the uncertainty of the extraction is assumed to increase. So, as obtained from the final step of the whole extraction procedure, the data on residual metals inherit all uncertainties from the previous steps and is integrated into the highest uncertainty [Citation110]. Furthermore, the SCE procedure has drawbacks such as alterations in chemical structures and loss of metal samples. Consequently, outcomes are not comparable and the data are not reliable. More importantly, SEC cannot provide the chemical forms of metals [Citation1].
5. Chemical forms of metal(loid)s in soil-plant system
Multiple forms of metal(loid)s are present in the natural soil-plant system, these metal(loid)s continue to migrate and transform. Metal(loid)s have multiple effects on plants caused by elements such as Zn, Cu, Al, Pb, Cd, and As [Citation108]. According to geochemical features, metals commonly present in soils as free cations, hydroxylates, carbonates, bicarbonates, sulfates, chlorides, fulvic salts, and complexes of humic acids. Metals in soil are fractionated into four fractions such as acid-soluble fractions, reducible fractions, oxidizable fractions, and residual fractions. The third fractions in BCR, organic-bonded fraction, persist for a long time and are difficult to be released into the environment [Citation122,Citation127]. Metals can be found in plants in a number of different forms, as shown in , including ions, sulfides, organic acids, and proteins. The speciation of metals in plants depends upon their mechanisms of translocation [Citation40]. The bioavailability and toxicity of Cd in plant roots are different from soil because of biological and biochemical transformation [Citation17,Citation136]. Ueno et al. identified the organic form of Cd in Arabidopsis halleri, from which 85.7% of Cd exists in the free state, 7.7%, 3.2%, and 0.1% of Cd are found in complexes with sulfate, citrate, and histidine, respectively [Citation40]. Schreck et al. identified lead within or on leaves in PbSO2+ , PbO+, and PbCO+ and Pb forms [Citation139]. Organic acids, proteins, polyphenols, and polysaccharides complexes are other forms of manganese other than free metal ions [Citation151,Citation152]. Further information revealed that most of the copper found in spinach leaves was bound to proteins, with only a small amount linked to oils [Citation153]. In the Datura plant, zinc was found to complexed with organic acids, histidine, and phyto-chelate [Citation154].
6. Sequential chemical extraction methods for speciation analysis of metal(loid)s in plants
Based on the difference in physio-chemical properties among different forms of metals, a series of methods were proposed to extract metals by using chemical extractants with enhanced selectivity [Citation155] as shown in . In sequential chemical extraction, as term indicates, a series of extraction agents is sequentially introduced to the same sample to fractionate the total metal content [Citation2]. According to prior research and published data, the six-step SCE method is frequently used and in some studies the four-step method is operated for metal speciation in plants.
6.1. Six-step sequential chemical extraction for metal(loid)s in plants
Chemical forms of metals in plants were successfully determined by the six-step SCE [Citation30–33]. The extraction results clearly characterize the metal fractions and metal forms in solution shown in .
Fraction 1—extracting metals bound to inorganic chelates, nitrates, chlorides, amino acid salts and amino phenols: The plant sample was incubated for 18 h at 30°C with 37.5 ml of 80% ethanol.
Fraction 2—extracting metals bound to organic acid: the residue of the fraction one was used to extract the metal complex with organic acid. The same volume of deionized water was added in residue and extract the water-soluble metal complex.
Fraction 3—extracting metals bound to protein and pectinate: The residue from second fraction was incubated for 18 hours at 30°C with 37.5 ml of 1.0 M/L NaCl and extract the metal complex with protein or pectin.
Fraction 4—extracting metals bound to phosphate: The residue from fraction 3 was soaked for 18 hours at 30°C with 37.5 ml 2% acetic acid and extract insoluble metal phosphate.
Fraction 5—extracting metals bound to oxalate: The residue from fraction 4 was incubated with 37.5 ml of 0.6 M/L HCl for 18 hours at 30°C mainly extracts metal combined with oxalic acid.
Fraction 6—residual metals: The residue from fraction 5 was digested with 5 ml HNO3/HClO4 (3:1 v/v). For determination of metal in residues.
Two grams of frozen plant tissues were cut into 1–2 mm2 small pieces and incubated with 37.5 ml of extraction agent for 18 hours at 30°C. After centrifugation at 5000 × g for 10 minutes, the supernatant of the extraction solution was collected in another tube and again soaked with the same volume of extractants for 2 hours at 30°C. Then, it was again centrifuged, and this step was repeated two times in the next 4 hours. The supernatant of the entire extraction solution was collected, making the total volume 150 ml. The residues of this procedure were used for the extraction of other chemical forms of metal by using the same steps and conditions. The extraction solution was evaporated at 70°C to a constant weight by a rotary evaporator and then digested using 5 ml of HNO3-HClO4 (3:1, v/v). At the end of sequential chemical extraction, HNO3/HClO4 (3:1 v/v) was used for the determination of metal content in residues.
6.2. Four-step sequential chemical extraction for metal(loid)s in plants
An operationally defined method to determine the exchangeable, oxidizable, reducible, and residual percentages is the regularly used four-step method based on the process first created by BCR [Citation156]. The metal load in the plant sample may also be speciated into different fractions using this method. Unfortunately, there have not been many attempts to get information on the chemical formation of plant metals. Majolagbe [Citation157] and colleagues used the four-step SCE approach, BCR, to characterize the metals in medicinal plants. The extractions were completed by the following four steps:
6.2.1. Step-1 exchangeable
In 250 mL flat bottom flask, 1.0 g of ground plant material and 40 mL of 0.1 M acetic acid was added as an aliquot. Then, the flask was shacked for 16 hours at room temperature on flask shaker at 400 rpm. The filtrate was used to determine the total content of the metals in solution.
6.2.2. Step-2 reducible
Twenty milliliters of double distilled deionized water was used to wash the residue from step 1 by shaking for 15 min. The supernatant was removed and washed residual solid plant material was stirred by adding 40 mL of 0.1 M hydroxylamine hydrochloride solution. The 2.0 pH was adjusted with nitric acid and the mixture was shaken for 16 hours at room temperature. The filtered extract was used to measure the concentration of metal in solution.
6.2.3. Step-3 oxidizable
Twenty milliliters of double distilled deionized water was used to wash the residue from step 2, and the supernatant was discarded and washed residue was shaken by adding 10 mL of 8 M hydrogen peroxide solution for 1 hour at room temperature. Then, the solution was placed on water bath at 85°C to evaporate to near dryness. This step was repeated with same conditions in next 1 hour. After evaporation, the residue was soaked with 50 mL of 0.1 M ammonium acetate solution (adjusted to pH 2 with nitric acid). After shaking the filtrate was used to analyse the metal in solution.
6.2.4. Step-4 residual
Twenty milliliters of double distilled deionized water was used to wash the residue from step 3, an aliquot of 5 mL deionized water and 12 mL of aqua-regia solution was added to the residue. Then, the solution was placed on water bath at 85°C to evaporate to near dryness. This step was repeated by adding 8 mL aqua-regia solution and 20 mL of 0.1 M nitric acid in residue. The filtrate was used to analyse the metals (Cr, Mn, Cu, and Zn) in solution.
6.3. Sequential chemical extraction (SCE) data analysis
The data obtained from SCE was analyzed using SPSS software [Citation158–160]. SPSS was used for multivariate ANOVA (one-way analysis of variance and two-way analysis of variance). ANOVA was used to analyze the effects of metal stress, tolerance, interaction effects, and differences among treatments. The least significant difference (LSD) model (p < .05) was used for the multiple comparisons between groups and performed a correlation analysis between the contents of the metal form fractions. Pearson correlation analysis was used to analyze the relationship between soil metals and plant metal concentrations.
