?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Sub-bituminous C coal stored in temporary stockpiles for long periods often causes spontaneous combustion. Intensive spontaneous combustion causes negative impacts such as methane gas emissions that are exposed to the air. This research aims to analyze the effect of differences in organic sulfur content in the form of carbon disulfide on the formation of methane gas emissions in the spontaneous combustion of sub-bituminous C coal in the temporary stockpile. The method used in this research uses field surveys and the data are analyzed using normality and regression tests. The results show that the spontaneous combustion of sub-bituminous C coal that occurs in the temporary stockpile is strongly influenced by the organic sulfur content in the form of carbon disulfide and the water content of the coal. Regression analysis shows BB52HS coal with high organic sulfur content and low water content produces methane gas emissions of 1,450 - 24,000 ppm.
1. Introduction
The temporary coal stockpile in the West Banko Mining Area is where sub-bituminous C coal is stored before it is mixed with high-rank coal to obtain the quality of coal required by the market, especially the international market [Citation1]. Low-rank coal stored in the temporary stockpile for an extended period often causes spontaneous combustion [Citation2,Citation3]. Cases of spontaneous combustion often occur in open mines in coal-producing countries worldwide, especially in mining areas, such as the mine front, dumping area, stockpile, and disposal area [Citation4,Citation5]. Intensive spontaneous combustion of low-rank coal causes negative internal and external impacts to the company, such as economic losses, decreased quality, decreased production, decreased health quality, and decreased environmental quality, especially from various polluting gases exposed to the air [Citation6–8].
Sulfur content is one of the leading causes of spontaneous combustion in open-pit coal mining areas. Many experts have put forward research and statements regarding the sulfur content of coal as an essential factor causing spontaneous combustion [Citation9–11]. Previous research on the relationship between organic sulfur content and the formation of methane gas emissions in spontaneous coal combustion still needs to be discussed. This research shows that the organic sulfur content in coal is an essential factor in the occurrence of spontaneous combustion. Research on organic sulfur shows that C – S bonds in the form of carbon disulfide play an essential role in methane gas emissions in the spontaneous combustion of coal, especially sub-bituminous C coal. Research on the influence of organic sulfur C – S bonds in the form of carbon disulfide shows that the organic sulfur content is low in the oxidation reaction in spontaneous combustion. It will break down and form hydrogen sulfide and sulfur dioxide [Citation12]. Research on organic sulfur in bituminous coal shows that spontaneous combustion will be intensive at high organic sulfur content [Citation13]. Other research on bituminous coal shows that organic sulfur plays a vital role in spontaneous combustion. Observations made include the increase in organic sulfur in sub-bituminous coal with increasing temperature, the role of carbon disulfide compounds, the formation of hydrogen gas and sulfur dioxide from bituminous coal, and the high organic sulfur content of bituminous coal will increase the potential for spontaneous combustion.
This research aims to analyze the influence of differences in the organic sulfur content of C – S bonds in the form of carbon disulfide in the formation of methane gas emission in the spontaneous combustion of sub-bituminous C coal in the West Banko Mining Area, especially the temporary stockpile based on previous studies and field surveys.
2. Materials and methods
2.1. Study area and research design
The research location is in the West Banko Mining Area, one of PT’s coal mining business permit areas. Bukit Asam (Persero) Tbk with an area of 4,500 ha located at coordinates 3°42’30’’- 4°47’30’‘South Latitude and 103°45’00’“−103°50’10”’ East Longitude. The research area in the temporary stockpile area of the West Banko Mine is approximately 3 ha. Coal mining activities use the strip mining method with a Shovel – Dump Truck system, which produces sub-bituminous C coal with a calorific value between 5,100 and 6,100 kcal/kg and a total sulfur content of 0.51% and 0.91%, which can be categorized as coal with low sulfur below 1% [Citation14]. When the research was carried out, there had been spontaneous coal combustion in 59 hotspots in the mining front, stockpile, and temporary stockpile in the West Banko Mining Area. This research took samples of ongoing spontaneous combustion of coal in the temporary stockpile in 46 hotspots, with details of 9 hotspots for coal with a sulfur content of 0.51%, referred to as BB52LS, and 37 hotspots with a sulfur content of 0.91%, referred to as BB52HS. Methane gas emission concentrations were measured using an Altair 4X multigas detector (Mine Safety Appliances Company 2011), which was connected to a small pipe measuring 5 cm long and 1 mm in diameter at the top of a modified closed-static chamber. The chamber is placed above each spontaneous combustion hotspot with an observation time limit until the maximum concentration of methane gas emission is reached.
