Abstract
Background
COPD is one of the leading causes of morbidity and mortality in both high- and low-income countries and a major public health burden worldwide. While cigarette smoking remains the main cause of COPD, outdoor and indoor air pollution are important risk factors to its etiology. Although studies over the last 30 years helped reduce the values, it is not very clear if the current air quality guidelines are adequately protective for COPD sufferers.
Objective
This systematic review was to summarize the up-to-date literature on the impact of air pollution on the COPD sufferers.
Methods
PubMed and Google Scholar were utilized to search for articles related to our study’s focus. Search terms included “COPD exacerbation”, “air pollution”, “air quality guidelines”, “air quality standards”, “COPD morbidity and mortality”, “chronic bronchitis”, and “air pollution control” separately and in combination. We focused on articles from 1990 to 2015. We also used articles prior to 1990 if they contained relevant information. We focused on articles written in English or with an English abstract. We also used the articles in the reference lists of the identified articles.
Results
Both short-term and long-term exposures to outdoor air pollution around the world are associated with the mortality and morbidity of COPD sufferers even at levels below the current air quality guidelines. Biomass cooking in low-income countries was clearly associated with COPD morbidity in adult nonsmoking females.
Conclusion
There is a need to continue to improve the air quality guidelines. A range of intervention measures could be selected at different levels based on countries’ socioeconomic conditions to reduce the air pollution exposure and COPD burden.
Introduction
COPD is one of the leading causes of mortality and morbidity worldwide. While cigarette smoking is the primary cause and risk factor, many other risk factors contribute to the development or exacerbation of COPD. Outdoor air pollution has been recognized for its impact on human health for centuries, and in the past 50–60 years, particularly in the past 30 years, its adverse impact on COPD sufferers has been intensively studied worldwide. Indoor air pollution using biomass fuel in low-income countries has also been found to contribute to the COPD prevalence, particularly in nonsmoking females. However, over the years, efforts have been made to regulate air pollution levels in many countries around the world, which significantly reduced exposure levels compared to earlier times. It is not very clear how these air quality standards and guidelines, particularly the current ones, impacted the COPD sufferers. This review intends to evaluate the impact of air pollution on COPD sufferers in general and the current air quality standards or guidelines on the COPD sufferers specifically. Our objective was to conduct a comprehensive and systematic literature search and review and summarize up-to-date information to present an overall picture.
Materials and methods
This article reviewed the literature on the epidemiology of COPD, air pollution and its impact on COPD sufferers, and how air quality guidelines can improve the health of COPD patients.
PubMed and Google Scholar were the main databases utilized to search for articles related to our study’s focus. Search terms included “COPD exacerbation”, “air pollution”, “air quality guidelines”, “air quality standards”, “COPD morbidity and mortality”, “chronic bronchitis”, and “air pollution control” separately and in combination. We included articles from 1990 to 2015. We also used articles prior to 1990 if they provided historic background and were relevant in understanding air pollution and COPD epidemiologic studies. While articles written in English or with an English abstract were mostly considered, articles in other languages were occasionally used if relevant, and when online, an English translation was available.
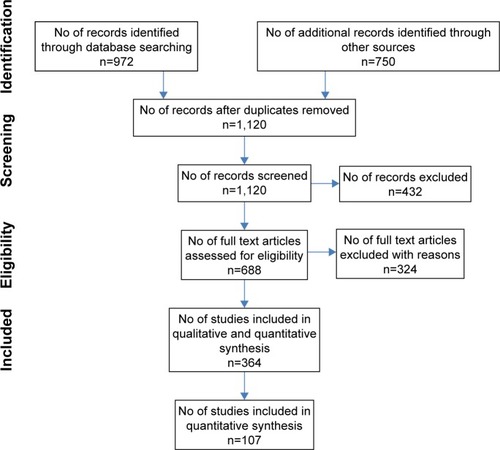
We identified 972 articles from the main databases and 750 from other sources such as Scopus and Global Health (EBSCOHost) or from the reference lists of the searched articles. We removed some duplicates and came up with 1,120 articles. These articles were further screened for relevance. We then excluded 432 articles that were irrelevant. The final full text articles further assessed were 688. We then focused on studies that addressed outdoor air pollution related to COPD mortality, hospital admissions or emergency room visits, incidence, prevalence, respiratory symptoms and lung functions, exacerbation of COPD patients in both high- and low- to middle-income countries, and indoor biomass cooking and the risk of COPD prevalence in low-income countries. As a result, 324 articles were removed, leaving us with 364 articles. We further removed 257 articles based on the following reasons: 1) animal or human subject experimental studies; 2) studies on active and passive smoking; 3) occupational exposure to dust and fumes (although some were mentioned in the introduction); 4) studies on dust storms, haze, bushfires or wildfires, and volcanoes; 5) reviews, updates, reports, and meta-analysis studies; 6) studies where COPD cases were combined with asthma or other diseases such as interstitial disease as a single category; 7) studies with pollutants measured in exhaled air; 8) farm and agricultural area exposure studies; 9) studies with both mortality and hospital admission cases combined; 10) studies on mortality and morbidity of all diseases or cardiorespiratory diseases without a specific category for COPD; 11) irrelevant genetic studies; 12) indoor air pollution studies in high-income countries; and 13) negative studies where no relationships between air pollution and mortality and morbidity of COPD were found, although a few representative studies were discussed in the text. Articles in earlier studies and indoor air pollution studies often used chronic bronchitis, while later studies focused more on COPD with or without bronchitis. This final selection left us with eleven studies on COPD mortality in both high- and low- to middle-income countries (); 27 studies on COPD hospital admissions and emergency room visits in high-income countries (); 12 studies on COPD hospital admissions and emergency room visits in low- to middle-income countries (); 15 studies on respiratory symptoms, lung functions, and prevalence and incidence of COPD (); ten panel studies conducted with COPD patients to specifically evaluate their exacerbations (); 21 studies on indoor air pollution in low- to middle-income countries (); and eleven studies on intervention effectiveness (a total of 107 studies). shows a summary of the article screening and selection process. Additionally, other studies are cited in the text when necessary.
Table 1 Outdoor air pollution and COPD-related mortality in both high- and low- to middle-income countries
Table 2 Outdoor air pollution and COPD-related hospital admissions or emergency room visits in high-income countries
Table 3 Outdoor air pollution and COPD-related hospitalizations or emergency room visits in low- to middle- income countries
Table 4 Outdoor air pollution and respiratory symptoms, lung function, and COPD prevalence and incidence
Table 5 Outdoor air pollution on exacerbation of COPD patients
Table 6 Indoor air pollution and COPD incidence or prevalence in low-income countries
Results
Introduction to the epidemiology of COPD
Definition of COPD
In 1997, a Global Initiative for Chronic Obstructive Lung Disease (GOLD) was launched in collaboration with the US National Heart, Lung, and Blood Institute; National Institutes of Health; and the World Health Organization (WHO). GOLD works with health care professionals and public health officials around the world to raise awareness of COPD and develop and regularly update evidence-based strategy documents to guide COPD diagnosis, treatment, management, and prevention.Citation1 In its most recent update document (2014),Citation1 GOLD defines COPD as
a preventable and treatable disease characterized by persistent airflow limitation that is usually progressive and associated with enhanced chronic inflammatory response in the airways and the lung to hazardous particles and gases. Exacerbations and comorbidities contribute to the severity in individual patients.