7. SCE method for bivalent metals and non-bivalent metals
The SCE protocol can be adjusted according to plant materials and the different metal(loid)s to be analyzed. When 2% HAc is used to dissolve the phosphate-bound state, bivalent metals can be extracted in the liquid phase. However, the non-bivalent metal(loid)s in plants are insoluble in 2% HAc, and metal phosphate precipitation will occur [Citation161], resulting in experimental error. According to , different metal phosphates have different solubility products, and during extraction, different results indicate that solubility products depend on the valency of the metal(loid)s. Thus, the result shows that the SCE protocol used to extract bivalent metal phosphates needs improvement for multivalent metals.
Table 6. Phosphate solubility product of some metal elements.
The cadmium was used as a control for speciation analysis in plants, and the common valence state is bivalent. The cadmium was compared with lead, gadolinium, and chromium. Both Cd and Pb are bivalent, and the valences of Gd and Cr are +3. The chemical formula and solubility products of phosphates for Cd, Pb, Gd, and Cr are Cd3(PO4)2, KSP Cd = 8 × 10˗39 [Citation182], Pb3(PO4)2, KSP Pb = 8 × 10˗43 [Citation183], GdPO4, KSP Gd = 1.4 × 10˗26 [Citation184], and CrPO4, KSP Cr = 2.4 × 10˗23 [Citation45], respectively. Assuming that the activity of each metal in its solution is 1 μM, the maximum phosphate activity α(PO43-) in the Cd, Pb, Gd, or Cr solutions can be calculated as follows:
The difference in phosphate activities concluded that phosphates of Cd and Pb are easily soluble in solution and dissolved in the extractants, but the solubility products of Gd and Cr phosphates are less than those of Cd, so these are still in the solid phase. Therefore, the results of Wang et al. for phosphate forms of Gd, Ce, and Y in the plants obtained from the six-step SCE deserve to be deliberated [Citation63–65].
The elements arsenic (As) and antimony (Sb) are either positive trivalent or pentavalent. It seems that the studies on the chemical forms of Sb in Ficus [Citation185] or As in Eucalyptus [Citation186] by the six-step SCE are not so reliable. It can also not be approved that the six-step SCE was used to study the forms of chromium with the valences of positive trivalent or hexavalent [Citation113,Citation187]. As the SCE method is used to extract Ag from plants, the extractants 1 M NaCl and 0.6 M HCl will cause silver chloride (AgCl2) precipitation and would fail in the speciation analysis of silver in plants. It can be seen that the properties and valences are different, so the usage of traditional six-step SCE for the forms of non-bivalent metals should be re-evaluated. Some scholars have tried the new reagents and SCE methods for the formation of non-bivalent metals in plants. To investigate the aluminium (Al) speciation in cabbage and radish leaves, deionized water, hydrochloric acid (HCl = 1:3), and concentrated nitric acid were used, and as a result, the soluble free, inorganic, organic aluminum was obtained [Citation129], as shown in . Pan et al. performed a fractional analysis of aluminum in maize roots using reagents of 0.1 M, 0.05 M EDTANa2, 0.01 M HCl, and concentrated nitric acid to extract the exchangeable aluminum, complexed aluminum, precipitated aluminum, and residual aluminum, respectively [Citation48].
Table 7. Literature on sequential chemical extraction (SCE) for speciation analysis of non-bivalent metals in plants.
8. Comparison of sequential chemical extraction with the other methods used for speciation analysis of metal(loid)s in plants
Spectral methods, electrochemical methods, and separation techniques including high performance liquid chromatography (HPLC), Capillary Electrophoresis can be used to detect metal forms in plants [Citation46,Citation188]. Despite the high detection accuracy, the requirements of green, speedy and large-scale detection cannot be reached by these methods, since the time consumption, arduous, difficult sample pre-treatment, and restricted detection range [Citation49,Citation52].
Single chemical extraction such as ultrasonic assisted solvent extraction has gained popularity over the past few years as an attractive alternative to microwave-assisted extraction technology. The single extraction is much easier to control, and the precision and accuracy of the method are suitable for batch sample analyses [Citation53,Citation54]. However, one drawback of this method is that it cannot extract all forms of plant metals and is not suitable for metal speciation analysis. The SCE can do metal speciation analysis, and by SCE one can analyse all the forms of metals, and understand the mobility, binding states, solubility, bioavailability, potential phyto-toxicity, tolerance, detoxification mechanism, and the accumulation of all forms of metal (loids). The SCE is easy to operate and is low cost, flexible method with a wide range of applications. Up to now, the six-step SCE method is the most popular for plant sample analysis, but this method has relatively longer procedure, so, in the future, the four-step SCE or even shorter procedure will be more attractive.
9. Application status of sequential chemical extraction for speciation analysis of metal(loid)s in plants
Sequential extraction has large-scale promotion and more flexible applications, so it is an extremely useful method for the speciation analysis of metals. For a better understanding of chemical and biochemical reactions in plants, it is necessary to understand chemical morphology. This can provide us with information on the mobility, toxicity, bioavailability, and detoxification mechanisms of metals through plants, facilitating the risk assessment of various metals in the environment or food chain. At present, the common view is that the ethanol extraction state and the deionized water extraction state of the metals are liable for migration, while the sodium chloride extraction state and the acetic acid extraction state are of low toxicity and mobility [Citation55–58]. Therefore, many studies use this point of view to study the resistance and detoxification mechanisms of plants to specific metals. In a study of a ramie (Bechmeria nivea L.), Wang et al. found that the Cd level in inorganic and water-soluble forms is much higher, but Cd levels in pectin and protein-bound forms are much lower in the Cd-sensitive barley genotype as compared to anti-Cd genotypes [Citation39]. Therefore, they hypothesized that the high Cd levels in both NaCl and 2% HAc extraction states should be responsible for the cadmium-tolerant mechanisms of ramie [Citation59]. Hu et al. studied the lead-tolerant Salsola passerina Bunge by SCE; in the root, 2% HAc extractable Pb, F4, was found to be the main form, followed by the ethanol extractable Pb, F1, indicating that the main Pb form in the root is insoluble with low activity. The sodium chloride extraction state is the dominant form in the stem; the form of Pb in the stem is mainly complex with relatively low toxicity. In addition, hydrochloric acid extractable lead forms in the stem were high, indicating that Pb was stable in this part. In addition, the water-extracted Pb is the lowest form both in the root and stem, indicating that there are almost no free lead ions in plants, and free plant Pb ions have been transformed into stable forms [Citation148]. The SCE can also help in seed selection and phytoremediation designation. Wang et al. compared the chemical forms of cadmium in soy-bean seedlings of four genotypes and found that there were significant genotype differences in the chemical forms of Cd in both root and shoot. Results showed that the Cd levels of four chemical forms (F1, F2, F3, and F4) are high for the sensitive variety BX10, and the Cd levels of three chemical forms (F3, F4, and F5) are high for the tolerant variety HX3[Citation60]. Stable Cd forms help soy bean-tolerant varieties tolerate Cd, and specific chemical forms of Cd in different varieties may be a trait that contributes to genotypic differences and determines cadmium accumulation, binding, and transport.
The SCE method can also be used to evaluate the effects of exogenous factors on plants, such as earthworm mucus and amino acids [Citation61], exogenous nitric oxide [Citation53], and prolonging the growth period [Citation62]. Liu et al. found through SCE that the addition of exogenous melatonin increased the proportion of sodium chloride extract state of Cd in rice, while the proportion of water-soluble state and ethanol extracted state was significantly reduced [Citation144]. This indicates that the addition of melatonin can promote the transfer of cadmium from strong mobility to weak mobility in rice, reducing the absorption and transport of cadmium in rice. The SCE has also been used to combine chemical speciation analysis with subcellular analysis [Citation63–65]. It will help in the selection of appropriate measures to improve phytoremediation efficiency and promote phytoremediation techniques [Citation66]. The common applications of SCE mainly includes: 1) studying the resistance and detoxification mechanisms; 2) evaluating the effects of exogenous factors on plants; and 3) helping with seed selection and improvement of phytoremediation technique.