2.2. Chamber specifications and sampling techniques
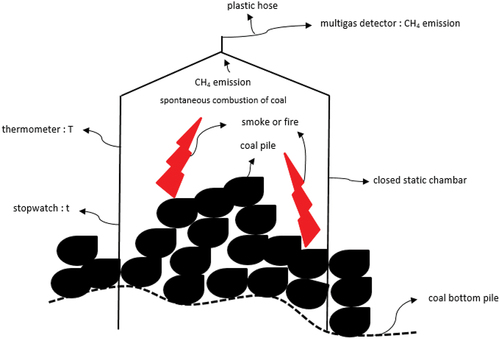
Modifications to the closed static chamber were made to the shape, size, and materials to suit the average area of coal spontaneous combustion hotspots in the temporary stockpile in the West Banko Mine Area. The size of the chamber is divided into two parts, namely: the lower part is a rectangular box [Citation15] with dimensions of 45 cm x 45 cm x 50 cm with a volume of 101,250 cm3 and the upper part is a rectangular pyramid with dimensions of 45 cm x 45 cm x 10 cm with a volume of 6,750 cm3 and the total chamber volume is 108,000 cm3. The size of the chamber and coal pile is adjusted to the self-heating and spontaneous combustion hotspot of 2,025 cm2 in accordance with the bottom area of the chamber of 45 cm x 45 cm. The average capacity of coal samples in self-heating and spontaneous combustion hotspots is 8,375 cm3 or cone-shaped with a height of 20 cm and a diameter of 20 cm. Samples were taken at a self-heating and spontaneous combustion hotspot in the temporary stockpile in the West Banko mining area. The choice of the top of the chamber in the form of a rectangular pyramid is intended so that the concentration of methane gas emission during spontaneous combustion of coal can go to one exit point at the top of the pyramid where a small pipe is installed, which is connected to a multigas detector. The chamber material uses stainless steel with a thickness of 0.5 mm to make the material easy to move, resistant to spontaneous combustion temperatures up to above 100°C, the gas is not chemically reactive, the gas does not react with the material, and contamination does not occur [Citation16]. The system in the chamber is an adiabatic process where no heat enters and exits so that all walls are covered with fiber material. Other equipment used is a multigas detector to measure the concentration of methane gas emission, a thermometer to measure temperature, and a stopwatch to measure the duration of spontaneous combustion.
shows a schematic of measuring the concentration of methane gas emissions from a closed static chamber modified using a multigas detector. shows the use of a modified closed-static chamber to measure methane gas emissions in the field. A closed static chamber connected to a multigas detector is placed above the coal spontaneous combustion hotspot. Determination of the concentration of methane gas emission, temperature and time is carried out at each spontaneous combustion hotspot until the gas emissions reach a maximum in a certain time period. Data will be obtained on the concentration of methane gas emission, temperature and time, each in units of % LEL converted to ppm, oC, and minutes. Measurements of methane gas emission concentrations were carried out on sub-bituminous coal with a calorific value between 5,100 and 6,100 kcal/kg according to the codes BB52LS and BB52HS.
2.3. Multigas calibration of detectors and chambers
Methane gas emission concentration measurements at coal spontaneous combustion hotspots in the field experience several obstacles, including relatively high and undulating topography, spontaneous combustion temperatures reaching above 100°C, hotspot positions spread out, slow survey movements, and sampling in the field for mobilization for laboratory examination. They were using slow and old gas chromatography equipment. One way to overcome this obstacle is to calibrate by directly measuring the concentration of methane gas emission, temperature, and time in the field using an Altair 4X multigas detector connected to a small pipe at the top of the closed static chamber. Calibration of methane gas emission concentration measurements, temperature, and time before spontaneous combustion is only carried out on multigas detectors based on the rules set by the equipment manufacturer (MSA Altair 4X Multigas Detector Bump Test Procedure). Initial measurements were carried out under standard conditions where the chamber was placed on coal where spontaneous combustion did not occur, and the methane gas concentration, temperature, and time readings were by the regulations set by the company. Next, methane gas emission concentrations, temperature, and time were measured at each coal spontaneous combustion hotspot, and the data obtained were subjected to statistical tests and regression analysis. The infrared thermometer used, the GM 550 type, has a temperature range of −50°C − 550°C with an accuracy level of 1.5°C or 1.5%, while the accuracy level of the Altair 4X multigas detector has been calibrated before measuring methane gas emissions in the field according to the instructions or equipment manual. shows an infrared thermometer for measuring self-heating and spontaneous combustion while shows the Altair 4X multigas detector used to measure methane gas emissions in the field. The correlation between time, temperature, and concentration of methane gas emissions during the spontaneous combustion of coal is significant, especially to see its effect on the differences in the organic sulfur content of sub-bituminous C coal between BB52LS and BB52HS [Citation17].