This definition is similar to that in the updated position paper by the American Thoracic Society and the European Respiratory Society.Citation2 COPD is not a single disease, but several lung diseases combined. Emphysema and chronic bronchitis are the most important conditions that compose COPD. They frequently coexist,Citation3 but they are no longer used as separate disease categories and now are included within the COPD diagnosis.Citation4 Although some patients with asthma also develop poorly reversible airflow limitations and are indistinguishable from patients with COPD, asthma is considered a separate entityCitation2 not included in the diagnosis and treatment of COPD.
The significant airflow limitation in COPD patients is indicated by the value of forced expiratory volume in 1 second (FEV1) that does not return to normal and frequently worsens over time, but responds largely to bronchodilators.Citation2 GOLD recommends that any patient with dyspnea, chronic cough or sputum production, and a history of exposure to risk factors such as tobacco smoke or occupational dusts or chemicals should be considered for a diagnosis of COPD, but spirometry is required to make the clinical diagnosis. The presence of a postbronchodilator ratio of FEV1 and forced vital capacity (FVC) <0.7 is the confirmation of obstructive airflow limitation.Citation1 GOLD also classifies the severity of airflow limitation in COPD into four categories in patients with FEV1/FVC <0.7: GOLD 1 (mild) − FEV1 ≥80% predicted, GOLD 2 (moderate) − 50%≤ FEV1 <80% predicted, GOLD 3 (severe) − 30%≤ FEV1 <50% predicted, and GOLD 4 (very severe) − <30% predicted.Citation1,Citation5
COPD prevalence and disparity
COPD remains a major public health problem worldwide, and is one of the leading causes of morbidity and mortality in both high- and low-income countries. Estimated prevalence rates varied a great deal among different regions and countries possibly due to different methods used in different studies.Citation5 In the US, based on the National Health Interview Survey conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention and analysis conducted by the American Lung Association,Citation3 12.7 million US adults have been diagnosed with COPD. The actual number could be as high as 24 million if using the lung function test result, which indicates that there is an underdiagnosis. For chronic bronchitis, >10 million Americans reported a physician diagnosis in 2011. The total prevalence rate was 4.4%, whereas in 1999, the total number was 8.8 million although the prevalence was similar. For emphysema, 4.7 million Americans reported ever being diagnosed and the prevalence rate was 2.0% in 2011. This is a significant increase from 1999 where 2.8 million people were reported representing a prevalence rate of 1.4%.Citation3
The prevalence rate of COPD was strikingly variable among different races, sexes, and age groups. The rate for chronic bronchitis in 2011 (NCHS)Citation3 was much higher in non-Hispanic whites (4.7%) and blacks (4.9%) than in Hispanics (2.9%) and other non-Hispanics (2.4%). The rate was twice as high in females (5.7%) as in males (3.0%). Prevalence rates were the highest among those 65 years or older (6.4%) and the lowest among those 18–44 years (2.9%) with 70% of cases occurring in those older than 45 years. For emphysema, the prevalence rate in 2011 followed a similar pattern among ethnic groups, which was the highest for non-Hispanic whites (2.4%) followed by blacks (1.8%), other non-Hispanics (1.3%), and Hispanics (0.7%). Females surpassed males in the prevalence rate (2.1% vs 1.9%), although historically, the rate was lower in females. Similarly, prevalence rates for emphysema were the highest among those 65 years or older (5.5%) and the lowest among those 18–44 years (0.3%) with the rate in between (2.7%) for the age group 45–64.Citation3
Geographically, COPD prevalence rates in the US also varied a great deal among different states as surveyed by the Behavioral Risk Factor Surveillance System in 2011. Kentucky had the highest age-adjusted rate at 9.7%, followed by Alabama at 9.4%, while Minnesota (4.0%) and Washington (4.1%) had the lowest. This geographical difference in COPD prevalence by state parallels the difference in smoking rates where Kentucky was on the top (30.2%) and Washington (17.0%) and Minnesota (15.8%) were on the lowest end.Citation6 COPD prevalence rate was also the highest for males in Kentucky (8.4%), while the lowest for males in Washington (3.3%) and Washington DC. Among females, Tennessee had the highest age-adjusted rate (11.5%) and Minnesota the lowest (4.3%). Rates tend to be higher in the Midwest and Southeast.Citation3
Worldwide, 65 million people have moderate-to-severe COPD,Citation4 and the prevalence is also highly variable. Mannino and BuistCitation5 summarized the rates from 12 sites in the Burden of Obstructive Lung Disease (BOLD) studyCitation7 and four sites in the Latin American Project for the Investigation of Obstructive Lung Disease (PLATINO) studyCitation8 and showed that in both males and females, the highest rate was in South Africa and the lowest in Mexico. The rate for the US was the fifth highest. In the BOLD study for females, the highest rate was in Cape Town, South Africa (16.7%) and the lowest in Guangzhou, People’s Republic of China (5.1%). For males, the highest rate again was in Cape Town, South Africa (22.2%) and lowest in Reykjavik, Iceland (8.5%). In the PLATINO study, crude rates of COPD ranged from 7.8% in Mexico City to 19.7% in Montevideo.Citation5
COPD Mortality and disparity
According to Antó et al,Citation9 50% of patients are expected to live 10 years post-diagnosis with more than one-third of patients dying due to respiratory insufficiency. COPD is the third leading cause of death in the US after cancer and heart disease.Citation3 Based on the data from NCHS, the total number has increased from 119,524 in 1999 to 133,965 in 2009. The number of deaths was consistently higher in females than in males from 2000 to 2009. Approximately 80% of COPD deaths are in non-Hispanic whites; Hispanics had the least number of deaths counting 3,724 in 2009. The overall age-adjusted death rate was 41.2/100,000 in 2009 with the rate the highest (46.0/100,000) for non-Hispanic whites than for other ethnic groups. Overall, non-Hispanic white males had the highest age-adjusted death rates (53/100,000), while other non-Hispanic females had the lowest age-adjusted death rates (11.0/100,000 population).Citation3
WHO estimated that globally, more than 3 million people died of COPD in 2005, which corresponds to 5% of all deaths.Citation4 It was known that almost 90% of COPD deaths occurred in low- and middle-income countries. In 2001, WHO estimated that COPD was the fifth leading cause of death in high-income countries and the sixth leading cause of death in low- and middle-income countries.Citation4 In 2004, WHO updated their findings and concluded that COPD was the fourth leading cause of death for all ages, resulting in 3.0 million deaths worldwide.Citation10 WHO also estimated that total deaths from COPD are projected to increase by >30% in the next 10 years and will become the third leading cause of death worldwide by 2030.Citation4 In terms of disability-adjusted life years, COPD is currently seventh and is expected to rise to the fifth leading cause of burden of disease by 2030.Citation10
Causes and risk factors
The primary cause of COPD is tobacco smoke, including secondhand smoke or environmental tobacco smoke.Citation4 Most smokers develop some respiratory impairment due to COPD.Citation11 WHO estimates that 73% of mortality is related to smoking in high-income countries and 40% to low-to-middle-income countries.Citation5 In a population cohort study conducted in North Sweden,Citation12,Citation13 it was reported that 50% of smokers would develop COPD based on GOLD guidelines.Citation11
Many other risk factors have been identified in past researchCitation9,Citation14 that contributed to the development or exacerbation of COPD and have been well summarized in previous reviews.Citation5,Citation14,Citation15 These include genetic and phenotypic traits, occupational exposures to dust and fumes, indoor and outdoor air pollutants, aging, infections, asthma, sex, and socioeconomic status. These risk factors can act singly or synergistically.