10. Developmental trends and future directions
With the continuous application of SCE to plants, new research directions have emerged. This review paper elaborates on the SCE methods for metal speciation analysis in plants, which is one of the emerging research trends. In addition, the combination of SCE with high-precision instruments, such as AAS [Citation67], ICP-MS [Citation64,Citation65], etc., has become a common practice, but there is a lack of the combination of SCE with other elemental analysis methods, which can be a future research direction. The development trend of SCE is mainly the combination of analysis and auxiliary extraction methods, dynamic extraction methods, and stoichiometric methods. Moreover, the SCE is easy to operate, has low equipment costs, is flexible with a wide range of sample applications, and can present information about metals in the form of batches.
Another way to achieve fast results is to load samples into suitable containers and perform continuous extraction in continuous flow mode. This method has been used in the speciation analysis of soil or sediment [Citation2] but is rare in plants and awaits excavation. Flow injection analysis (FIA) and sequential injection analysis (SIA) have recently rapidly developed the trend of continuous chemical extraction, which is conducive to the determination of metal speciation and helps to improve the selectivity and sensitivity of analytical methods [Citation68]. In many other areas of analytical science, the use of multivariate data analysis procedures as tools for interpreting SCE results has increased significantly over the past decade [Citation14]. Some researchers have used SCE to combine hierarchical analysis and subcellular analysis; e.g. Zhang et al. believe that the results of cluster analysis show that Cd is mostly in the form of undissolved oxalates and phosphates in stem and root cell walls. Different forms of Cd are distributed in the organelles of stems and roots [Citation64]. As Cd concentrations increase, the intracellular location of Cd closely relates to the chemical form of Cd, and a correlation lies between the migration activity and the toxicity of Cd.
11. Concluding remarks
The SCE is a crucial and frequently used approach for learning about the possible mobility of metals in the environment and their potential bioavailability and toxicity. This method involves the extraction of metal inorganic salts, amino acid salts, and water-soluble organic salts with 80% ethanol and deionized water. Elements bound to pectin and protein were extracted by 1 M sodium chloride solution, and poorly soluble phosphate and oxalate were extracted with 2% acetic acid solution and 0.6 M hydrochloric acid, and the residue metals were left for digestion. It should be noted that the SCE must be adjusted according to the plants or metals under study, especially for non-bivalent metals due to the different solubility products. This work suggests seven directions for future research, including improving sample preparation, preservation, and experimental operation; combining the SCE with mathematics and instrumental methods, and using the speciation data for environmental risk assessment and environmental management. The application of these possibilities will make it easier to comprehend how the metal speciation in plants has resulted from the soil metals and will affect the environmental health through the food chain, indicating that SCE will have a bright future in the upcoming years.
Acknowledgments
This work was supported by The National Programs for High Technology Research and Development of China (2007AA10Z406), and The High Level Talent Project of “Six Talents Summit” in Jiangsu Province (JNHB-55).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Yang J, Sun L, Shen X, et al. An overview of the methods for analyzing the chemical forms of metals in plants. Int J Phytoremedi. 2022;24(13):1418–505. doi: 10.1080/15226514.2022.2033687
- Bacon JR, Davidson CM. Is there a future for sequential chemical extraction. Analyst. 2008;133(1):25–46.
- Walker CH, Hopkin SP, Sibly RM, Peakall DB. Principles of Ecotoxicology. 3rd ed. Phoenix (AZ): Taylor & Francis Group; 2006.
- Marchese M, Gagneten AM, Parma MJ, et al. Accumulation and elimination of chromium by freshwater species exposed to spiked sediments. Arch Environ Contam Toxicol. 2008;55(4):603–609. doi: 10.1007/s00244-008-9139-0
- Stanislawska M, Janasik B, Wasowicz W. Application of high performance liquid chromatography with inductively coupled plasma mass spectrometry (HPLC-ICP-MS) for determination of chromium compounds in air at the workplace. Talanta. 2013;117:14–19.
- Álvarez C R, Jiménez Moreno M, Guzmán Bernado FJ, et al. Mercury methylation, uptake and bioaccumulation by the earthworm Lumbricus terrestris (Oligochaeta). Appl Soil Ecol. 2014;84:45–53.
- Machado A, Šlejkovec Z, Van Elteren JT, et al. Arsenic speciation in transplanted lichens and tree bark in the framework of a biomonitoring scenario. J Atmos Chem. 2006;53(3):237–249. doi: 10.1007/s10874-006-9013-2
- Manceau A, Marcus MA, Tamura N, et al. Quantitative speciation of heavy metals in soils and sediments by synchrotron X-ray techniques. Cosmochim Acta. 2002;68:2467.
- Isaure MP, Laboudigue A, Manceau A, et al. Quantitative Zn speciation in a contaminated dredged sediments by µ-PIXE, µ-SXRF, EXAFS spectroscopy and principal component analysis. Geochim Cosmochim Acta. 2002;66:1549.
- Bang JS, Hesterberg D. Dissolution of trace elements contaminants from two coastal plain soil as affected by pH. J Environ Qual. 2004;33:891.
- Taylor MP, MacFarlane GR, Merrington G. Advances in metal speciation in plants, algae and terrestrial and aquatic systems. Environ Chem. 2019;16(2):73–88.
- Klinkenberg M, Scheckel KG. The role of synchrotron-based X-ray absorption spectroscopy in advancing understanding of metal behavior in the environment. Curr Opin Environ Sci Health. 2018;4:42–48.
- Donner E, Ryan CG, Howard DL. Nuclear magnetic resonance spectroscopy in speciation analysis of arsenic, antimony, and thallium. Anal Bioanaly Chem. 2016;408(20):5421–5433.
- Michalke B. Element speciation definitions, analytical methodology, and some examples. Ecotoxicol Environ Saf. 2003;56(1):122–139. doi: 10.1016/S0147-6513(03)00056-3
- Ure AM. Trace element speciation in soil, soil extracts and solutions. Microchim Acta. 1991;2:49.
- Hlavay J, Prohaska T, Weisz M, et al. Determination of trace elements bound to soil and sediment fractions (IUPAC technical report). Pure Appl Chem. 2004;76(2):415. doi: 10.1351/pac200476020415
- Kroukamp EM, Wondimu T, Forbes PBC. Metal and metalloids speciation in plants: overview, instrumentation, approaches and commonly assessed elements. Trends Analyt Chem. 2016;77:87–99.
- Tessier AP, Campbell PGC, Bisson M. Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem. 1979;51(7):844–851. doi: 10.1021/ac50043a017
- Rauret G, López-Sánchez JF, Sahuquillo A, et al. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monitor. 1999;1:57–61.
- Ure AM, Quevauviller P, Muntau H, et al. Speciation of heavy metals in soils and sediments, an account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the commission of the European communities. Int J Environ Anal Chem. 1993;51:135.
- Larios R, Fernández-Martínez R, LeHecho I, et al. A methodological approach to evaluate arsenic speciation and bioaccumulation in different plant species from two highly polluted mining areas. Sci Total Environ. 2012;414:600–607. doi: 10.1016/j.scitotenv.2011.09.051
- Kaplan O, Kaya G, Yaman M. Sequential and selective extraction of copper in different soil phases and plant parts from former industrialized area. Commun Soil Sci Plant Anal. 2011;42(19):2391–2401. doi: 10.1080/00103624.2011.605497
- Gleyzes C, Tellier S, Astruc M. Fractionation studies of trace elements in contaminated soils and sediments: a review of sequential extraction procedures. Trends Analyt Chem. 2002;21(6):451–467.