2.4. Statistical analysis
This research is included in parametric statistics, a part of inferential statistics that considers the value of one or more population parameters. Inferential statistics describes the activities of collecting, compiling, processing, and presenting data in tables, graphs, or diagrams to provide an orderly, concise, and clear picture based on a population with a normal distribution [Citation18,Citation19]. Data normality testing is essential to determine whether the data is usually distributed, especially for regression analysis. Good regression analysis requires data on dependent variables and independent variables that are usually distributed. The normality test for sample data on methane gas emission concentration, temperature, and spontaneous combustion time for BB52LS and BB52HS coal using the Shapiro – Wilk test showed that it was usually distributed where the significance of p count > p table.
3. Results and discussion
3.1. Analysis of rank and sulfur content of coal in the West Banko Mining Area
Analysis of coal rank and type of coal sulfur content at the research location in the West Banko Mine Area was carried out using a literature review and field survey approach. Low-rank coal, such as lignite and sub-bituminous, is intensively used as fuel for industry and power plants. This coal is characterized by high total water content (20–50%), low calorific value (5,100–6,100 kcal/kg), high volatile matter (30–60%), low thermal efficiency, low oxidation temperature, and frequent spontaneous combustion [Citation20,Citation21]. The coal in the research location in the West Banko Mine Area is low rank (sub-bituminous). The characteristics of low-rank coal can be seen in , which shows that sub-bituminous coal at the research location has high water content (27.10–30.50%), low calories (5,051–5,099 kcal/kg), and high volatile matter (33.98–34.06%). Some researchers classify low-rank coal using variable calorific values. The calorific value of coal is between 5,051 and 5,099 kcal/kg in the West Banko Mining Area, which is sub-bituminous coal, included in sub-bituminous C coal [Citation22–24]. The characteristics of sub-bituminous C coal are that it is very susceptible to spontaneous combustion at low oxidation temperatures.
Table 1. Proximate and ultimate analysis of BB52LS and BB52HS coal.
The West Banko Mining Area is located in the South Sumatra Basin, one of Indonesia’s most crucial coal basins [Citation25]. The West Banko Mining Area includes the Muara Enim Formation, or the Central Palembang Formation, from the Late Miocene to the Pliocene. The coal produced in the West Banko Mining Area is sub-bituminous coal, which is included in sub-bituminous C. The total sulfur content of sub-bituminous C coal is between 0.51% and 0.91%, which is dominated by organic sulfur content and a small portion of pyrite sulfur and sulfate [Citation26,Citation27]. The organic sulfur content in sub-bituminous C coal is dominated by C – S bonds in the form of carbon disulfide, although there is still a small amount of pyrite and sulfate sulfur [Citation28].
shows the composition of organic sulfur, pyrite, and sulfate in the West Banko Mine Area, where organic sulfur is dominant in BB52LS coal and BB52HS coal. Other factors that need to be considered in the spontaneous combustion process of coal are the water content, calorific value, and volatile matter of coal. The high water and volatile matter content of coal will cause the coal combustion process to be faster. At the same time, the lower calorific value of coal will also stimulate the coal oxidation process with oxygen [Citation29–32].
Table 2. Composition of organic sulfur, pyrite, and sulfate of sub-bituminous C coal in the West Banko Mining Area.
3.2. Analysis of the formation of methane gas emission from the spontaneous combustion of coal
The debate regarding the spontaneous combustion process of coal is still ongoing, especially regarding the initial and final processes, which consist of combustion and drying, devolatilization and pyrolysis, and charcoal combustion [Citation33,Citation34]. These processes can be grouped as self-heating and spontaneous combustion of coal. The process of spontaneous coal combustion in the West Banko Mine Area is greatly influenced by the organic sulfur content, especially C – S bonds in the form of carbon disulfide, which is very intensive in forming hydrogen sulfide gas. The reversible reaction of carbon disulfide bonds in the formation of hydrogen sulfide gas can be written as follows:
Reactions in EquationEquations 1(1)
(1) -Equation3
(3)
(3) is a reversible reaction of organic sulfur, which is dominated by C – S bonds in the form of carbon disulfide reacting with sub-bituminous C coal with high water content to form hydrogen sulfide gas in the spontaneous combustion of coal in the West Banko Coal Mine Area. In the further process of spontaneous combustion of coal with increasing temperatures, the reaction between carbon disulfide and hydrogen sulfide and hydrogen produces methane gas emissions with the following reaction [Citation35,Citation36]:
Reactions in EquationEquations 4(4)
(4) -Equation5
(5)
(5) occur from the spontaneous combustion of coal, which forms methane gas emission, which will reach a maximum until the carbon disulfide bonds are broken in the reaction. This reaction shows that the spontaneous combustion of coal in the formation of methane gas emission in the West Banko Mine Area is strongly influenced by intrinsic and extrinsic properties, especially the organic sulfur content in the form of disulfide bonds and the water content of sub-bituminous C coal [Citation37]. Therefore, the formation of methane gas emission from the spontaneous combustion of sub-bituminous C coal in the West Banko Mine Area with different organic sulfur content in the form of sulfur disulfide can be seen from the correlation between the variables of methane gas emission in ppm, temperature in °C, and time in minutes.
