Abstract
Asthma–COPD overlap syndrome (ACOS) is a commonly encountered chronic airway disease. However, ACOS is still a consensus-based clinical phenotype and the underlying inflammatory mechanisms are inadequately characterized. To clarify the inflammatory mediatypical for ACOS, five biomarkers, namely interleukin (IL)-13, myeloperoxidase (MPO), neutrophil gelatinase-associated lipocalin (NGAL), chitinase-like protein (YKL-40), and IL-6, were selected. This study hypothesized that sputum biomarkers relevant for airway inflammation in asthma (IL-13), COPD (MPO, NGAL), or in both asthma and COPD (YKL-40, IL-6) could be used to differentiate ACOS from COPD and asthma. The aim of this study was to characterize the inflammatory profile and improve the recognition of ACOS. Induced sputum levels of IL-13, MPO, NGAL, YKL-40, and IL-6 were measured by enzyme-linked immunosorbent assay/Luminex assay in a Finnish discovery cohort (n=90) of nonsmokers, smokers, and patients with asthma, COPD, and ACOS and validated in a Japanese cohort (n=135). The classification accuracy of potential biomarkers was compared with area under the receiver operating characteristic curves. Only sputum NGAL levels could differentiate ACOS from asthma (P<0.001 and P<0.001) and COPD (P<0.05 and P=0.002) in the discovery and replication cohorts, respectively. Sputum NGAL levels were independently correlated with the percentage of pre-bronchodilator forced expiratory volume in 1 second predicted in multivariate analysis in the discovery and replication cohorts (P=0.001 and P=0.002, respectively). In conclusion, sputum biomarkers reflecting both airway inflammation and remodeling of the tissue show potential in differentiation between asthma, COPD, and ACOS.
Keywords:
Introduction
The asthma–COPD overlap syndrome (ACOS) has recently been recognized as a consensus-based clinical phenotype sharing the features of asthma and COPD.Citation1–Citation3 Approximately 20%–50% of patients with irreversible airway obstruction show airflow variability characteristic to asthma.Citation4–Citation7 Differential diagnosis of ACOS from asthma and COPD is increasingly important, since ACOS has a poor prognosis and different treatment guidelines. ACOS is associated with low health-related quality of life,Citation6 increased exacerbation rate and hospital admissions, a rapid decline in lung function, and high mortality.Citation5,Citation6,Citation8 However, ACOS cannot be diagnosed unambiguously on the basis of lung function tests, patients’ demographics, and sputum cell counts or by imaging of the lungs.Citation6,Citation9,Citation10 There is an unmet need to develop novel diagnostic biomarkers for clinical assessment of ACOS.
Although the inflammatory pathways of asthma and COPD are markedly different,Citation11,Citation12 they coincide in ACOS, and both eosinophils and neutrophils in sputum are characteristic for ACOS. Clinically, the main interest would be to identify those patients with ACOS who could be responsive to inhaled corticosteroids.Citation13 For this, the assessment of fractional exhaled nitric oxide or immunoglobulin E in COPD patients could be useful.Citation14 For the current study, interleukin (IL)-13,Citation15,Citation16 which is a T-helper 2 type marker known to be important in eosinophilic inflammation and asthma, was analyzed. In addition, chitinase-like protein (YKL-40),Citation17,Citation18 which is elevated in both asthma and COPD, was selected.Citation19 IL-6 was selected because its serum and sputum levels are associated with impaired lung function,Citation15,Citation20,Citation21 and elevated serum IL-6 levels were detected in patients with ACOS.Citation22 In addition, myeloperoxidase (MPO) and neutrophil gelatinase-associated lipocalin (NGAL) were assessed based on the authors’ earlier findings that suggested enhanced neutrophil-mediated inflammation and/or airway epithelial injury in ACOS.Citation9
This study hypothesized that sputum biomarkers relevant for airway inflammation in asthma (IL-13), COPD (MPO, NGAL), or in both asthma and COPD (YKL-40, IL-6) could be used to differentiate ACOS from COPD and asthma and further could be used to improve the determination and recognition of ACOS.