- Cao XD, Wang XR, Zhao GW. Assessment of the bioavailability of rare earth elements in soils by chemical fractionation and multiple regression analysis. Chemosphere. 2000;40(1):23–28.
- Xu J, Bao Z, Yang J, et al. Chemical forms of Pb, Cd and Cu in crops. Chin J Appl Ecol. 1991;3:244–248.
- Yasuda O, Kazuko Y, Masao E. Effects of differences in calcium supply, leaf position, and individual growth stage on the distribution of chemistry morphology of calcium in paddy rice organism. Japanese J Of Fertilizer Sci. 1970;41(1):19–26.
- Koji A, Kozo K, Yoshio K. Manganese absorption and migration in Malvacaido and Mitsubakaidō: Studies on manganese over absorption in fruit trees (part 1). Nipp J Fertilizer Sci. 1980;51:405–410.
- Hui-Mei WU, Fei-Li LI, Mou HQ, et al. Analysis of heavy metal fractions in plants by two steps sequential extraction procedure. Environ Sci Technol. 2012;35:133–137.
- Ota A, Yamamoto K, Deguqi, M. The difference in the supply of calcium, the leaf position, and the growth stage of the individual is reflected in the chemistry and shape of the calcium in the paddy rice leaf. Japanese Soc Soil Sci and Plant Nutrition. 1970;41:19–26.
- Wu FB, Dong J, Qian QQ, et al. Cellular distribution and chemical form of Cd and Cd–Zn interaction in different barley genotypes. Chemosphere. 2005;60:1437–1446.
- Wang X, Liu Y, Zeng G, et al. Cellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) Gaud. Environ Exp Bot. 2008;62:389–395.
- Qiu Q, Wang Y, Yang Z, et al. Effects of phosphorus supplied in soil on subcellular distribution and chemical forms of cadmium in two Chinese flowering cabbage (Brassica parachinensis L.) cultivars differing in cadmium accumulation. Food Chem Toxicol. 2011;49(9):2260–2267. doi: 10.1016/j.fct.2011.06.024
- Huang RZ, Jian YB, Jia CH, et al. Cellular distribution and chemical forms of cadmium in Morus alba L. Int J phytoremedi. 2018;20(5):448–453.
- Wu H, Li F, Mou H, et al. Analysis of heavy metal fractions in plants by two steps sequential extraction procedure. Environ Sci Technol. 2012;35(7):133–137.
- Perronnet K, Schwartz C, Morel JL. Distribution of cadmium and zinc in the hyperaccumulator Thlaspi caerulescens grown on multicontaminated soil. Plant Soil. 2003;249(1):19–25. doi: 10.1023/A:1022560711597
- Xue Y, Wang JQ, Huang J, et al. The response of duckweed (Lemna minor L.) roots to Cd and its chemical forms. J Chem. 2018;2018:1–9. doi: 10.1155/2018/7274020
- Pan G, Yan WD, Zhang HP, et al. Cellular distribution and chemical forms involved in manganese accumulation and detoxification for Xanthium strumarium L. Chemosphere. 2019;237:124531.
- Juneja S, Prakash S. The chemical form of trivalent chromium in xylem sap of maize (Zea mays L.). Chem Speciat Bioavailab. 2005;17(4):161–169. doi: 10.3184/095422906783438820
- Weng B, Xie XY, Weiss DJ, Kandelia Obovata S. Yong tolerance mechanisms to cadmium: subcellular distribution, chemical forms and thiol pools. Mar Pollut Bull. 2012;64(11):2453–2460.
- Ueno D, Iwashita T, Zhao FJ, et al. Characterization of Cd translocation and identification of the Cd form in xylem sap of the Cd-hyper accumulator Arabidopsis halleri. Plant Cell Physiol. 2008;49(4):540–548. doi: 10.1093/pcp/pcn026
- Zhou XY, Qiu RL, Ying RR, et al. Effects of zinc on subcellular distribution and chemical forms of cadmium in Potentilla griffithii var. Velutina. J Agro-Environ Sci. 2008;6(3):1066–1071.
- Zeng P, Li X, Wang X, et al. Cadmium and lead mixtures are less toxic to the Chinese medicinal plant Ligusticum chuanxiong hort, than either metal alone. Ecotoxicol Environ Saf. 2020;193:110342.
- Tao X, Xu Y, Wang L, et al. Effects of foliar zinc application on bioavailability and morphology of cadmium zinc in rape. J Agro-Environ Sci. 2022;41(4):735–745.
- Zou J, Wang Y, Wang S, et al. Ca alleviated Cd-induced toxicity in Salix matsudana by affecting Cd absorption, translocation, subcellular distribution, and chemical forms. J Plant Physiol. 2023;281:153933. doi: 10.1016/j.jplph.2023.153926
- Du JW, Zeng L, Zhang SL, et al. Complete recycling of valuable metals from electroplating sludge: green and selective recovery of chromium. Chem Eng J. 2003;467:143484.
- Wang K, Tang SF, Hou X. Molecular mechanism investigation on the interactions of copper (II) ions with glutathione peroxidase 6 from Arabidopsis thaliana. Spectrochimica. Acta part A.: Mol. Biomole Spectrosc. 2018;203:428–433.
- Morabito R. Extraction techniques in speciation analysis of environmental samples. Fresen J Anal Chem. 1995;351(4–5):378–385. doi: 10.1007/BF00322906
- Yu H, Yang ZY, Yang ZJ, et al. Chemical forms and subcellular and molecular distribution of Cd in two Cd-accumulation rice genotypes. Chin J Appl Ecol. 2008;19(10):2221–2226.
- Bian F, Zhong Z, Zhang X, et al. Bamboo - an untapped plant resource for the phytoremediation of heavy metal contaminated soils. Chemosphere. 2020;246:125750.
- Sajida M, Alhooshanib K. Dispersive liquid-liquid micro extraction based binary extraction techniques for chromatographic analysis: a review. Trends Analyt Chem. 2018;108:167–182.
- Kersten M, Förstner U. Speciation of trace elements in sediments. In: Batley GE, editor. Trace Element Speciation: Analytical Methods and Problems. Boca Raton: CRC Press; 1989. p. 245–317.
- Shojaei S, Jafarpour A, Shojaei S, et al. Heavy metal uptake by plants from waste water of different pulp concentrations and contaminated soils. J Cleaner Pro. 2021;296:126345.
- Dong YX, Wang XF, Cui XM. Exogenous nitric oxide involved in subcellular distribution and chemical forms of Cu2+ under copper stress in tomato seedlings. J Integr Agric. 2013;12(10):1783–1790. doi: 10.1016/S2095-3119(13)60367-6
- Yang H, Tang Q. Utilization of ultrasonic vibration extraction-ICP-MS for metal soil chemical speciation. Environ Monit Manage and Tech. 2015;27(4):51–53.
- Gong Y, Xu J, Lu R. Studies on the content of different forms of calcium compound and their change in the fruits of pear. Acta Horticultural Sinica. 1992;19(2):129–134.
- Li WQ, Qing T, Li CC, et al. Integration of subcellular partitioning and chemical forms to understand silver nanoparticles toxicity to lettuce (Lactuca sativa L.) under different exposure pathways. Chemosphere. 2020;258:258. doi: 10.1016/j.chemosphere.2020.127349
- Montes-Bayón M, Molet MJD, González EB, et al. Evaluation of different sample extraction strategies for selenium determination in selenium-enriched plants (Allium sativum and Brassica juncea) and Se speciation by HPLC-ICP-MS. Talanta. 2006;68(4):1287–1293. doi: 10.1016/j.talanta.2005.07.040
- Yin XQ, Wang CZ, Yi L, et al. Study on the relationship between Pb chemical forms and enzyme activity in Brassica chinensis. Agri Res Arid Areas. 2010;28(5):133–137.
- Wang X, Liu YG, Zeng GM, et al. Cellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) Gaud. Environ Exp Bot. 2007;62(3):398–395.