3.3. Relationship between methane gas emission, temperature, and the spontaneous combustion time in different organic sulfurs
Self-heating of sub-bituminous C coal occurs if the coal and oxygen (solid-gas) undergo oxidation at low temperatures below 100°C [Citation38]. The main trigger for self-heating is the high water and sulfur content in low-rank coal, such as lignite and sub-bituminous [Citation39]. The physical and chemical processes of heating itself will occur due to exothermal reactions and adiabatic processes where very little of the heat is absorbed by the external environment, and most of it is absorbed by coal, which has wide pores; there will be an increase in temperature. An uncontrolled increase in temperature and a decrease in the water content of coal results in the spontaneous combustion of coal, characterized by the presence of smoke and fire in the temporary stockpile [Citation40]. The effect of water content on coal self-heating can be explained into two processes: The first process is where the interaction between coal and water content at low temperatures becomes latent heat.
In contrast, the second process is the adsorption of water vapor in coal into the air, which will cause an increase in temperature [Citation41]. If the heat produced is greater than the heat lost to the air, the temperature of the coal will increase, causing self-heating. The continued increase in temperature will cause spontaneous heating of the coal. The self-heating and spontaneous heating processes of sub-bituminous C coal in the West Banko Mine Area involve both processes. Research on the organic sulfur content in spontaneous combustion shows that organic sulfur plays a role in the self-heating process at low temperatures. A low organic sulfur content will cause low spontaneous heating; conversely, if the organic sulfur content is high, the possibility of spontaneous heating will be higher [Citation42].
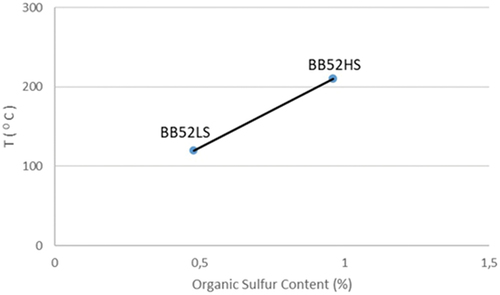
shows that the higher the organic sulfur content in carbon disulfide bonds, the higher the spontaneous combustion temperature of sub-bituminous C coal will occur. Therefore, organic sulfur in the form of carbon disulfide bonds plays a significant role in the spontaneous combustion of sub-bituminous C coal in the West Banko Mine Area. Organic sulfur in the form of carbon disulfide is one of the primary triggers for the spontaneous combustion of sub-bituminous C coal, which produces methane gas emissions [Citation43]. The self-heating stage of sub-bituminous C coal is primarily determined by the total water content, where BB52LS coal is more significant than BB52HS coal, which is 30.50% and 27.10%, respectively. The inherent water content in BB52LS and BB52HS coal is 15.70% and 12.60%, respectively. shows that BB52LS coal will self-heat faster than BB52HS because BB52LS coal has a lower organic sulfur content in carbon disulfide. Increasing the temperature of the oxidation of sub-bituminous C coal in the self-heating process will peak before the organic sulfur in the form of carbon disulfide bonds is broken.
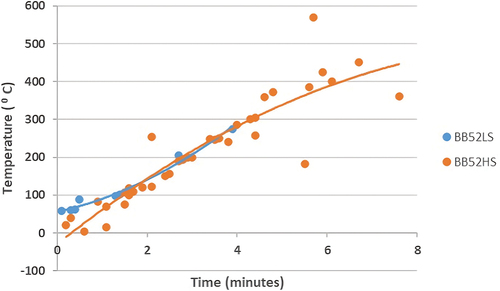
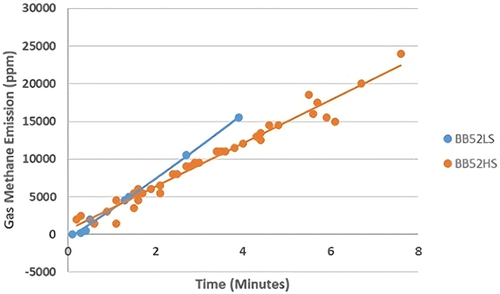
Increasing the time in the oxidation process in self-heating and spontaneous coal combustion will increase the temperature (). The temperature increase in self-heating and spontaneous combustion of BB52LS coal occurs at 59–275°C, while BB52HS coal occurs at 4–570°C. The time required for the self-heating process and spontaneous combustion of BB52LS coal is faster than BB52HS coal, with a time between 0.1 and 3.9 minutes, while BB52HS coal is between 0.2 and 7.6 minutes. The pattern of increasing temperature on the formation of methane gas emissions during self-heating and spontaneous combustion shows the same pattern as previous researchers. Therefore, the influence of organic sulfur content in the form of carbon disulfide and water content of coal dramatically influences the time and temperature of self-heating and spontaneous combustion.