Subjects and methods
Subjects
The discovery cohort consisted of 90 volunteer individuals who were a part of the cohort previously reported in Iwamoto et al.Citation9 The cohort size was smaller than the original one because of the consumption of sputum samples. The patients, who were a part of the longitudinally followed cohort of Finnish asthma and COPD patients (FinnCADStudy),Citation6,Citation9,Citation23 were recruited from the Helsinki University Hospital. Based on their medical history and self-reported questionnaire data, the subjects were categorized into five groups: healthy nonsmokers (NSs, n=14), healthy smokers (HSs, n=14), and patients with asthma (n=24), COPD (n=20), and ACOS (n=18). Spirometry with a bronchodilator test and the diffusing capacity of the lung for carbon monoxide were performed on all participants. The control subjects (NSs and HSs) consisted of responders to an advertisement in the Helsinki University Hospital and the local media. These participants were asymptomatic, never, or current smokers with no history of lung disease confirmed by a normal lung function with post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ≥0.7 in spirometry.
The diagnosis of asthma was based on the British Guidelines on Asthma ManagementCitation24 and defined as reversible airflow obstruction with a post-bronchodilator FEV1/FVC ≥0.7, with one or more of the following findings: a post-bronchodilator increase in FEV1 of ≥12%, a bronchodilator response of ≥15% or diurnal variation of ≥20% in peak expiratory flow recording, moderate-to-severe bronchial hyperreactivity, or a decrease in FEV1 of ≥15% in the exercise test. COPD was diagnosed according to the American Thoracic Society/European Respiratory Society Task Force recommendationsCitation23,Citation25,Citation26 and defined by incompletely reversible airflow obstruction with a post-bronchodilator FEV1/FVC <0.7.Citation1 ACOS was identified by the features that it shares with both asthma and COPD according to the Global Initiative for Asthma guidelinesCitation2 and Global Initiative for Obstructive Lung Diseases strategy.Citation1 In the ACOS group, the age of onset was >40 years and all the patients had history of smoking. The patients with ACOS had increased airflow variability and incompletely reversible airflow obstruction. Patients with COPD and ACOS had no history of alpha-1-antitrypsin deficiency in their family. All patients had respiratory symptoms and were current or former smokers (>10 pack-years) when developed their lung disease. None of the patients received oral glucocorticoid treatment or experienced an exacerbation during the study period. Signs of atopy were not included in the diagnostic criteria.
The Japanese replication cohort (n=135) consisted of NSs (n=22), HSs (n=40), and patients with asthma (n=21), COPD (n=35), and ACOS (n=17). The diagnosis of asthma was assessed by specialists in respiratory medicine using the Global Initiative for Asthma guidelines.Citation2 COPD diagnosis was based on long-term smoking (>10 pack-years) and a pre-bronchodilator FEV1/FVC <0.7.Citation1,Citation27,Citation28. The individuals in the ACOS group consisted of patients who had been diagnosed with both asthma and COPD. All the patients with asthma, COPD, and ACOS were in stable condition and were using regular inhaled medications. For healthy control subjects, only prebronchodilator spirometries were performed, and their prebronchodilator FEV1/FVC values were >0.7.
This study was approved by the Ethics Committees of Helsinki University Central Hospital and Hiroshima University and conducted in accordance with the ethical standards established in the 1975 Declaration of Helsinki. All participants provided written informed consent.