It has been suggested that susceptibility to COPD is, at least in part, genetically determined.Citation16 While the best described genetic factor in COPD is alpha-1 antitrypsin deficiency (PiZZ genotype), present in 1%–3% of COPD patients,Citation15 several genes have been studied for their associations with COPD.Citation16,Citation17 For example, five single nucleotide polymorphisms in ADAM33 gene were associated with COPD and lung function in long-term smokers.Citation18 The MSR1-coding single nucleotide polymorphism P275A was associated with susceptibility to COPD in smokers and a lower percent predicted FEV1, FEV1/FVC, and percent predicted forced expiratory flow (25%–75%).Citation19 Smokers who are carriers of the surfactant protein D AG and AA polymorphic genotypes may be at a higher risk of developing COPD.Citation16 Retinoic acid receptor-related orphan receptor-α has been implicated in the development of COPD.Citation20 The hedgehog-interacting protein gene and family with sequence similarity 13, member A (FAM13A1) gene, were suggested to be involved in COPD susceptibility in Chinese Han population.Citation21,Citation22
Occupational exposure may make a substantive contribution to the etiology of COPD, particularly, in nonsmokers, females, and young people.Citation23 Exposed agents include cotton dust,Citation24,Citation25 grain dust,Citation26 western red cedar dust,Citation27,Citation28 coal dust,Citation29 cement dust,Citation30 gasesCitation31 and metal fumes,Citation32,Citation33 or a mixture of them. Most studies reported relative risk (RR) or odds ratio (OR), and a few studies directly reported the percentage of attributable population risk (PAR%).Citation34 For chronic bronchitis, reported PAR% varied from 11% to 26% with a median at 19%. For lung function impairment, the reported PAR% varied from 12% to 34% with a median at 19%. The reported PAR% also varied for different symptoms.Citation34 Overall, the PAR% due to occupational exposure was estimated to be 15% in smokers and 20% in nonsmokers.Citation11,Citation23
It is suggested that up to 20% of cases of COPD worldwide can be attributed to indoor air pollution from exposure to smoke from cooking and heating with biomass fuels in poorly ventilated dwellings.Citation11 Age contributing to the risk of COPD was due to the decline in lung function.Citation5 Infection can predispose individuals for COPD development, and socioeconomic factors represent a combination of risk factors that contribute to the susceptibility for COPD, including poor nutrition and closer proximity to hazardous pollutants.Citation5 This review focused on air pollution as an etiological factor or risk factor for the development and exacerbation of COPD; for other risk factors, the readers are directed to other review papers in this journal or other journals.
Review of the effects of air pollution on COPD sufferers
Outdoor air pollution and COPD mortality
Although outdoor air pollution can occur naturally (eg, volcanoes and forest fires), anthropogenic activities are the major cause of environmental air pollution.Citation35 The concern of outdoor air pollution on human health has been recognized for centuries.Citation36 The effects of outdoor air pollution have caused a spectrum of responses, such as irritation of the upper respiratory systems, increased prevalence of respiratory infections, and symptoms and clinical signs. Symptoms and signs of respiratory responses include coughing, phlegm production, chest tightness, wheezing, and chronically reduced pulmonary function in FVC and FEV1. These symptoms lead to increased incidences in exacerbation of cardiopulmonary diseases, asthma attacks, cancer, and mortality.Citation2 While air pollution may affect all ages of the population, the elderly, particularly those with preexisting cardiopulmonary diseases such as COPD, are the most susceptible group.
Air pollution causing COPD-related mortality was well presented when air pollution catastrophes significantly increased death rates. For example, in the UK historically, the burning of coal in homes for domestic heat often created very high levels of air pollution and caused death rates to dramatically rise. One of the most well-known pollution events was the 1952 London Smog incident that resulted in 4,000 extra deaths, with 80%–90% of the deaths due to cardiorespiratory causes. The greatest relative increase was in deaths due to bronchitis, which rose ninefold.Citation37,Citation38 The pollutant involved in the London Smog incident was black smoke, defined as visual blackness of particles collected on a white filter expressed as equivalent mass concentration of standard coal smokeCitation39 and sulfur dioxide (SO2). A later estimation indicated that 12,000 extra deaths occurred from December 1952 through February 1953 because of acute and persisting effects of the 1952 London Smog incident.Citation40 A time series analysis conducted for the data from 1958 to 1972 indicated that particulates were strongly associated with mortality rates in London even at much lower levels, and the relation was likely causal.Citation41 A more recent study on the health effects of an air pollution episode in London, December 1991, in which concentrations of nitrogen dioxide (NO2) rose to record levels with moderate increases in black smoke showed a 23% increase in COPD mortality.Citation42 Earlier, before the London Smog incident in 1930, the Meuse Valley, Belgium, experienced a period of intense fog in a heavy industrial area resulting in the death of 60 people.Citation43 In October 1948, a lethal haze enveloped the town of Donora, PA, US. Over 5 days, approximately half of the town’s 14,000 residents experienced severe respiratory and cardiovascular problems. The death toll rose to ~40 people.Citation44