3.4. Relationship between methane gas emission and the spontaneous combustion temperature
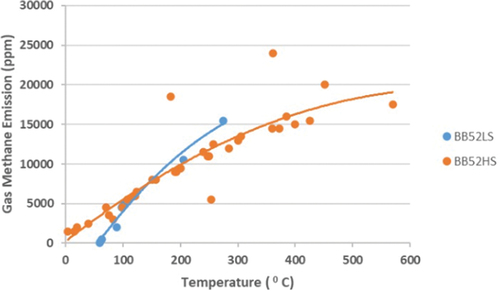
The pattern of influence of temperature on the formation of methane gas emissions in self-heating and spontaneous combustion of sub-bituminous C coal is very consistent with previous researchers where the higher the temperature, the higher the formation of methane gas emissions [Citation44–48]. BB52LS coal, which has a low organic sulfur content in the form of carbon disulfide and high water content, shows the formation of different methane emissions compared to BB52HS coal. The self-heating and spontaneous combustion temperature of BB52LS coal is between 59°C and 275°C, resulting in the formation of methane gas emissions between 50 and 15,500 ppm, while BB52HS coal is between 4°C and 570°C, which results in the formation of methane gas emissions between 1,450 and 24,000 ppm. The pattern of increasing temperature and the formation of methane gas emissions from coal BB52LS and BB52HS shows the same pattern, but the values for the formation of methane gas emissions differ. The process of forming methane gas emissions from BB52HS coal occurs first at a temperature of 4°C compared to BB52LS coal at 59°C. The organic sulfur content greatly influences this difference in the form of carbon disulfide and will continue to increase until the sulfur content is reduced. Other factors that also greatly influence the formation of methane gas emissions during self-heating are coal rank, calorific value, water content, and volatile matter. shows the correlation of temperature with the formation of methane gas emissions.
3.5. Relationship between methane gas emissions and the spontaneous combustion time
The effect of coal spontaneous combustion time is intended to see the stages of the process in forming methane gas emissions in the West Banko Mining Area (). The stages of the coal spontaneous combustion process can be divided into three stages, namely: combustion and drying, devolatilization and pyrolysis, and charcoal combustion, each of which will produce methane gas emissions. The three stages can be grouped into just two: the self-heating stage (combustion and drying) and spontaneous combustion (devolatilization and pyrolysis and charcoal burning). In the combustion and drying stage, an oxidation reaction occurs between coal and oxygen, followed by water evaporation. The combustion and drying stages occur at 30°C, releasing methane gas and carbon oxide. An intense increase in temperature between 30°C and 80°C will result in increased methane and carbon dioxide gas emissions and produce small amounts of alkane gas. In the devolatilization and pyrolysis stages, volatile substances are released into the air at temperatures between 80°C and 170°C, producing increasing amounts of carbon oxide, alkane, and ethylene gas while methane gas emissions are slowing down. The charcoal combustion stage occurs when the remaining carbon reacts with oxygen and leaves ash from the spontaneous combustion process, which occurs at temperatures above 170°C with the production of carbon oxide gas, alkanes, ethylene, and methane gas decreasing [Citation49–51].