Sampling of sputum
Sputum was induced by inhalation of hypertonic saline and treated with dithioerythritol (Sigma-Aldrich Co., St Louis, MO, USA) as recommended by the European Respiratory Society Task Force and described earlier in detail.Citation9,Citation29,Citation30 The supernatant was frozen at −80°C for biochemical analyses. Cell viability was studied with Trypan blue in a Burker chamber.Citation31 Cytocentrifuge preparations were made by Cytospin (Shandon Cytospin 3) and centrifuged at 450 rpm for 6 minutes. The slides were stained with May–Grunwald–Giemsa solution (EMD Millipore, Billerica, MA, USA) for cell differential counts. Detailed cell profiles were assessed based on 400 cells counted from each slide. Only the representative samples with <70% of squamous epithelial cells were accepted for the assessments.Citation9,Citation31 The slides were frozen at −20°C. Induction of the sputum samples in the Japanese cohort has been described earlier.Citation32
Enzyme-linked immunosorbent assay and Luminex assays
YKL-40 was measured by Quantikine ELISA (R&D Systems, Inc., Minneapolis, MN, USA), IL-6 by Magnetic Human High Sensitivity Luminex assay (R&D Systems, Inc.), IL-13 by enzyme-linked immunosorbent assay (ELISA; USCN Life Science Inc., Wuhan, People’s Republic of China), MPO by ELISA (Abnova Inc., Walnut, CA, USA), and NGAL by ELISA (USCN Life Science Inc.) according to the manufacturers’ instructions. The minimal detectable dose for YKL-40, IL-6, IL-13, MPO, and NGAL were 3.55 pg/mL, 0.14 pg/mL, 5.7 pg/mL, 0.78 ng/mL, and 17 pg/mL, respectively. The analysis of MPO and NGAL in the discovery cohort has been performed earlier but added in the present analysis to identify the most informative panel of biomarkers.Citation9
Statistical analysis
The demographics are expressed as mean ± standard deviation, and the biomarker concentrations as medians with interquartile range. All statistical analyses were performed with the SPSS 20.0 software program (IBM Corporation, Armonk, NY, USA). Comparisons between groups were evaluated by the Kruskal–Wallis test followed by the Mann–Whitney U test, when appropriate. Sputum biomarkers were further analyzed by the area under the curve (AUC) statistics for their predictive capability to distinguish ACOS from asthma, and ACOS and asthma from COPD or NSs. Spear-man’s correlations of the induced sputum marker levels with age, lung function, and sputum cell counts were calculated. Multivariate stepwise regression analysis was performed to obtain the variables to model ACOS. Due to the differences in sputum processing, such as a single versus three times inhalation of hypertonic saline in the discovery and replication cohort, respectively, the concentrations of sputum biomarkers were not directly comparable between the two cohorts. To make the comparison feasible, raw data was used in the comparisons within each cohort and standardized data (Z-scores) in the comparisons between the two cohorts. P-values of <0.05 were considered statistically significant.
Results
Subject characteristics
The characteristics of the study subjects in the discovery cohort are shown in . Healthy controls were ∼10 years younger than the patients. Patients with COPD and ACOS had more pack-years and significantly lower FEV1, FEV1/FVC ratio, and diffusing capacity of the lung for carbon monoxide compared with those in the NS and asthma groups (). The patients with ACOS had significant reversibility in the bronchodilation test and diurnal variation of peak expiratory flow compared with those among the COPD patients. None of the participants had experienced an exacerbation or a respiratory tract infection within a month prior the study.
Table 1 Clinical characteristics and biomarker concentrations of the study subjects across the discovery cohort (n=90)
Successfully induced sputum samples were obtained from 90 individuals. The representativeness of samples was confirmed by cytospin centrifugation cell profiles. As expected, patients in the asthma (n=24) and ACOS (n=18) groups had a higher percentage of sputum eosinophil compared with that in the HS group (n=14). In addition, COPD (n=20) and ACOS groups had a higher percentage of sputum neutrophils compared with that in the NS (n=14) and HS groups ().
The characteristics of the study subjects in the replication cohort are shown in . There were no differences in pre-FEV1/FVC, pack-years, neutrophil%, and eosinophil% between the patients with ACOS in the replication and in the discovery cohorts. However, patients with ACOS had greater pre-FEV1% and pre-FVC% values in the replication cohort than in the discovery cohort (P=0.001 and P<0.001, respectively).