The 1952 London Smog and other air pollution events symbolized the beginning of the modern air pollution epidemiologic studies. They also prompted governments to pass legislation to reduce air pollution levels. As legislation over the years has led to a decrease in traditional air pollutants particulate matter (PM) and SO2 from stationary sources, today’s major air pollutants come from motor vehicle traffic, and the main perpetrators include PM, ozone (O3), and NO2.Citation37 A commentary and review by DockeryCitation39 well described how studies on the health effects of particulate air pollution evolved and helped improve the air quality standards and regulations in the US. The year 1970 was a milestone year when Congress passed the Clean Air Act Amendments that required the Environmental Protection Agency (EPA) to set up the first National Ambient Air Quality Standards (NAAQS) that included six types of air pollutants: carbon monoxide (CO), lead, NO2, O3, PM, and SO2. NAAQS was promulgated in 1971. The particles used then were total suspended particles (TSP) with aerodynamic diameter between 20 µm and 50 µm, which was set up as maximum allowable ambient concentration.Citation39 The Clean Air Act also encouraged scientists to identify pollutants that may reasonably be anticipated to endanger public health and welfare.Citation39
One of the earliest and largest air pollution studies in the US was the Harvard prospective cohort study of the respiratory health effects of respirable particles and SO2 on a sample of adults and children in six US cities, that began in 1974. The particles measured in this study included two classes: fine particles (aerodynamic diameter <2.5 µm [PM2.5]) and inhalable particles (aerodynamic diameter <15 µm [PM15] before 1984 and <10 µm [PM10] starting in 1984).Citation45 Over the 16-year follow-up, the study found a positive association of air pollution with both mortalities from lung cancer and cardiopulmonary causes, after adjusting for smoking and other risk factors. The adjusted mortality rate ratio for the most polluted of the cities as compared with the least polluted was 1.26 (95% confidence interval [CI], 1.08–1.47) or 26% of excess mortality. Mortality was most strongly associated with air pollution with fine particulates, including sulfates.Citation45 This study and othersCitation46–Citation48 provided scientific evidence that supported the US EPA’s replacement of the TSP standard with a standard for PM10 in 1987.Citation49 In 1997, EPA further amended the particle standard and added PM2.5 to recognize the potentially different health effects.Citation49
This switch to a more health-related exposure metric stimulated studies of the associations between ambient air PM and mortality, morbidity, and cardiopulmonary function indices. Over the past 30 years, a great number of studies, particularly time series studies, were conducted around the world to evaluate daily air pollution concentrations (short-term exposure) and the daily increased mortality with or without a few days of lag of exposure.Citation36 PM was the focus of these studies, but gaseous criteria air pollutants were often included for their independent effects or interaction with and modification of PM effect. PM size evaluation evolved from TSP to PM10 and then to PM2.5. Earlier studies mostly focused on a single city, and many evaluated total mortality or mortality due to cardiorespiratory diseases or respiratory diseases,Citation50–Citation56 and the effect size was often provided with higher units of exposure (100 µg/m3 or quartile increase of the studies).Citation55,Citation57,Citation58 More recent studies tend to focus on COPD as a separate categoryCitation59–Citation65 where the effect size was based on 10 µg/m3.Citation59–Citation62,Citation64,Citation65
These studies in a single city or multi cities around the world described earlier have repeatedly shown the increase in mortalities for all causes, for cardiopulmonary diseases, and for COPD associated with both short-term and long-term exposures to air pollution, although the exact percentage of increase (effect size) was variable among studies, time periods, pollutants, and cities. It was suggested that particles are the major culprit among the air pollutants and the role of other pollutants, if any, was additive and not multiplicative.Citation38
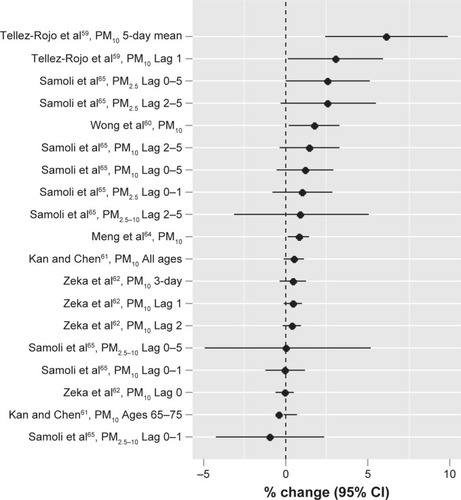
summarizes main studiesCitation55–Citation65 published on outdoor air pollution and COPD mortality thus far. To be comparable, recent studiesCitation59–Citation62,Citation64,Citation65 that used the same exposure unit (10 µg/m3) for particles are summarized in . The mean percent of change in mortality per 10 µg/m3 increase in particle exposure ranged from −1 for PM2.5–10 with a 0–1-day lag in a study conducted by Samoli et alCitation65 in ten European Mediterranean metropolitan areas to 6.1 for PM10 with a 5-day mean exposure in a study conducted by Tellez-Rojo et alCitation59 in Mexico City, Mexico. The effect sizes for other studiesCitation60–Citation62,Citation64 are in between with an average effect size at 1.12. The effect size ranged from 1.0 to 3.5 for SO2 and 1.8 to 3.2 for NO2 per 10 µg/m3 and 3.4 per 10 µg/m3 to 8.3 per 80 µg/m3 for O3.
Figure 2 Outdoor air pollution and COPD-related mortality in both high- and low- to middle-income countries: increased risk for COPD per increase in particle exposure (10 µg/m3).