4. Conclusion
The study concluded that the formation of methane gas emissions that occur has different values between BB52LS coal and BB52HS coal. The initial measurement stage of methane gas emissions in BB52LS coal occurs between 0.1 and 3.9 minutes, temperatures between 59°C and 275°C, with the formation of methane gas emissions between 50 and 15,500 ppm. Initial measurements of methane gas in BB52HS coal took place between 0.2 and 7.6 minutes and temperatures between 4°C and 570°C with the formation of methane gas emissions between 1,450 and 24,000 ppm. The formation of methane gas emissions in BB52LS coal occurs more quickly due to the influence of the relatively high water content and the relatively low value of organic sulfur content in the form of carbon disulfide compared to BB52HS coal. Observations in the field did not observe the initial process but were measurements at the hotspot directly when spontaneous coal combustion occurred. The relationship pattern between methane gas emissions, temperature, and time in the West Banko Mining Area with the stages of the self-heating process and spontaneous combustion of coal carried out by other researchers is very suitable. BB52LS coal produces methane gas emissions at temperatures between 59°C and 275°C with a spontaneous combustion time of between 0.1 and 3.9 minutes, including the stage between devolatilization and pyrolysis with charcoal combustion. BB52LS coal has a high water content of 30.5% and a low organic sulfur content in carbon disulfide of 0.48%. Meanwhile, BB52HS coal produces methane gas emissions at temperatures between 4°C and 570°C with a combustion time of 0.2–7.6 minutes, including the combustion and drying, devolatilization and pyrolysis, and charcoal burning stages. BB52HS coal has a lower water content of 27.1% and a high organic sulfur content of carbon disulfide of 0.85%. Therefore, organic sulfur content in the form of carbon disulfide and water content plays a vital role in the process of self-heating and spontaneous combustion of coal in the West Banko Mine Area. The self-heating and spontaneous combustion of sub-bituminous C coal that occurs in the temporary stockpile area of the West Banko Mine is greatly influenced by the organic sulfur content in the form of carbon disulfide and the water content of the coal. The higher the organic sulfur content of coal in the form of carbon disulfide, the higher the methane gas emission formation in the self-heating and spontaneous combustion of sub-bituminous C coal. Regression analysis shows that BB52LS coal with low organic sulfur content in the form of carbon disulfide and high water content produces methane gas emissions of 50–15,500 ppm, while BB52HS coal with high organic sulfur content in the form of carbon disulfide and lower water content produces methane gas emissions amounting to 1,450–24,000 ppm.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data underlying this article are available in the article. If you cannot find it, contact the corresponding author.
References
- Yulianto HD, Maulana FI, Wijaya DI. Performance analysis of the temporary stockpile reclaimer system in the mining industry using the Overall Throughput Effectiveness (OTE) method. E3S Web of Conf. 2023;426(2079):1–274. doi: 10.1051/e3sconf/202342602079
- Thabari JA, Auzani AS, Nirbito W, et al. Modeling of coal spontaneous fire in a large-scale stockpile. Int J Technol. 2022;14(2):257–266. doi: 10.14716/ijtech.v14i2.5367
- Chandralal N, Mahapatra D, Shome D, et al. Behaviour of low rank high moisture coal in large stockpile under ambient conditions. Am J Appl Sci Res Form App Nat Sci. 2014;6(1):19–26.
- Wan-Xing R, Zeng-Hui K, De-Ming W. Causes of spontaneous combustion of coal and its prevention technology in the tunnel Fall of ground of extra-thick coal seam. Procedia Eng. 2011;26:717–724. doi: 10.1016/j.proeng.2011.11.2228
- Govender S, du Plessis JJL, Webber-Youngman RCW. A critical investigation into spontaneous combustion in coal storage bunkers. J South Afr Inst Min Metall. 2021;121(5):251–259. doi: 10.17159/2411-9717/16-478/2021
- Gao D, Guo L, Wang F, et al. Study on the spontaneous combustion tendency of coal based on grey relational and multiple regression analysis. ACS Omega. 2021;6(10):6736–6746. doi: 10.1021/acsomega.0c05736
- Rendana M, Idris WMR, Rahim SA. Changes in air quality during and after large-scale social restriction periods in Jakarta city, Indonesia. Acta Geophysica. 2022;70(5):2161–2169. doi: 10.1007/s11600-022-00873-w
- Yu Z, Xueqing Z, Wen Y, et al. Pore structure and its impact on susceptibility to coal spontaneous combustion based on multiscale and multifractal analysis. Sci Rep. 2020;10(1):1–14. doi: 10.1038/s41598-020-63715-z
- Protasov S, Seregin E, Portola V, et al. Study of the parameters of spontaneous fire seats in coal pit rock dumps. E3S Web Of Conf. 2021;315(2007):1–8. doi: 10.1051/e3sconf/202131502007
- Su G, Jia B, Wang P, et al. Risk identifcation of coal spontaneous combustion based on COWA modifed G1 combination weighting cloud model. Scien Rep. 2022;12(2992):1–7. doi: 10.1038/s41598-022-06972-4
- Wang W, Liang R, Qi Y, et al. Study on the prediction model of coal spontaneous combustion limit parameters and its application. Fire. 2023;6(381):1–15. doi: 10.3390/fire6100381
- Zhang L, Li Z, He W, et al. Study on the change of organic sulfur forms in coal during low-temperature oxidation process. Fuel. 2018;222:350–361. doi: 10.1016/j.fuel.2018.02.157
- Onifade M, Genc B, Wagner N. Influence of organic and inorganic properties of coal-shale on spontaneous combustion liability. Int J Min Sci Technol. 2019;29(6):851–857. doi: 10.1016/j.ijmst.2019.02.006
- Sari SL, Rahmawati MA, Triyoga A, et al. Impact of sulphur content on coal quality at delta plain depositional environment: case study in geramat district, lahat regency, South Sumatra. J Geosci Eng Environ Tech. 2017;2(03):183–190. doi: 10.24273/jgeet.2017.2.3.301
- Leitea FFGD, Alves BJR, Nobrega GNN, et al. Checking the progress of using the static chamber method for themeasurement of greenhouse gases in Latin America. Carbon Manage. 2021;12(6):649–661. doi: 10.1080/17583004.2021.1995503
- Ortega OAC, Beltran PEP, Pineda GSH, et al. Construction and operation of a respiration chamber of the head-box type for methane measurement from cattle. Animals. 2020;10(227):1–11. doi: 10.3390/ani10020227
- Zhang Y, 2022. Experimental and Theoretical Investigation on the Initiation Mechanism of Low-Rank Coal’s Self-Heating Process [ Dissertation]. Department of Mining Engineering West Virginia University.