Table 2 Clinical characteristics and biomarker concentrations of the study subjects across the replication cohort (n=135)
Sputum MPO and IL-13 were elevated in ACOS when compared with healthy controls
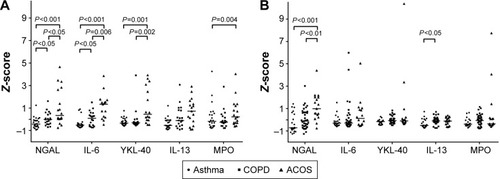
The sputum levels of MPO and IL-13 were significantly increased in the ACOS groups when compared with the levels in the NS (P=0.012 and P<0.001, respectively) and HS groups (P=0.008 and P<0.001, respectively; ). There was also a significant difference in sputum MPO levels between the ACOS and asthma groups (P=0.004). However, the sputum MPO and IL-13 levels could not distinguish ACOS from COPD (P=0.128 and P=0.088, respectively).
Sputum YKL-40, IL-6, and NGAL were elevated in ACOS when compared with COPD and asthma
The sputum levels of YKL-40, IL-6, and NGAL were significantly increased in ACOS when compared with the NS (P<0.001, P<0.001, P<0.001, respectively) and HS groups (P<0.001, P=0.001, and P=0.001, respectively; ). In addition, the sputum levels of YKL-40, IL-6, and NGAL were significantly increased in ACOS when compared with the asthma (P=0.001, P<0.001, and P<0.001, respectively) and COPD groups (P=0.002, P=0.006, and P=0.026, respectively). Sputum IL-6 and NGAL were also significantly increased in the COPD group when compared with the asthma group (P=0.034 and P=0.025, respectively), while sputum YKL-40 levels were not significantly different between the COPD and asthma groups (P>0.05).
Sputum YKL-40, IL-6, and NGAL could differentiate ACOS from COPD and asthma
A receiver operating characteristic curve analysis was carried out to evaluate the sensitivity, specificity, and diagnostic accuracy of the classical variables and the novel biomarkers (). All tested sputum biomarkers (MPO, NGAL, YKL-40, IL-6, and IL-13) could distinguish ACOS significantly from the other non-ACOS groups (AUC >0.7). Importantly, sputum biomarkers YKL-40, IL-6, and NGAL could distinguish ACOS significantly from both asthma and COPD (AUC >0.7). In addition, sputum MPO levels could differentiate ACOS from asthma, but not ACOS from COPD. Sputum IL-13 levels did not significantly separate ACOS from asthma or ACOS from COPD.
Table 3 ROC analysis for sputum biomarkers in the discovery cohort
Relationships between the sputum biomarkers and clinical characteristics
The study subjects in the asthma, COPD, and ACOS groups were pooled to analyze the correlations between biomarker concentrations as the dependent factor and clinical variables. Multivariate analysis revealed pack-years and age to be the independent predictors for sputum MPO, whereas age and pre-FEV1% were independent predictors for YKL-40 (). Sputum IL-6 was an independent predictor for pre-FEV1% predicted and pack-years, and sputum NGAL was independently associated with pre-FEV1% predicted ( and ).
Table 4 Multivariate stepwise analysis of all subjects with sputum biomarker as the dependent variable in patients with asthma, COPD, and ACOSTable Footnotea
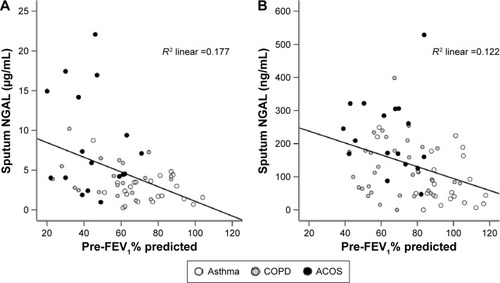
Figure 1 Correlation of sputum NGAL with pre-FEV1% predicted in patients with asthma, COPD, and ACOS in the discovery (A) and in the replication cohorts (B).