Alteration in coagulation and the autonomic control, and pollutant-related inflammation that enhances atherogenesis, are mechanisms of deaths triggered by increased air pollution.Citation66,Citation67 These mechanisms are relevant to patients with COPD who frequently have comorbid diseases.Citation5 Increased susceptibilities to respiratory infections, increased airflow obstruction, and deranged gas exchange are also important contributors. Patients who die during air pollution episodes include not only those with a very short life expectancy (this mechanism has been called “harvesting”) but also patients and subjects with a much longer life expectancy.Citation68
Outdoor air pollution and COPD morbidity
While mortality due to air pollution represents the extreme outcome for COPD sufferers, there is a continuum of health effects that also include the impact on the morbidity such as increased acute respiratory symptoms, reduced lung functions, exacerbation of COPD conditions that may be severe enough to require physician visits, use of ambulance, hospital respiratory admissions, and emergency room visits.Citation69 COPD sufferers are particularly vulnerable to additional stress on the respiratory system caused by the toxic effects of inhaled pollutants. The London Smog incident of December 5–9, 1952, caused total hospital admissions to rise by 50% and respiratory admissions to rise by 160%.Citation37 The later Smog event in 1991 caused a 43% increase in hospital admission.Citation42
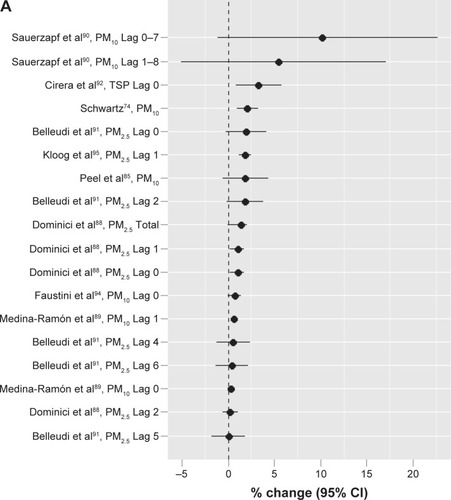
Similarly in the past 30 years, particularly since the early 1990s, many epidemiologic studies have been conducted around the world to evaluate short-term exposure to air pollution and the morbidity of respiratory diseases overall or COPD specifically. These studies that assessed the mortality often also evaluated the morbidity. summarizes the 27 studiesCitation70–Citation96 conducted in high-income countries that specifically evaluated the increased risk of hospital admission or emergency room visits due to COPD cause. Exposures assessed included both PM and gaseous pollutants, and the exposure unit used to assess the effect size of particles varied among studies and often was 50 µg/m3 and 100 µg/m3 or in the interquartile range (IQR) of the measured data in earlier studies. Recent studies tended to use 10 µg/m3. summarizes the effect sizes from different authors with different particle sizes and lag times per increase in 10 µg/m3.Citation74,Citation85,Citation88–Citation92,Citation94,Citation95 The percent increases ranged from 0.02 for PM2.5 in Lag 5 in the study conducted by Belleudi et alCitation91 in Rome, Italy, to 10.1 for PM10 in Lag 0–7 in the study conducted by Sauerzapf et alCitation90 in Norfork, UK. The average percent increase was 1.89. In 2006, a study in 204 counties in the US by Dominici et alCitation88 found a total percent increase at 1.4 for PM2.5. In the same year, another study by Medina-Ramón et alCitation89 with 36 US cities identified percent increases at 0.29 (Lag 0) and 0.59 (Lag 1) for PM10 based on per 10 µg/m3 increase in concentration. The most recent study by Kloog et alCitation95 in the Mid-Atlantic region of the US identified a percent increase of 1.83 (Lag 1) for PM2.5. The effect sizes (percent increases) for gaseous pollutants were also variable among the studies. For SO2, the percent change ranged from 2 in a study by Sunyer et alCitation70 in Barcelona, Spain, to as high as 39 in a study by Pönkä and VirtanenCitation72 in Helsinki, Finland, although these studies were not directly comparable as they used different exposure units. For NO2, recent comparable studiesCitation90,Citation92,Citation94 showed an increase in risk from 1.2%Citation94 to 22%.Citation90 For O3, different studiesCitation74–Citation76,Citation78,Citation81,Citation83,Citation89,Citation90,Citation92,Citation96 using different exposure units showed a range of percent change from 0.034Citation76 to as high as 27.Citation96 For CO,Citation82,Citation83,Citation85,Citation86,Citation90 the percent change ranged from 1.5Citation90 to 8,Citation86 although again these numbers were not directly comparable because the exposure units used were different.
Figure 3 Outdoor air pollution and COPD-related hospital admissions or emergency room visits: increased risk for COPD per increase in particle exposure (10 µg/m3).
Abbreviations: PM10, particulate matter with aerodynamic diameter ≤10 µm; TSP, total suspended particles; PM2.5, particulate matter with aerodynamic diameter ≤2.5 µm; CI, confidence interval; BS, black smoke.
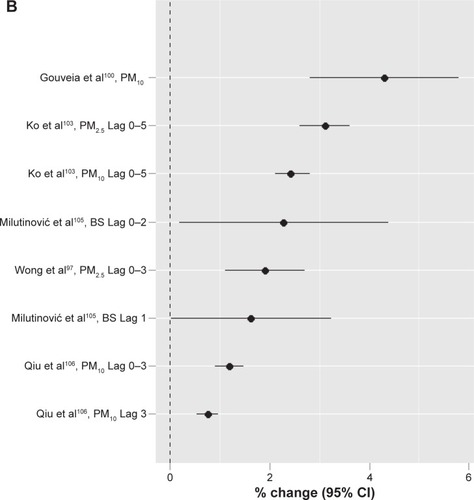
summarizes 12 studiesCitation97–Citation108 conducted in the low-to middle-income countries. With different exposure units used, the percent increase for particles in these studies was from 0.74 in a study conducted by Qiu et alCitation106 in Hong Kong, People’s Republic of China, to 18.6 in a study conducted by Arbex et alCitation104 in São Paulo, Brazil. When the same exposure unit of 10 µg/m3 was used, five studiesCitation97,Citation100,Citation103,Citation105,Citation106 showed a range of percent increase from 0.74 in Hong Kong, People’s Republic of China,Citation106 to 4.3 in São Paolo, Brazil,Citation100 with an average of 2.19 (). This is similar in magnitude to that in high-income countries. For SO2, the reported percent increase with different exposure units ranged from 0.44 in Tabriz, Iran,Citation108 to 19 in Kaohsiung, Taiwan, when the temperature was <25°C.Citation102 For NO2, the lowest reported was 0.38 in Tabriz, Iran,Citation108 and the highest was 97.5 again in Kaohsiung, Taiwan.Citation102 For O3, the percent change was 1.22 in Hong Kong, People’s Republic of China,Citation106 to 26.6Citation102 in Kaohsiung, Taiwan, when the temperature was <25°C. For CO, the percent change ranged from 4.9 in São Paolo, Brazil,Citation100 to 39.8Citation102 also in Kaohsiung, Taiwan, when the temperature was <25°C.
The above studies provided strong evidence that both particles and gaseous air pollutants can increase the hospital admissions or visits to emergency departments due to COPD exacerbation in both high-income and low- to middle-income countries, although the effect size is variable among study locations and different pollutants. The effect size also seems to be higher in gaseous pollutants, particularly in low-income countries.
Outdoor air pollution on respiratory symptoms and lung function
Several studies have also been conducted to evaluate the prevalence and incidence of COPD or chronic bronchitis and/or respiratory symptoms and lung functions (FVC, FEV1, or both) due to short-term or long-term exposure to outdoor air pollution. summarizes 15 of such studies.Citation109–Citation123 Exposures often were measured qualitatively as how close the home was to a major road with high traffic, although air pollutants were also measured in some of the studies. The prevalence of COPD ranged from 0.26% in Pietracupa, Italy,Citation112 to 4.5% in Rhine-Ruhr basin, Germany,Citation113 and the prevalence of chronic bronchitis was 4%.Citation115 Increased risks with different effect sizes were observed for symptoms such as cough and phlegm,Citation114,Citation117,Citation118 reduced lung functions,Citation110,Citation112,Citation113,Citation118,Citation121 and prevalence or incidence of COPD or chronic bronchitisCitation113,Citation116,Citation118–Citation120,Citation122 in different populations and various regions of the world. The amount of decreased FEV1 was reported to be from 2.1 mL/year per increase in PM2.5 (2 µg/m3) or 5.0 mL/year if living in <100 m distance to a major road in Framingham, US,Citation121 to 23.6 mL/year in males in a Los Angeles study.Citation110 These effect sizes delineated the risks of chronic exposure to outdoor air pollution in the general population.