- Wang P, Deng XJ, Huang Y, et al. Comparison of subcellular distribution and chemical forms of cadmium among four soybean cultivars at young seedlings. Environ Sci Pollut Res Int. 2015;22(24):19584–19595. doi: 10.1007/s11356-015-5126-y
- Zhang SJ, Hu F, Li HX. Effects of earthworm mucus and amino acids on cadmium subcellular distribution and chemical forms in tomato seedlings. Biores Technol. 2009;100(17):4041–4046. doi: 10.1016/j.biortech.2009.03.028
- Lai HY, Cai MC. Effects of extended growth periods on subcellular distribution, chemical forms, and the translocation of cadmium in Impatiens walleriana. Int J Phytoremediation. 2016;18(3):228–234. doi: 10.1080/15226514.2015.1073677
- Zhang CY, Liu DW, Shi KL, et al. Gadolinium accumulation, distribution, chemical forms, and influence on the growth of rice seedlings. Ecotoxicol Environ Saf. 2019;179:265–271. doi: 10.1016/j.ecoenv.2019.04.074
- Wang X, Liu DW. Integration of cerium chemical forms and subcellular distribution to understand cerium tolerance mechanism in the rice seedlings. Environ Sci Pollut Res. 2017;24(19):16336–16343. doi: 10.1007/s11356-017-9274-0
- Zheng SN, Zhang CY, Shi KL, et al. Bioaccumulation, subcellular distribution and chemical forms of yttrium in rice seedling. J Rare Earths. 2018;36(3):331–336. doi: 10.1016/j.jre.2017.09.006
- Zhou XY, Qiu RL, Li QF, et al. Effects of zinc on the distribution and chemical forms of lead in potentilla griffithii var. Velutina. Acta Sci Circumstantiae. 2008;(10):2064–2071.
- Wang WJ, Zhang MZ, Liu JN. Cellular distribution and chemical forms of Cd in Bougainvillea spectabilis Willd. As an ornamental phytostabilizer: an integrated consideration. Int J Phytoremediation. 2018;20(11):1087–1095.
- Xiao L. Application of sequential injection technique coupled with spectrophotometer in element speciation analysis [dissertation]. Shengyang: Northeastern University; 2010.
- Pan XY, Dong G, He X, et al. Effects of al stress on the growth and nitrogen uptake of maize varieties with different al tolerance as related with al chemical forms on root surfaces. J Soils Sedi. 2020;20:1–11.
- Hou M, Huo Y, Yang XH, et al. Chemical form and subcellular distribution of vanadium in corn seedlings. Microchem J. 2020;153:104468. doi: 10.1016/j.microc.2019.104468
- Liu Y, Wang CQ, Li B, et al. Effect of selenium-zinc interaction on the chemical forms of selenium of tea leaves in spring season. J Tea Sci. 2010;30(5):343–349.
- Hou M, Gou Z, He JL. Accumulation and chemical forms of vanadium in different rice cultivars. J Agro-Environ Sci. 2013;32(9):1738–1744.
- Fu XP, Dou CM, Chen YX. Cellular distribution and chemical forms of cadmium in Phytolacca americana L. J Hazard Mater. 2011;186(1):103–107.
- Li B, Wang SE, You XS, et al. Effect of foliar spraying of gibberellins and brassinolide on cadmium accumulation in rice. Toxics. 2023;11(4):364. doi: 10.3390/toxics11040364
- Li KT, Peng SY, Zhang B, et al. Exopolysaccharides from Lactobacillus plantarum reduces cadmium uptake and mitigates cadmium toxicity in rice seedlings.World. J Microb & Biotech. 2022;38(12):243.
- Wang JB, Su LY, Yang JZ, et al. Comparisons of cadmium subcellular distribution and chemical forms between low-Cd and high-Cd accumulation genotypes of watercress (Nasturtium officinale L.). Plant Soil. 2015;396(1/2):325–337.
- Niu H, Wang ZL, Song JN, et al. Cadmium subcellular distribution and chemical form in Festuca arundinacea in different intercropping systems during phytoremediation. Chemosphere. 2021;276:130137.
- Zhu JJ, Zhao P, Nie ZJ, et al. Selenium supply alters the subcellular distribution and chemical forms of cadmium and the expression of transporter genes involved in cadmium uptake and translocation in winter wheat (Triticum aestivum). BMC Plant Biol. 2020;20(1):550. doi: 10.1186/s12870-020-02763-z
- Wang JC, Chen XF, Chu SH, et al. Influence of Cd toxicity on subcellular distribution, chemical forms, and physiological responses of cell wall components towards short-term Cd stress in Solanum nigrum. Environ Sci Pollut Res. 2020;28(11):13955–13969.
- Li ZW, Lin WJ. Synergetic effects of DA-6/24-EBL and NTA on uptake, subcellular distribution and chemical form of Cd in Amaranthus hybridus L. Soil Sci Plant Nutr. 2020;66(4):653–661. doi: 10.1080/00380768.2020.1786721
- Li GX, Li QS, Wang L, et al. Cadmium tolerance and detoxification in Myriophyllum aquaticum: physiological responses, chemical forms, and subcellular distribution. Environ Sci Pollut Res Int. 2020;27(30):1–12.
- Feng DY, Huang CR, Xu WH, et al. Difference of cadmium bioaccumulation and transportation in two rye grass varieties and the correlation between plant cadmium concentration and soil cadmium chemical forms. Wireless Pers Commun. 2020;110(1):291–307. doi: 10.1007/s11277-019-06727-x
- Lam CM, Chen KS, Lai HY. Chemical forms and health risk of cadmium in water spinach grown in contaminated soil with an increased level of phosphorus. Int J Environ Res Public Health. 2019;16(18):3322.
- Peng Q, Li T, Xu WH, Jiao LC, Deng JB. Differences in the cadmium-enrichment capacity and cellular distribution and chemical form of cadmium in different varieties of pepper. Environ Sci. 2019;40(7):3347–3354.
- Zhang XF, Hu ZH, Yan TX, et al. Arbuscular mycorrhizal fungi alleviate Cd phytotoxicity by altering Cd subcellular distribution and chemical forms in Zea mays. Ecotoxicol Environ Saf. 2019;171:352–360. doi: 10.1016/j.ecoenv.2018.12.097
- Xu GP, Deng CB, Wang J, et al. Lead bioaccumulation, subcellular distribution and chemical form in sugarcane and its potential for phytoremediation of lead-contaminated soil. Hum Ecol Risk Assess: Int J. 2020;26(5):1175–1187. doi: 10.1080/10807039.2018.1543016
- Li G, Li Q, Wang L, et al. Effects of variable sulfur supply on the accumulation, subcellular distribution, and chemical forms of cadmium in Hydrilla verticillata. Polish J Environ Stud. 2018;28(3):1255–1265.
- Yang LP, Zeng J, Wang P, et al. Sodium hydrosulfide alleviates cadmium toxicity by changing cadmium chemical forms and increasing the activities of antioxidant enzymes in salix. Environ Exp Bot. 2018;156:161–169. doi: 10.1016/j.envexpbot.2018.08.026
- Xin JP, Zhang Y, Tian RN. Tolerance mechanism of Triarrhena sacchariflora (Maxim.) Nakai. Seedlings to lead and cadmium: translocation, subcellular distribution, chemical forms and variations in leaf ultrastructure. Ecotoxicol Environ Saf. 2018;165:611–621. doi: 10.1016/j.ecoenv.2018.09.022
- Yang LP, Zhu J, Wang P, et al. Effect of Cd on growth, physiological response, Cd subcellular distribution and chemical forms of Koelreuteria paniculata. Ecotoxicol Environ Saf. 2018;100(160):10–18.