Validation of the findings in an independent replication data set
The clinical data and the levels of sputum biomarkers (MPO, NGAL, YKL-40, IL-6, and IL-13) in the replication cohort are shown in . Sputum neutrophil% count was significantly different between the groups (P=0.008), and eosinophil% count showed a tendency of differences between the groups (P=0.051).
In the replication cohort, sputum IL-6, NGAL, and IL-13 levels had statistically significant differences between the study groups (). The sputum levels of IL-6 and NGAL were significantly increased in ACOS when compared with the NS (P=0.001 and P<0.001, respectively) and the HS groups (P=0.018 and P<0.001, respectively) (). Importantly, only sputum NGAL levels were elevated in ACOS when compared with the COPD (P=0.002) and asthma (P<0.001) groups () and had an increasing trend in COPD when compared with those in the asthma group (P=0.068). Other sputum biomarkers did not differ significantly between ACOS and asthma or between ACOS and COPD in the replication cohort, but there was a significant difference in sputum IL-13 levels between asthma and COPD (P=0.025).
Figure 2 Expression levels of sputum biomarkers in patients with asthma, COPD, and ACOS in the discovery (A) and replication cohorts (B).
Abbreviations: ACOS, asthma–COPD overlap syndrome; IL, interleukin; MPO, myeloperoxidase; NGAL, neutrophil gelatinase-associated lipocalin; YKL-40, chitinase-3-like protein 1.
NGAL levels could differentiate ACOS from the other study participants (non-ACOS) with an AUC value of 0.827 (95% CI: 0.727–0.926, P<0.001) in the replication cohort. In addition, sputum NGAL could differentiate ACOS from the COPD and asthma groups with AUC values of 0.794 (95% CI: 0.629–0.924, P=0.002) and 0.840 (95% CI: 0.717–0.964, P<0.001), respectively.
The asthma, COPD, and ACOS groups in the replication cohort were pooled to analyze the correlations in the univariate and multivariate analyses. Sputum YKL-40 and sputum IL-6 did not correlate with any clinical parameters. Multivariate analysis revealed that sputum IL-13 correlated with pack-years and sputum MPO correlated with neutrophil% count. In agreement with the results from the discovery cohort, NGAL levels independently correlated with pre-FEV1% predicted in the replication cohort (P=0.002, ).
Discussion
This study analyzed the biomarkers related to asthma or COPD or implicated in both diseases to discover inflammatory profiles typical for ACOS in two independent cohorts. The levels of sputum IL-13 and MPO were higher in the ACOS patients than in the healthy controls in the discovery cohort, but this was not apparent in the replication cohort. In both cohorts, the sputum levels of NGAL, IL-6, and YKL-40 were elevated in ACOS when compared with those of NSs. However, only sputum NGAL could differentiate ACOS from COPD and asthma in both cohorts.
The most recent treatment guidelines present features that characterize ACOS rather than provide a formal definition of ACOS.Citation33 Four distinct pathways have been proposed for ACOS.Citation34 Airflow limitation in early life can persist through adolescence into adulthood and ACOS is more likely than severe asthma if other risk factors, such as smoking, are present. A second pathway is represented in COPD patients with substantial smoking histories or other exposures and late-onset features of asthma. A third asthma-dominant pathway is represented by asymptomatic adults with airway hyperresponsiveness who progress to chronic airflow limitation compatible with a diagnosis of COPD. Finally, a fourth pathway recognizes the link between early-life risk factors and small lungs, with an increased risk of development of fixed airflow limitation and asthma. Thus, from the molecular genetic point of view, ACOS can represent a very heterogeneous group of patients. All patients with ACOS in the discovery cohort were either current or former smokers. There were eleven patients who mostly represent the second COPD-dominant pathway in the discovery cohort, and seven patients who developed fixed airway limitation after asthma diagnosis. There was more variation in the biomarker levels in the replication cohort than in the discovery cohort suggesting that there may be more etiological variability between the patients. The final goal would be to identify a panel of markers with the optimal capability to dissect the endotypes valid for prognosis and in treatment choices.