- Gerald B, Patson TF. Parametric and nonparametric tests: a brief review. Int J Stat Distrib Appl. 2021;7(3):78–82. doi: 10.11648/j.ijsd.20210703.12
- Rendana M, Idris WMR, Rahim SA. Atmospheric methane condition over the South Sumatera peatland during the COVID-19 pandemic. Aerosol Air Qual Res. 2021;21(10):210072. doi: 10.4209/aaqr.210072
- Lee S, Yoo J, Umar DH. Low-rank coal and poly fatty acid distillate characterization as a preparation of coal upgrading palm oil technology. IOP conf Ser: Earth Environ Sci. 2021;882(120380):1–11. doi: 10.1088/1755-1315/882/1/012038
- Daulay B, Santoso B, Sodikin AI. Indonesian low rank coal resources to which ubc technology is commercially applicable. Indones Min J. 10(80):18–23.
- Schweinfurth SP. An introduction to coal quality. In: Pierce BS Dennen KO, editors Chapter C of the National Coal Resource Assessment Overview, U.S. Geological survey. Reston, Virginia; 2009.
- Belkin HE, Tewalt SJ, Hower JC, et al. Geochemistry and petrology of selected coal samples from Sumatra, Kalimantan, Sulawesi, and Papua, Indonesia. Int J Coal Geol. 2009;77(3–4):260–268. doi: 10.1016/j.coal.2008.08.001
- Monika I, Sulistyohadi F. Ash deposit characteristics of blended coal in coal combustion process. Indones Min J. 2019;22(1):49–60. doi: 10.30556/imj.Vol22.No1.2019.675
- Gautama RS, Kusuma GJ, Pujiantoro E, et al., 2018. On the spatial variation of geochemical rock characteristics in coal mining: case bukit asam coal mine in South Sumatra. Indonesia, Conference: International Conference of Acid Rock Drainage & IMWA2018 Annual, Pretoria, South Africa (Tshwane University of Technology), Risk to Opportunity, II, 604–611
- Santoso B, Daulay B. Coalification trend in South Sumatera Basin. Indones Min J. 2006;9(6):9–21.
- Rich AL, Patel JT. Carbon disulfide (CS2) mechanisms in formation of atmospheric carbon dioxide (CO2) formation from unconventional shale gas extraction and processing operations and global climate change. Environ Health Insights. 2015;9(s1):35–39. doi: 10.4137/EHI.S15667
- Gao F, Jia Z, Qin M, et al. Effects of organic sulfur on oxidation spontaneous combustion characteristics of coking coal. Energy Explor Exploit. 2021;40(1):193–205. doi: 10.1177/01445987211049045
- Qu Z, Sun F, Gao J, et al. A new insight into the role of coal adsorbed water in low-temperature oxidation: enhanced OH radical generation. Combust Flame. 2019;208:27–36. doi: 10.1016/j.combustflame.2019.06.017
- Li Z, Zhang M, Yang Z, et al. Exothermic characteristics of coal during low-temperature oxidation based on grey correlation method. Energy Rep. 2022;8:6744–6752. doi: 10.1016/j.egyr.2022.05.029
- Naifu Cao N, Gang Wang G, Yuntao Liang Y, et al. Study on the microscopic mechanism of spontaneous combustion and oxidation kinetics of water-leached coal. J Chem. 2021;2021(5564290):1–15. doi: 10.1155/2021/5564290
- Onifade M, Genc B. Spontaneous combustion of coals and coal-shales. Int J Min Sci Technol. 2018;28(6):933–940. doi: 10.1016/j.ijmst.2018.05.013
- Silva J, Castro C, Teixeira S, et al. Evaluation of the gas emissions during the thermochemical conversion of eucalyptus woodchips. Processes. 2022;10(2413):1–12. doi: 10.3390/pr10112413
- Wang JR, Chen QW, Deng CB, et al. Reaction mechanism of coal spontaneous combustion producing methane. J China Coal Soc. 2009;34(12):1660–1664.