- Guan MY, Zhang HH, Pan W, et al. Sulfide alleviates cadmium toxicity in Arabidopsis plants by altering the chemical form and the subcellular distribution of cadmium. Sci Total Environ. 2018;627:663–670. doi: 10.1016/j.scitotenv.2018.01.245
- Xie H, Liao ZS, Li J, et al. Effects of exogenous calcium on cadmium accumulation in amaranth. Chemosphere. 2023;326:138435. doi: 10.1016/j.chemosphere.2023.138435
- Xiao QQ, Wang S, Chi YH. Accumulation and chemical forms of cadmium in tissues of different vegetable crops. Agronomy-Basel. 2023;13(3):680. doi: 10.3390/agronomy13030680
- Huang WL, Niu YT, Li Y, et al. Effects of free amino acids, Metallothionein, and chemical forms of heavy metals on the accumulation and detoxification of cadmium and chromium in Chinese Goldthread (Coptis chinensis Franch). J Sensor. 2022;2022:8070016 .
- Sari SHJ, Chien MF, Inoue C. Subcellular localization and chemical speciation of Cd in Arabidopsis halleri ssp. gemmifera to reveal its hyperaccumulating and detoxification strategies. Environ Exp Bot. 2022;203:105047. doi: 10.1016/j.envexpbot.2022.105047
- Gu SG, Zhang W, Wang F, et al. Particle size of biochar significantly regulates the chemical speciation, transformation, and ecotoxicity of cadmium in biochar. Environ Pollut. 2023;320:121100. doi: 10.1016/j.envpol.2023.121100
- Jiang Y, Han JH, Xue WX, et al. Over expression of SmZIP plays important roles in Cd accumulation and translocation, subcellular distribution, and chemical forms in transgenic tobacco under Cd stress. Ecotoxicol Environ Saf. 2021;214:112097–112097. doi: 10.1016/j.ecoenv.2021.112097
- Zhu M, Jiang S, Fu G, et al. Morphology of heavy metals in process of pyrolysis of rape stalk. J Cent South Univ (Sci And Tech). 2019;50(9):1672–7207.
- Li CC, Dang F, Cang L, et al. Integration of metal chemical forms and subcellular partitioning to understand metal toxicity in two lettuce (Lactuca sativa L.) cultivars. Plant Soil. 2014;384(1–2):201–212. doi: 10.1007/s11104-014-2194-6
- Yang J, Zha Y, Liu H. Distribution and chemical forms of Cd, Cu and Pb in polluted seeds. China Environ Sci. 1999;6:500–504.
- Liu Y, Li ZY, Xu RK. Distribution of manganese (II) chemical forms on soybean roots and manganese (II) toxicity. PEDOSPHERE. 2019;29(5):656–664. doi: 10.1016/S1002-0160(17)60413-2
- Cheng HK, Zhang B, Jing, XX, Yang SQ, Zhao P, Sun XX, Zhou ZY. Response of maize to lead stress and chemical forms of lead. Environ Sci. 2015;36(4):1468–1473.
- Sun XB, Li YC, Wang N. Comparisons on active chemical forms and distribution of lead in wheat and corn. J Agro-Environ Sci. 2005;24(4) :666–669.
- Chen YH, Zhang FY, Wu XF, et al. Effects of different modifier concentrations on lead-zinc tolerance, subcellular distribution and chemical forms for four kinds of woody plants. Environ Sci. 2015;36(10):3852–3859.
- Zhou XY, Qiu RL, Hu PJ, et al. Effects of cadmium and lead on subcellular distribution and chemical forms of zinc in Potentilla griffithii var. Velutina. Environ Sci. 2008;29(7):2028–2036.
- Xu J, Yu MG, Chen YX, et al. Characteristics of distribution and chemical forms of Pb in tea plant varieties. Chin J Appl Ecol. 2011;22(4):891–896.
- Cheng YR, Wang C, Chai SY, et al. Ammonium N influences the uptakes, translocations, subcellular distributions and chemical forms of Cd and Zn to mediate the Cd/Zn interactions in dwarf polish wheat (Triticum polonicum L.) seedlings. Chemosphere. 2018;193:1164–1171. doi: 10.1016/j.chemosphere.2017.11.058
- Angulo-Bejarano PI, Puente-Rivera J, Cruz-Ortega R. Metal and metalloid toxicity in plants: an overview on molecular aspects. Plants. 2021;10(4):635. doi: 10.3390/plants10040635
- Bian WL, Yan JP, Cui L, et al. The effect of selenium on cadmium accumulation,chemical forms and the resistance of peanuts. J Agro-Environ Sci. 2018;37(6):1094–1101.
- Peijnenburg WJGM, Zablotskaja M, Vijver MG. Monitoring metals in terrestrial environments within a bioavailability framework and a focus on soil extraction. Ecotoxicol Environ Saf. 2007;67(2):163–179. doi: 10.1016/j.ecoenv.2007.02.008
- Huang RZ, Jiang YB, Jia CH, et al. Cellular distribution and chemical forms of cadmium in Morus alba L. Int J Phytoremediation. 2018;20(5):448–453.
- Xin J, Zhao XH, Tan QL, et al. Comparison of cadmium absorption, translocation, subcellular distribution and chemical forms between two radish cultivars (Raphanus sativus L.). Ecotoxicol Environ Saf. 2017;145:258–265. doi: 10.1016/j.ecoenv.2017.07.042
- Zeng F. Physiological and molecular mechanism of chromium toxicity and tolerance in rice [dissertation]. Hangzhou: Zhejiang University; 2010.
- Zhu GX, Xiao HY, Guo QJ, et al. Cellular distribution and chemical forms of heavy metals in three types of compositae plants from lead-zinc tailings area. Environ Sci. 2017;38(7):3054–3060.
- Mwamba TM, Li L, Gill RA, et al. Differential subcellular distribution and chemical forms of cadmium and copper in Brassica napus. Ecotoxicol Environ Saf. 2016;134(Pt 1):239–249. doi: 10.1016/j.ecoenv.2016.08.021
- Zhou Q, Liu ZD, Liu Y, et al. Relative abundance of chemical forms of Cu(II) and Cd(II) on soy bean roots as influenced by pH, cations and organic acids. Sci Rep. 2016;6(1):513–524. doi: 10.1038/srep36373
- You SH, Teng Y, Ma LL, et al. Characteristics of Cd uptake and chemical forms in Typhaan gustifolia. Environ Eng. 2016;34(8):58–61.
- Zhan FD, He YM, Li Y, et al. Cellular distribution and chemical forms of cadmium in a dark septate endophyte (DSE. Exophiala Pisciphila Environ Sci And Pollut Res. 2015;22(22):17897–17905.
- Wali M, Fourati E, Hmaeid N, et al. NaCl alleviates Cd toxicity by changing its chemical forms of accumulation in the halophyte Sesuviumbportulacastrum. Environ Sci Pollut Res Int. 2015;22(14):10769–10777. doi: 10.1007/s11356-015-4298-9
- Fu HB, Zeng Y, Chen JA, et al. Content and chemical forms distribution of Cd in farmland soil and potato in zinc smelting area. J Henan Agri Sci. 2014;43(9):66–71.
- Liu J, Zhou K, Xu WH, et al. Effect of exogenous iron on accumulation and chemical forms of cadmium, and physiological characterization in different varieties of tomato. Environ Sci. 2013;34(10):4126–4131.
- Yu H, Zhang Z, Zhang Y, et al. Metal type and aggregate micro-environment govern the response sequence of speciation transformation of different heavy metals to micro plastics in soil. Sci Total Environ. 2021;752:141956.
- Zhang CL, Zhang P, Mo CG, et al. Cadmium uptake, chemical forms, subcellular distribution, and accumulation in Echinodorus osiris Rataj. Environ Sci Processes Impacts. 2013;15(7):1459–1465. doi: 10.1039/c3em00002h
- Lu ZY, Liu ZQ, Song ZG, et al. Subcellular distribution and chemical forms of Cd and the synthesis of phytochelatins(PCs)in different barley genotypes. J Agro-Environ Sci. 2013;32(11):2125–2131.