Elevated IL-6 and YKL-40 levels were observed in patients with ACOS in both cohorts although the differences between patients with ACOS, asthma, and COPD were not statistically significant in the replication cohort. This could be explained by the higher etiological variability between the patients or by the milder airway limitation. In a recent study, elevated serum IL-6 levels were detected in patients with ACOS, although systemic IL-6 levels did not differentiate COPD from ACOS.Citation22 Circulating biomarkers may also not be specific for airway disease unlike sputum samples and there is no direct link between systemic and airway inflammatory mediators.Citation35
The important finding of this study was that sputum NGAL levels were significantly increased in patients with ACOS in comparison with the levels in the asthma and COPD groups in two independent cohorts. Therefore, although ACOS might be a complex and heterogeneous disease, the results of the current study further support that increased sputum NGAL might be a characteristic feature of ACOS. Although MPO and NGAL are both COPD-related biomarkers, MPO is associated with a local activation of neutrophils,Citation9,Citation36,Citation37 whereas NGAL is not only attributed to activated neutrophils but could also be secreted by the respiratory epithelial cells in response to inflammatory stimuliCitation9,Citation38 and by myeloid and epithelial cells in response to toll-like receptor activation during bacterial infections.Citation39,Citation40 Patients with ACOS seem to have more frequent exacerbations,Citation41,Citation42 and microorganisms are one of the main etiologic factor involved in exacerbations of COPD. Therefore, the sputum NGAL levels might be related to airway inflammation and low-grade microbial colonization, which predispose patients with ACOS to acute viral infections and exacerbations.
In addition, earlier reports have shown that NGAL expression in lung can be induced by reactive oxygen species.Citation43,Citation44 Because the pathophysiology of both asthma and COPD is associated with oxidative processes induced by environmental exposures and airway inflammation,Citation45 ACOS might have augmented oxidative stress that could result in airway reactivity, injury, and remodeling and might also relate to increased sputum NGAL levels. Finally, it is important that increased sputum NGAL was independently correlated with degree of airflow limitation in both the discovery and replication cohorts. This finding indicates that sputum NGAL may be a candidate marker for airway remodeling, but the clinical utility of this finding should be tested in a future prospective study.
Although many biomarkers examined in this study are likely to be associated with other morbidities, the use of sputum may improve their specificity for airway disease. The changes in sputum biomarker levels are likely to reflect the changes of cell composition and lung function during the development of chronic airway disease. Therefore, this study results suggest that ACOS could be characterized by mediators related to airway inflammation and airway remodeling. Future studies with high-throughput design are required to fully reveal the transcriptomic or proteomic differences in chronic airway disease.
The strength of this study design was that the control subjects did not have any other exposures, and the diagnosis criteria for asthma, COPD, and ACOS were based on international guidelines, sputum cell counts, and clinical characteristics of the disease. The sample size was limited, but the obtained results were replicated in another independent cohort.
Conclusion
The results from the current study indicate that ACOS is associated with sputum biomarkers that drive both airway inflammation and tissue remodeling, and these biomarkers could have diagnostic value in differentiation of ACOS from asthma and COPD.