Outdoor air pollution on COPD patients
Another type of time series study is the panel study with COPD patients to evaluate the daily variations of air pollution directly on their exacerbation (increased symptoms and reduced lung functions). However, relatively fewer of such studies have been conducted, and the results on the effects are inconsistent. presents the details for ten panel studies.Citation124–Citation133 One of the earliest panel studies was conducted by Lawther et al.Citation124 They used diary cards to assess the symptoms of bronchitis related to the change in air pollution levels in patients and found a 28% overall increase in worsened symptom rates. The panel studies in the past 25 years showed a variety of exacerbations on the COPD patients ranging from increased respiratory symptoms, blood pressure, and inhaler and nebulizer use, reduced lung function, and limits in physical activities to death, although the effect sizes were variable among different studies in different regions. Most of these studies used a small number of COPD patients. The largest study identified was conducted by Sunyer and BasagañaCitation128 in Barcelona, Spain, with 2,305 COPD patients >35 years of age. An IQR increase of 27 µg/m3 in PM10 resulted in an all-cause mortality increase of 11%.
The above review indicated that outdoor air pollution, especially particulate air pollution, has been consistently linked to various health effects on COPD sufferers ranging from increased respiratory symptoms, decreased pulmonary function, exacerbation leading to increased hospitalization admissions and emergency room visits, and mortality due to cardiopulmonary disease. These health effects are observed at levels common to many US cities, including levels below the maximum set by the US NAAQS at the time of the studies.Citation134
Indoor air pollution and impact on COPD sufferers
Human beings spend a large part of their time indoors such as in homes, workplaces, libraries, shopping malls, school classrooms, and daycare centers and inside vehicles. For example, Americans spend ~90% of their time indoors, where the concentrations of some pollutants are often two to five times higher than typical outdoor concentrations.Citation135 According to WHO, almost three million people or 50% of the households worldwide use biomass as the main source of energy for cooking, heating, and other household needs, such as wood, crop residues, and animal dungs in addition to coal. Biofuels have higher emission factors for PM and other pollutants, especially during incomplete combustion at lower temperatures.Citation136 The burning of biofuels generates indoor airborne particles at levels much higher than those of cleaner fuelsCitation137 or outdoor levels,Citation138 and well above levels in most polluted cities.Citation139 Such particles also have small aerodynamic diameters (eg, ranging from 0.05 µm to 1 µm for wood smoke)Citation140 and can penetrate deep into the alveolar region to induce adverse pulmonary effects.
Indoor air pollution studies dated back to as early as the 1960s when wood smoke exposure and chronic lung diseases were investigated in Papua and New Guinea.Citation140–Citation142 Since the early 1980s, there have been quite a few studies conducted to evaluate indoor exposure to biomass air pollution and the odds of increased chronic bronchitis or/and COPD, particularly in low-income countries where only lower grade energy resources are available and affordable. summarizes 21 studiesCitation143–Citation163 conducted in low-income countries identified through this search in which exposed populations were largely nonsmoking females, but exposed to smoke from cooking and heating using biomass fuels since early childhood with their mothers or later as housewives. Most studies 1) were population-based cross-sectional surveysCitation143–Citation145,Citation149–Citation154,Citation157–Citation163 or case–control design,Citation146–Citation148,Citation156 and only one study was identified as a (retrospective) cohort study;Citation155 2) used standard questionnaires such as questionnaires from American Thoracic Society and the British Medical Research Council with adaptations appropriate to local culture, along with or without the lung function testing to identify cases, but few studies used the GOLD standard for diagnosis; 3) assessed exposure using fuel type, stove type, poor ventilation, or time spent cooking and rarely measured actual exposure levels to particles and gasses; and 4) measured the prevalence of chronic bronchitis and/or respiratory symptoms with COPD in most earlier studies or COPD only in recent studies, analyzed OR for indoor biofuel use, or conducted a crude dose–response relationship analysis using cooking time per year as a cumulative exposure measurement. Unit air concentration-based effect sizes were not available. However, a consistent relationship between indoor exposure to biomass cooking and excess risk was found from different countries. The prevalence (%) of chronic bronchitis in study villages with indoor biomass cooking varied from 1.79 in IndiaCitation163 to 28.5 in Turkey,Citation153 which is overall higher than in high-income countries. The prevalence for urban control area homes, outdoor cooking practice, and cleaner fuels such as gas and electricity tended to be much lower. The prevalence of COPD varied from 2.4 in IndiaCitation161 to 12 in a rural community in Guangzhou, People’s Republic of China.Citation158 The significantly increased OR for biomass cooking ranged from 1.86 (95% CI 1.16–2.99) in BrazilCitation145 to 28.7 (95% CI 8.7–95.9) in TurkeyCitation152 for chronic bronchitis, 1.2 (95% CI 0.4–4.2) in IndiaCitation161 to 15.0 (95% CI 5.6–40.0) in MexicoCitation148 for COPD, 9.7 (95% CI 3.7–27.0) overall to 75 (95% CI 18–306) when cooking was >200 hour-years in MexicoCitation148 for chronic bronchitis and COPD combined, and 2.3 (95% CI 1.2–4.4) to 2.9 (95% CI 1.7–5.1) for various respiratory symptoms.Citation162 It was reported that if cumulative exposure is >60 hour-years, the OR for chronic bronchitis is significantly increased.Citation163 Reported attributable portion of risk was 23.1%.Citation153 These results indicated that overall, evidence supporting an association between biomass smoke exposure and COPD in adult females in rural areas is fairly robust.Citation164
Impact of current air quality guidelines on COPD sufferers
Epidemiologic studies worldwide have provided strong evidence to link air pollution, especially particulate air pollution to the mortality, morbidity, and socioeconomic burden of cardiorespiratory disease in general and COPD in particular. This has prompted the legislation around the world to continuously modify the air quality standards or guidelines to reduce the disease burden over time such as in the US.Citation49,Citation165 WHO provides the basis for global standards in environmental quality and effective investments for public health.Citation166 WHO published its air quality guidelines in 1987 and revised them in 1997. Based on the research developments thereafter, they updated the guidelines for PM, O3, NO2, and SO2 in 2005.Citation166 The values in the WHO guidelines are much lower than in the US NAAQS.Citation167,Citation168 We focused this part of the review on studies conducted in the last 10 years to specifically evaluate if the current air quality guidelines are protective of COPD sufferers.