- Ma P, Zang J, Shao T, et al. Cadmium distribution and transformation in leaf cells involved in detoxification and tolerance in barley. Ecotoxicol Environ Saf. 2023;249:114391. doi: 10.1016/j.ecoenv.2022.114391
- Bai X, Chen YH, Geng K, et al. Accumulation,subcellular distribution and chemical forms of cadmium in Viola Tricolor L. Acta Scientiae Circumstantiae. 2014;34(6):1600–1605.
- Gujre N, Mitra S, Soni A, et al. Speciation, contamination, ecological and human health risks assessment of heavy metals in soils dumped with municipal solid wastes. Chemosphere. 2021;262:262. doi: 10.1016/j.chemosphere.2020.128013
- Xu PX, Wang ZL. Physiological mechanism of hypertolerance of cadmium in Kentucky bluegrass and tall fescue: chemical forms and tissue distribution. Environ Exp Bot. 2013;96:35–42. doi: 10.1016/j.envexpbot.2013.09.001
- Tang X, Li C, Yin J, et al. Analysis of the existing forms of calcium and aluminium in vegetables. Trace Elements And Health Res. 2001;18(4) :49–50.
- Nuza’iti A, Ayinul A, Dilinur M. Effects of phosphorus on chemical forms and physiological properties of Cd in Fragaia ananassa D. Chin J Soil Sci. 2013;44(6):1460–1464.
- Wang YP, Huang J, Gao YZ, et al. Arbuscular mycorrhizal colonization alters subcellular distribution and chemical forms of cadmium in Medicago sativa L. And resists cadmium toxicity. PLoS One. 2012;7(11):e48669. doi: 10.1371/journal.pone.0048669
- Du YP, Li HJ, Yin KL, et al. Cadmium accumulation, subcellular distribution and chemical forms Vitis Vinifera Cv. Chardonnay Grapevine. Chin J Appl Ecol. 2012;23(6):1607–1612.
- Qiu Q, Wang YT, Yang ZY, et al. Effects of phosphorus supplied in soil on subcellular distribution and chemical forms of cadmium in two Chinese flowering cabbage (Brassica parachinensis L.) cultivars differing in cadmium accumulation. Food Chem Toxicol. 2011;49(9):2260–2267. doi: 10.1016/j.fct.2011.06.024
- Zheng MM, Feng D, Liu HJ, et al. Subcellular distribution, chemical forms of cadmium and rhizosphere microbial community in the process of cadmium hyperaccumulation in duckweed. Sci Total Environ. 2023;859:160389. doi: 10.1016/j.scitotenv.2022.160389
- He SY, Wu QL, He ZL. Effect of DA-6 and EDTA alone or in combination on uptake, subcellular distribution and chemical form of Pb in Lolium perenne. Chemosphere. 2013;93(11):2782–2788. doi: 10.1016/j.chemosphere.2013.09.037
- Hu L, McBride MB, Cheng H, et al. Root-induced changes to cadmium speciation in the rhizosphere of two rice (Oryza sativa L.) genotypes. Environ Res. 2001;111:356–361.
- Zhang YQ, Tai CF, Li PJ, et al. Effect of plant growth inhibitors on accumulation and chemical form of Cd in Tagetes erecta L. J Agro-Environ Sci. 2010;29(2):258–263.
- Yang WD, Chen YT, Qu MH. Subcellular distribution and chemical forms of cadmium in Salix officinalis. Acta Bot Boreal Occident Sin. 2009;29(7):1394–1399.
- Schreck E, Foucault Y, Sarret G, et al. Metal and metalloid foliar uptake by various plant species exposed to atmospheric industrial fallout: mechanisms involved for lead. Sci Total Environ. 2012;427-428:253–262. doi: 10.1016/j.scitotenv.2012.03.051
- Li HH, Yang XE. Effects of sulfur on accumulation subcellular distribution and chemical forms of cadmium in hyperaccumulator Sedum alfredii Hance. Plant Nutrit And Fertilizer Sci. 2009;15(2):395–402.
- Qin JQ, Xia BC, Zhao P, et al. Subcellular distribution and chemical forms of cadmium in two Miscanthus floridulus populations. Ecol Environ Sci. 2009;18(3):817–823.
- Tang CF, Zhang RQ, Wen SZ, et al. Effects of exogenous spermidine on subcellular distribution and chemical forms of cadmium in Typha latifolia L. under cadmium stress. Water Sci Technol. 2009;59(8):1487–1493. doi: 10.2166/wst.2009.170
- Zhao YF, Shang DR, Ning JS, et al. Cellular distribution and chemical forms of lead in the red algae, Porphyra yezoensis. Chemosphere. 2019;227:172–178.
- Liu SX, Huang YZ, Luo ZJ, et al. Effect of exogenous melatonin on accumulation and chemical form of Cd in rice. Chin J Appl Ecol. 2017;28(5):1588–1594.
- Zhang X, Liu J, Xu W, et al. Effects of phosphorus on cadmium accumulation, chemical form and physiological characteristics of different pepper cultivars. Environ Sci. 2011;32(4):1171–1176.
- Zhou JM, Dang Z, Chen NC, et al. Influence of NTA on accumulation and chemical form of copper and zinc in maize. J Agro-Environ Sci. 2007;26(2):453–457.
- Zeng FR, Ali S, Qiu BY, et al. Effects of chromium stress on the subcellular distribution and chemical form of Ca, Mg, Fe, and Zn in two rice genotypes. J Plant Nutr Soil Sci. 2010;173(1):135–148. doi: 10.1002/jpln.200900134
- Hu R, Sun K, Su X, et al. Physiological responses and tolerance mechanisms to Pb in two xerophils: Salsola passerina Bunge and Chenopodium album L. J Hazard Mater. 2012;205-206:131–138. doi: 10.1016/j.jhazmat.2011.12.051
- Liu XW, Qi CM, Li Y, et al. Subcellular distribution and chemical forms of lead in Eupatorium adenophorum at different lead levels. Guihaia. 2016;36(3):335–341.
- Gong YC, Xu J, Lv RJ. Study on the content and different forms of calcium compound and their change in the fruit of pear. Acta Horticultural Sinica. 1992;19(2):129–134.
- Kramer U, Pickering IJ, Prince RC, et al. Cellular localization and speciation of nickel in hyperaccumulator and non-accumulator Thlaspi species. Plant Physiol. 2000;122(4):1343–1353.
- Schaumloffel D, Ouerdane L, Bouyssiere B, et al. Speciation analysis of nickel in the latex of a hyperaccumulating tree Sebertia acuminata by HPLC and CZE with ICP-MS and electrospray MS-MS detection. J Anal Spectrum. 2003;18(2):120–127.
- Erdemir US, Gucer S. Assessment of copper bioavailability in spinach (Spinacia oleracea L) leaves by chemical fractionation. Food Anal Methods. 2014;7(5):994–1001. doi: 10.1007/s12161-013-9704-7
- Kelly R, Andrews J, DeWitt J. An X-ray absorption spectroscopic investigation of the nature of the zinc complex accumulated in Datura innoxia plant tissue culture. Microchem J. 2002;71(2–3):231–245. doi: 10.1016/S0026-265X(02)00015-2
- Wu J, Yang D, Wang L, et al. Research progress on the analysis of the occurrence and distribution characteristics of heavy metals in plants. China Environ Monit. 2018;34(4):141–149.