Acknowledgments
The authors would like to thank Professor Vuokko Kinnula for her contribution prior to her death on November 17, 2012. Ms Tinja Kanerva, Tiina Lapinkari, Kerstin Alhskog, Kirsi Elorinne, Sari Tillander, Yukari Iyanaga, Kakuhiro Yamaguchi, Naoko Higaki, and Shuai Ni are acknowledged for their help and excellent technical assistance. The authors also thank Doctor Päivi Piirilä for her help at accessing lung function tests. This work was financially supported by the EVO funding of the Helsinki University Central Hospital, Research Funds of the University of Helsinki, Sigrid Jusé-lius Foundation, Finnish Anti-Tuberculosis Association Foundation, the Jalmari and Rauha Ahokas Foundation, the Finnish Cultural Foundation, and the SalWe Research Program for IMO (Tekes – the Finnish Funding Agency for Technology and Innovation grant 648/10). Jing Gao was further supported by the China Scholarship Council, CIMO, the Research Foundation of the Pulmonary Diseases, Ida Montin Foundation, and Väinö and Laina Kivi Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
- VestboJHurdSSAgustiAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- ReddelHKBatemanEDBeckerAA summary of the new GINA strategy: a roadmap to asthma controlEur Respir J201546362263926206872
- PostmaDSRabeKFThe asthma-COPD overlap syndromeN Engl J Med2015373131241124926398072
- GibsonPGMcDonaldVMAsthma-COPD overlap 2015: now we are sixThorax201570768369125948695
- GibsonPGSimpsonJLThe overlap syndrome of asthma and COPD: what are its features and how important is it?Thorax200964872873519638566
- KauppiPKupiainenHLindqvistAOverlap syndrome of asthma and COPD predicts low quality of lifeJ Asthma201148327928521323613
- MarshSETraversJWeatherallMProportional classifications of COPD phenotypesThorax200863976176718728201
- AndersenHLampelaPNevanlinnaASaynajakangasOKeistinenTHigh hospital burden in overlap syndrome of asthma and COPDClin Respir J20137434234623362945
- IwamotoHGaoJKoskelaJDifferences in plasma and sputum biomarkers between COPD and COPD-asthma overlapEur Respir J201443242142923794464
- HardinMSilvermanEKBarrRGCOPDGene InvestigatorsThe clinical features of the overlap between COPD and asthmaRespir Res20111212721951550
- BarnesPJThe cytokine network in asthma and chronic obstructive pulmonary diseaseJ Clin Invest2008118113546355618982161
- BarnesPJImmunology of asthma and chronic obstructive pulmonary diseaseNat Rev Immunol20088318319218274560
- BarrechegurenMEsquinasCMiravitllesMThe asthma – chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challengesCurr Opin Pulm Med2015211747925405671
- TamadaTSugiuraHTakahashiTBiomarker-based detection of asthma-COPD overlap syndrome in COPD populationsInt J Chron Obstruct Pulmon Dis2015102169217626491283
- DenteFLBacciEVagagginiBPaggiaroPCytokines in induced sputum: a role for the ratio of IL-6/IL-13 in the differentiation of asthma and chronic obstructive pulmonary disease?Respiration20128429810022488352
- Wills-KarpMLuyimbaziJXuXInterleukin-13: central mediator of allergic asthmaScience19982825397225822619856949
- TangHFangZSunYYKL-40 in asthmatic patients, and its correlations with exacerbation, eosinophils and immunoglobulin EEur Respir J201035475776020356987
- OtsukaKMatsumotoHNiimiASputum YKL-40 levels and pathophysiology of asthma and chronic obstructive pulmonary diseaseRespiration201283650751921968467
- JamesAJReiniusLEVerhoekMIncreased YKL-40 and chi-totriosidase in asthma and chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2016193213114226372680
- Grubek-JaworskaHPaplinskaMHermanowicz-SalamonJIL-6 and IL-13 in induced sputum of COPD and asthma patients: correlation with respiratory testsRespiration201284210110722311051