- Khairulin S, Kerzhentsev M, Salnikov A, et al. Direct selective oxidation of hydrogen sulfide: laboratory, pilot and industrial tests. Catalysts. 2021;11(1109):1–45. doi: 10.3390/catal11091109
- Ma H, Sichen L, Zhou L, et al. Detailed kinetic modeling of H2S formation during fuel-rich combustion of pulverized coal. Fuel Process Technol. 2020;199(106276):1–10. doi: 10.1016/j.fuproc.2019.106276
- Gao F, Jia Z, Shan Y, et al. Influence of organic sulfur on low-temperature oxidation of coal and its transition characteristics. ACS Omega. 2022;7(44):39830–39839. doi: 10.1021/acsomega.2c03824
- Nelson MI, Chen XD. Survey of experimental work on the self-heating and spontaneous combustion of coal. Geol Soc Am Rev Eng Geol. 2007;XVIII:31–83.
- Zhafira HK, Widiani AS, Nugroho YS. Control of spontaneous combustion of sub-bituminous coal by means of heat exchanger submersion inside the piles, IOP conf. Ser: J Phys Conf Ser. 2018;1107(62004):1–6. doi: 10.1088/1742-6596/1107/6/062004
- Dong Z, Sun L, Jia T, et al. Rapid contrastive experimental study on the adiabatic spontaneous combustion period of loose lignite. ACS Omega. 2021;6(50):34989–35001. doi: 10.1021/acsomega.1c05667
- Xi Z, Xi K, Lu L, et al. Study on oxidation characteristics and conversion of sulfur-containing model compounds in coal. Fuel. 2023;331(1):125756. doi: 10.1016/j.fuel.2022.125756
- Zheng H, Li Y, Zhang L, et al. Study on the effect of organic sulfur on coal spontaneous combustion based on model compounds. Fuel. 2021;289(119846):1–10. doi: 10.1016/j.fuel.2020.119846
- Yusuf M. The role of organic sulfur in the formation of methane emissions on the spontaneous combustion of coal. J Ecol Eng. 2023;24(4):192–201. doi: 10.12911/22998993/159632
- Dong X, Wen Z, Wang F, et al. Law of gas production during coal heating oxidation. Int J Min Sci Technol. 2019;29(4):617–620. doi: 10.1016/j.ijmst.2019.06.011
- Guo J, Quan Y, Cai G, et al. Meticulous graded and early warning system of coal spontaneous combustion based on index gases and characteristic temperature. ACS Omega. 2023;8(7):6801–6812. doi: 10.1021/acsomega.2c07401
- Deng J, Lei C, Xiao Y, et al. Determination and prediction on “three zones” of coal spontaneous combustion in a gob of fully mechanized caving face. Fuel. 2018;211:458–470. doi: 10.1016/j.fuel.2017.09.027
- Gui X, Xue H, Zhan X, et al. Measurement and numerical simulation of coal spontaneous combustion in goaf under Y‑type ventilation mode. ACS Omega. 2022;7(11):9406–9421. doi: 10.1021/acsomega.1c06703
- Zhao J, Ming H, Guo T, et al. Semi‑enclosed experimental system for coal spontaneous combustion for determining regional distribution of high‑temperature zone of coal fire. Int J Coal Sci Technol. 2022;9(62):1–14. doi: 10.1007/s40789-022-00535-8
- Marangwanda GT, Madyira DM, Babarinde TO. Coal combustion models: an overview. J Phys. 2019;1378(32070):1–13. doi: 10.1088/1742-6596/1378/3/032070
- Armand CT, Akong O, Bonoma B. Numerical study of burning of biomass in fixed bed. Energy Power Eng. 2019;11(2):35–57. doi: 10.4236/epe.2019.112003
- Schneider T, Muller D, Kar J. Effect of natural ilmenite on the solid biomass conversion of inhomogeneous fuels in small-scale bubbling fluidized beds. Energies. 2022;15(2747):1–21. doi: 10.3390/en15082747