- Mogwasi R, Zor S, Kariuki DK, et al. Sequential extraction as novel approach to compare 12 medicinal plants from Kenya regarding their potential to release chromium, manganese, copper, and zinc. Biol Trace Elem Res. 2018;182(2):407–422. doi: 10.1007/s12011-017-1083-2
- Majolagbe AO, Kuteyi V, Onwordi CT, et al. Concentration and bioavailability of iron in some selected blood building medicinal plants in Southwest Nigeria. J Environ. 2013;2:19–24.
- Chen S, Zhang GQ, Liang X, et al. A Dark septate endophyte Improves cadmium tolerance of maize by Modifying Root morphology and Promoting cadmium binding to the cell Wall and phosphate. J Fungi. 2023;9(5):531. doi: 10.3390/jof9050531
- Chen X, Yang S, Ma J, et al. Manganese and copper additions differently reduced cadmium uptake and accumulation in dwarf polish wheat (Triticum polonicum L.). J Hazard Mater. 2023;448:130998. doi: 10.1016/j.jhazmat.2023.130998
- Yao Q, Li WP, Liu Y, et al. FeCl3 and Fe-2(SO4)(3) differentially reduce Cd uptake and accumulation in polish wheat (Triticum polonicum L.) seedlings by exporting Cd from roots and limiting Cd binding in the root cell walls. Environ Pollut. 2023;317:120762.
- Wei Y. Study on the relationship between rare earth elements and phosphate in nutrient solution. J Chin Rare Earth Soc. 2002;19(1):91–93.
- Wang XL, Zhang BJ, Wu DS, et al. Chemical forms governing Cd tolerance and detoxification in duckweed (Landoltia punctata). Ecotoxicol Environ Saf. 2021;207:111553. doi: 10.1016/j.ecoenv.2020.111553
- Sha S, Cheng MH, Hu KJ, et al. Toxic effects of Pb on Spirodela polyrhiza L.: Cellular distribution, chemical forms, morphological and physiological disorders. Ecotoxicol Environ Saf. 2019;181:146–154.
- Huang L, Zhang HQ, Song YY, et al. Cellular compartmentalization and chemical forms of lead participate in lead tolerance of Robinia pseudoacacia L. with Funneliformis mosseae. Front Plant Sci. 2017;8:517.
- Zhao L, Li TX, Yu HY, et al. Changes in chemical forms, subcellular distribution, and thiol compounds involved in Pb accumulation and detoxification in Athyrium wardii (Hook.). Environ Sci Pollut Res. 2015;22(16):12676–12688. doi: 10.1007/s11356-015-4464-0
- Zu YQ, Li Y, Min H, et al. Cellular distribution and chemical form of Pb in hyperaccumulator Arenaria orbiculata and response of root exudates to Pb addition. Front Environ Sci Eng. 2015;9(2):250–258.
- Zhou FR, Wang JX, Zhang Q, et al. Chemical forms and distribution of lead in the leaves of Platycladus orientalis and Sophora japonica. J Agro-Environ Sci. 2012;31(11):2121–2127.
- Wang QY, Liu JS, Hu B. Integration of copper subcellular distribution and chemical forms to understand copper toxicity in apple trees. Environ Exp Bot. 2016;123:125–131. doi: 10.1016/j.envexpbot.2015.11.014
- Peng B, Huang XP, Zhang DW. Chemical forms and accumulation patterns of Cu in two sea grass species Thalassia hemprichii and Enhalus acoroides. Chin J Ecol. 2010;29(10):1993–1997.
- Liao B, Deng DM, Yang B, et al. Subcellular distribution and chemical forms of Cu in Commelina communis. Acta Sci Natural Univ Suny. 2004;43(2):72–75,80.
- Zhou SB, Xu LS, Wu LH, et al. Subcellular distribution and chemical forms of Cd and Zn in Sedum jinianum. Chin J Appl Ecol. 2008;11:2515–2520.
- Wu Q, Du SJ, Zeng XW, et al. Subcellular distribution and chemical forms of Potentilla grifithii hook. Ecolo And Environ. 2006;15(1):40–44.
- Zhang N, Wang W, Li Y. Morphological distribution of Pb in the reclaimed water-soil-vegetable System. Environ Prot Sci. 2015;41(6):38–43.
- Hu JZ, Zheng AZ, Pei DL, et al. Bioaccumulation and chemical forms of cadmium, copper and lead in aquatic plants. Braz Arch Biol Technol. 2010;53(1):235–240. doi: 10.1590/S1516-89132010000100029
- Tatsuya H, Yasushi S, Takefumi O, et al. Chemical forms and levels of calcium in turnip leaves in relation to harvest time and growing temperature. J Japanese Soci Hortic Sci. 2003;72(2):169–174.
- Xiao ZH, Pan G, Li XH, et al. Effects of exogenous manganese on its plant growth, subcellular distribution, chemical forms, physiological and biochemical traits in Cleome viscosa L. Ecotoxicol Environ Saf. 2020;198:110696. doi: 10.1016/j.ecoenv.2020.110696
- Pan G, Zhang HP, Liu WS, et al. Integrative study of subcellular distribution, chemical forms, and physiological responses for understanding manganese tolerance in the herb Macleaya cordata (Papaveraceae). Ecotoxicol Environ Saf. 2019;181:455–462. doi: 10.1016/j.ecoenv.2019.06.040
- He W, Chen YH, Liang X, et al. Screening for tolerant woody plants for improved manganese slag and research on sub-cellular distribution and chemical form of manganese. Environ Eng. 2018;36(9):154–160.
- Li G, Wang Y, Zhang S, et al. Investigation on entrance mechanism of calcium and magnesium into micro-arc oxidation coatings developed on Ti-6Al-4V alloys. Surf Coat Technol. 2019;378:124951. doi: 10.1016/j.surfcoat.2019.124951
- Zhao B, Zheng K, Liu CG. Bio-dissolution process and mechanism of copper phosphate hybrid nanoflowers by Pseudomonas aeruginosa and its bacteria-toxicity in life cycle. J Hazard Mater. 2021;419:126494. doi: 10.1016/j.jhazmat.2021.126494
- Abraham T, Priyanka RN, Joseph S, et al. Fabrication of zirconium ferrite doped Ag3PO4 composite for the degradation of refractory pollutants: visible light assisted Z-scheme insight. Mater Sci Semicond Process. 2021;130:105797. doi: 10.1016/j.mssp.2021.105797
- McGowen SL, Basta NT, Brown GO. Use of diammonium phosphate to reduce heavy metal solubility and transport in smelter-contaminated soil. J Environ Qual. 2001;30:493–500.
- Cao X, Wang WB, Ma R, et al. Solidification/Stabilization of Pb2+ and Zn2+ in the sludge incineration residue-based magnesium potassium phosphate cement: Physical and chemical mechanisms and competition between coexisting ions. Environ Pollut. 2019;253:171–180. doi: 10.1016/j.envpol.2019.07.017
- Ziya SC, Scott AW, Christopher HG. The aqueous geochemistry of the rare earth elements. Part XIV. The solubility of rare earth element phosphates from 23 to 150 8°C. Chem Geol. 2005;217:147–169.
- Wang Y, Chai LY, Yang ZH, et al. Cellular distribution and chemical forms of antimony in Ficus tikoua. Int J Phytoremediation. 2017;19(2):97–103.
- Wang WS, Meng M, Li L. Arsenic detoxification in Eucalyptus: subcellular distribution, chemical forms, and sulfhydryl substances. Environ Sci Pollut Res Int. 2019;26(24):24372–24379. doi: 10.1007/s11356-019-05701-1
- Zhang XH, Liu J, Wang DQ, et al. Bioaccumulation and chemical form of chromium in Leersia hexandra Swartz. Bull Environ Contam Toxicol. 2009;82(3):358–362.
- Brzezicha-Cirocka J, Grembecka M, Szefer P. Monitoring of essential and heavy metals in green tea from different geographical origins. Environ Monit Assess. 2016;188(3):183. doi: 10.1007/s10661-016-5157-y