- AttaranDLariSMTowhidiMInterleukin-6 and airflow limitation in chemical warfare patients with chronic obstructive pulmonary diseaseInt J Chron Obstruct Pulmon Dis2010533534021037957
- FuJJMcDonaldVMGibsonPGSimpsonJLSystemic inflammation in older adults with asthma-COPD overlap syndromeAllergy Asthma Immunol Res20146431632424991455
- LaitinenTHodgsonUKupiainenHReal-world clinical data identifies gender-related profiles in chronic obstructive pulmonary diseaseCOPD J Chronic Obstr Pulmonary Dis200964256262
- British Thoracic Society Scottish Intercollegiate Guidelines NBritish guideline on the management of asthmaThorax200863Suppl 4iv1iv12118463203
- MillerMRHankinsonJBrusascoVStandardisation of spirom-etryEur Respir J200526231933816055882
- EnrightPSklootGHerbertRStandardization of spirometry in assessment of responders following man-made disasters: World Trade Center worker and volunteer medical screening programMt Sinai J Med200875210911418500705
- IshikawaNHattoriNKohnoNKobayashiAHayamizuTJohnsonMAirway inflammation in Japanese COPD patients compared with smoking and nonsmoking controlsInt J Chron Obstruct Pulmon Dis20151018519225670894
- ViljanenAAHalttunenPKKreusKEViljanenBCSpirometric studies in non-smoking, healthy adultsScand J Clin Lab Invest Suppl19821595206957974
- DjukanovicRSterkPJFahyJVHargreaveFEStandardised methodology of sputum induction and processingEur Respir J Suppl2002371s2s12361359
- KellyMMKeatingsVLeighRAnalysis of fluid-phase mediatorsEur Respir J Suppl20023724s39s12361360
- LouhelainenNStarkHMazurWRytilaPDjukanovicRKinnulaVLElevation of sputum matrix metalloproteinase-9 persists up to 6 months after smoking cessation: a research studyBMC Pulm Med2010101320226090
- ShiotaNYokoyamaAHarutaYHattoriNKohnoNAssociation of airway inflammation with asthma control level evaluated by the asthma control testJ Asthma201148990791321942275
- BouletLPFitzGeraldJMReddelHKThe revised 2014 GINA strategy report: opportunities for changeCurr Opin Pulm Med20152111725405667
- BatemanEDReddelHKvan Zyl-SmitRNAgustiAThe asthma-COPD overlap syndrome: towards a revised taxonomy of chronic airways diseases?Lancet Respir Med20153971972826255108
- DonaldsonGCSeemungalTARPatelISAirway and systemic inflammation and decline in lung function in patients with COPDChest200512841995200416236847
- KeatingsVMBarnesPJGranulocyte activation markers in induced sputum: comparison between chronic obstructive pulmonary disease, asthma, and normal subjectsAm J Respir Crit Care Med199715524494539032177
- AaronSDAngelJBLunauMGranulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2001163234935511179105
- CowlandJBSorensenOESehestedMBorregaardNNeutrophil gelatinase-associated lipocalin is up-regulated in human epithelial cells by IL-1 beta, but not by TNF-alphaJ Immunol2003171126630663914662866
- FloTHSmithKDSatoSLipocalin 2 mediates an innate immune response to bacterial infection by sequestrating ironNature2004432701991792115531878
- ChanYRLiuJSPociaskDALipocalin 2 is required for pulmonary host defense against Klebsiella infectionJ Immunol200918284947495619342674
- NielsenMBarnesCBUlrikCSClinical characteristics of the asthma-COPD overlap syndrome – a systematic reviewInt J Chron Obstruct Pulmon Dis2015101443145426251584
- MenezesAMMontes de OcaMPerez-PadillaRIncreased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthmaChest2014145229730424114498
- RoudkenarMHKuwaharaYBabaTOxidative stress induced lipocalin 2 gene expression: addressing its expression under the harmful conditionsJ Radiat Res2007481394417229997
- SunilVRPatelKJMainelisGPulmonary effects of inhaled diesel exhaust in aged miceToxicol Appl Pharmacol2009241328329319729031
- ErzurumSCNew insights in oxidant biology in asthmaAnn Am Thorac Soc201613suppl 1S35S3927027950