A prospective cohort studyCitation119 in Copenhagen, Denmark, with 57,053 participants assessed the effect of exposure to traffic air pollution (NO2 and nitrogen oxides [NOx]) over 35 years on the incidence of COPD. The modeled 35-year mean of outdoor NO2 level was 17.0 µg/m3 or 9 ppb for the total population and 18.1 µg/m3 or 9.6 ppb for COPD patients. These levels were well below the current NAAQS NO2 standard of 53 ppb for annual mean. The study found that COPD incidence was associated with the 35-year mean NO2 level (hazard ratio 1.08; 95% CI 1.02–1.14, per IQR 5.8 µg/m3 or 3.1 ppb), with stronger associations in subjects with diabetes (hazard ratio 1.29; 95% CI 1.05–1.50) and asthma (hazard ratio 1.19; 95% CI 1.03–1.38)Citation119 (). Another cohort studyCitation63 followed up all residents in Oslo, Norway, aged 51–90 years from 1992 to 1998 to evaluate the mortality of COPD. The elevated risk was found at NO2 levels >40 µg/m3 in the youngest age group and with a linear effect in the interval 20–60 µg/m3 for the oldest. The effects were particularly strong for COPD, which appeared to have linear effects. The levels (µg/m3) in this study were 39 for NO2 (or 20.7 ppb) and 15 for PM2.5, again well below the current NAAQS standard (24 hour mean =35 µg/m3 for PM2.5). A recent mortality studyCitation169 enrolled 145,681 COPD patients aged 35 years or older from the residents of Rome with a comparison group of 1,710,557 subjects without COPD. The annual average daily concentrations were 36.4 µg/m3 for PM10 and 20.2 µg/m3 for PM2.5, both below the limits recommended by European Union (EU) legislation (40 µg/m3 and 25 µg/m3, respectively). The annual average concentration of NO2 (60 µg/m3) was higher than the EU limit (40 µg/m3), and the 8-hour running mean concentration of O3 was <100 µg/m3. It was found that PM10, PM2.5, and NO2 (0- to 5-day lag) were associated with daily mortality with stronger effects in people with COPD. The mortality associated with PM10 (per IQR 16 µg/m3) was five times more in COPD patients (3.5%, 95% CI −0.1%−7.2%) than in other subjects (0.7%, 95% CI −0.8%−2.2%). The effects on respiratory mortality among COPD subjects were particularly elevated for PM2.5 (IQR 11 µg/m3; 11.6%, 95% CI 2.0%–22.2%) and NO2 (IQR 24 µg/m3; 19.6%, 95% CI 3.5%–38.2%).Citation169 In Vancouver, Canada, a population-based studyCitation170 with 467,994 residents aged 45–85 years without COPD had a 5-year exposure period and a 4-year follow-up period. The 5-year average concentrations were 4.10 µg/m3 for PM2.5 and 32.2 µg/m3 (or 17 ppb) for NO2. In unadjusted single-pollutant models, PM2.5, NO2, and NO were associated with COPD hospitalization and mortality, although after adjustment for covariates, these air pollutants were not significantly associated with COPD hospitalization and mortality.Citation170 As described earlier, Schikowski et alCitation113 showed that chronic exposure to PM10, NO2, and living near a major road might increase the risk of developing COPD. The annual mean level was 39 µg/m3 (or 20.7 ppb) for NO2 and 44 µg/m3 for PM10 (). In a New Zealand study with COPD patients (),Citation125 SO2 and NO2 and most PM10 concentrations were well below their air quality guidelines, but increased risk of chest symptoms for PM10 in the night time and increased use of an inhaler and nebulizer for NO2 were observed.
SulzbachCitation171 commented that epidemiological studies have shown that sensitive populations are prone to exacerbated health effects even when the air quality measurements are within the EPA standards. Specifically, Sulzbach investigated Minnesota to determine the constituents of the air pollution and measure the level of air pollution in the Twin Cities. The result of the study showed that Minnesota was one of eleven states that met federal air quality health standards at the time. However, there were still a significant number of days when the air quality could trigger health problems in sensitive populations.Citation171
Bell et alCitation172 estimated a national average relative rate of mortality associated with short-term exposure to ambient O3 for 95 large US urban communities from 1987 to 2000. They found that a 10 ppb increase in the previous week’s O3 was associated with a 0.52% increase in daily mortality (95% posterior interval [PI], 0.27%–0.77%) and a 0.64% increase in cardiovascular and respiratory mortalities (95% PI, 0.31%–0.98%). They indicated that even though the US EPA’s 8-hour regulation was met every day in each community, there was still a 0.30% increase in mortality per 10 ppb increase in the average of the same and previous days’ O3 levels (95% PI, 0.15%–0.45%). Therefore, they suggested that interventions to further reduce O3 pollution levels should be implemented so as to benefit public health, even in regions that meet current regulatory standards and guidelines.Citation173
The WHO advised that due to the lack of thresholds of air pollutants at which adverse health effects occur, the guidelines proposed cannot fully protect human health.Citation166
It should be noted that there were also some studies that do not support the associations between outdoor and indoor air pollution and the burden on COPD sufferers. For example, Schikowski et alCitation174 used data from four cohort studies (10,242 subjects) participating in the European Study of Cohorts for Air Pollution Effects. The mean exposures varied from 9.5 µg/m3 to 17.8 µg/m3 for PM2.5, 15.7 µg/m3 to 26.7 µg/m3 for PM10, and 22.4 µg/m3 to 28.9 µg/m3 for NO2 among the cohorts. No association was found between NO2 and PM10 and COPD in individual cohorts. The meta-analysis with all the cohorts only found a nonsignificant association between NO2, NOx, PM10, and the traffic indicators and COPD, although a significant association was observed in females (1.57; 1.11–2.23 for prevalence and 1.79; 1.21–2.68 for incidence). Pujades-Rodríguez et alCitation175 analyzed data from 2,644 adults aged 18–70 in Nottingham, UK, and found no significant cross-sectional associations between home proximity to the roadside or NO2 levels and COPD or lung function measurements. Similarly, a prospective cohort study in Greece with 3,046 subjects found no association between air pollution and the development of COPD.Citation176
Although further research is needed to better assess the relationship, the majority of the literature has indicated that the impact on COPD suffers, including morbidity and mortality, due to air pollution is still detectable under the current air quality guidelines.
Discussion
Implications for future policy and decision-making
To reduce the impact of outdoor/indoor air pollution on COPD sufferers, a range of strategies and approaches need to be sought, which are summarized in the following categories based on this literature review.
Amendment to further lower current standards and guidelines
To evaluate whether improved air quality standards reduce the adverse health effects, the Harvard six cities study extended mortality follow-up for 8 years in a period of reduced air pollution concentrations.Citation177 They focused on the PM2.5 concentrations, which were measured between 1979 and 1988 and estimated for later years from publicly available data. It was found that an increase in overall mortality was associated with each 10 µg/m3 increase in PM2.5 modeled either as the overall mean (rate ratio 1.16; 95% CI 1.07–1.26) or as exposure in the year of death (rate ratio 1.14; 95% CI 1.06–1.22). Improved overall mortality was associated with a decreased mean PM2.5 (10 µg/m3) between periods (rate ratio 0.73; 95% CI 0.57–0.95).Citation177 This suggests that the mortality effects of long-term air pollution may be at least partially reversible.Citation39 Pope et al found that a decrease of 10 µg/m3 in the concentration of fine PM was associated with an estimated increase in mean (± standard error) life expectancy of 0.61±0.20 years (P=0.004). Reductions in air pollution accounted for as much as 15% of the overall increase in life expectancy in the study areas.Citation178
This indicates that it is beneficial to further tighten the current air quality guidelines around the world to reduce exposure levels and the effects on the general population and COPD sufferers.
Interventions to reduce sources of outdoor air pollution
The study conducted by Dockery et alCitation179 in the Republic of Ireland well illustrated that reducing the air pollution from the source might be the most effective way to improve the air quality. In Ireland, domestic coal burning was a major source of repeated severe pollution episodes. The government introduced sequential bans in 1990, 1995, and 1998 on the marketing, sale, and distribution of coal in different cities. The authors compiled records of daily black smoke, total gaseous acidity (SO2), and counts of cause-specific deaths from 1981 to 2004 for several cities and counties. They also compiled daily counts of hospital admissions for cardiovascular, respiratory, and digestive diagnoses. They compared the results with counties not affected by the bans. The mean black smoke concentrations fell in all affected population centers post-ban compared with the preban period, with decreases ranging from 4 µg/m3 to 35 µg/m3 (corresponding to reductions of 45% to 70%, respectively). Respiratory mortality was reduced in association with the bans in 1990, 1995, and 1998 (17%, 9%, and 3%, respectively). A 4% decrease in hospital admissions for cardiovascular disease associated with the 1995 ban and a 3% decrease with the 1998 ban were found, and admissions for pneumonia, COPD, and asthma were reduced.Citation179 Boogaard et alCitation180 found that implementing local traffic policies including low emission zones directed at heavy duty vehicles (trucks) in five Dutch cities reduced all pollutant levels, especially PM2.5 levels (20%–30%) and NO2 and NOx levels (25%–41%) in various areas. A recent review indicated that overall air pollution interventions have succeeded at improving air quality and also have been associated with health benefits, mainly reduced cardiovascular and/or respiratory mortality and/or morbidity.Citation181
These studies suggest that exposure control at the source can more efficiently reduce the air pollution level and therefore the human exposure and adverse outcomes.
Intervention to reduce indoor biomass air pollution in low-income countries
Since most countries probably do not have indoor air pollution standards and indoor air environments are generally not regulated, other measures to reduce indoor exposures to air pollutants from biomass or other solid fuels need to be developed, which could include a range of methods targeting the emission source (improved cook stoves or cleaner fuels), the indoor environment (improved ventilation and better design to separate the sources from main activity rooms), and the residents’ behaviors (to avoid direct exposure to the sources and for females not to carry young children on their back during cooking as this is a tradition in some rural areas in low-income countries). A recent review focusing on the People’s Republic of China as a typical case by Zhang and SmithCitation182 indicated that >180 million improved stoves with chimneys were introduced since the early 1980s. These stove programs have helped reduce the exposures. While randomized trials are difficult to do in the People’s Republic of China, natural experiments from Xuanwei County in Southwest People’s Republic of China indicated that installation of a chimney on the stove was associated with distinct reduction in the incidence of COPD.Citation155 The RR comparing stove users with or without a chimney was 0.58 (95% CI 0.49–0.70, P<0.001) in males and 0.75 (95% CI 0.62–0.92, P=0.005) in females. A 9-year prospective cohort study was conducted among 996 participants aged 40 years or older from November 1, 2002, through November 30, 2011, in 12 villages in southern People’s Republic of China by Zhou et al.Citation183 The intervention measures included improving kitchen ventilation (providing instruction or installing exhaust fans) and promoting the use of clean fuels (ie, biogas) instead of biomass for cooking (providing instruction and installing household biogas digesters). The study found that the combined intervention measures reduced the decline in FEV1, with a slowing rate of 16 mL/year (95% CI 9–23 mL/year). The longer the duration of the intervention measures used, the slower the decline of FEV1. The reduction in the overall risk of COPD was an OR of 0.28 (95% CI 0.11–0.73) for both intervention measures.
Intervention measures such as improved stoves, cleaner fuels, and other feasible and economical methods need to be tailored to the situation in each community based on affordability, effectiveness, and local culture so as to reduce the high exposure to biomass pollution and large COPD burden in nonsmoking females in low-income countries.
Integrated intervention and management program for COPD sufferers
A total of 1,062 subjects with or without COPD in a study in Guangdong, People’s Republic of China, by Zhou et alCitation184 randomly evaluated the effectiveness of integrated interventions, which included systematic health education, intensive and individualized intervention, treatment, and rehabilitation. The annual rate of decline in FEV1 was significantly lower in the intervention community than in the control community, with an adjusted difference of 19 mL/year (95% CI 3–36) and 0.9% (0.1%–1.8%) of predicted values (all P<0.05), as well as a lower annual rate of decline in FEV1/FVC ratio at 0.6% (0.1%–1.2%). Shofer et alCitation185 recommended that patients at increased risk for adverse effects of inhaled air pollutants, such as those who have been diagnosed with chronic lung disease and cardiovascular disease, including asthma, COPD, coronary artery disease, congestive heart failure, and peripheral vascular disease, should be educated regarding what symptoms may be related to poor air quality and how they can monitor the Air Quality Index to modify their activity to prevent symptoms and other adverse events. Heavy outdoor exertion should be avoided on days expected to have poor air quality or performed earlier in the day on days when outdoor activity cannot be avoided.
Conclusion and future directions
While air quality standards and guidelines have reduced human exposure overall and exposure of COPD sufferers in particular to PM and gaseous air pollutants around the world, health effects measured as mortality and morbidity still occur with COPD patients in the form of exacerbation or lead to the increased incidence of COPD in the general population. Further improvement in current air quality guidelines seems necessary at the government level, but other policy and exposure control measures could be implemented locally or at the personal level. Continued epidemiologic research, particularly long-term prospective cohort studies involving multiple countries or cities to evaluate the effects of multiple pollutants and their interactions on the COPD burden, is needed in both high-income and low- to middle-income countries. Additionally, more intervention studies targeting reduced exposures and improved outcomes specifically for COPD sufferers are needed.
Disclosure
Dr Liu is the recipient of a research grant (5R03OH009815) and a contract (200-2015-M-63768) from the National Institute for Occupational Safety and Health and a Clinical Scholars Award from Cook Children’s Health Care System. The authors report no other conflicts of interest in this work.
References
- Goldcopd.org [webpage on the Internet]Global Strategy for the Diagnosis, Management and Prevention of COPDNew York, NYGlobal Initiative for Chronic Obstructive Lung Disease Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report2014_Feb07.pdfAccessed August 10, 2015
- American Thoracic SocietyWhat constitutes an adverse health effect of air pollution? Official statement of the American Thoracic SocietyAm J Respir Crit Care Med200016166567310673213
- Lung.org [webpage on the Internet]Trends in COPD (Chronic Bronchitis and Emphysema): Morbidity and MortalityChicagoAmerican Lung Association Available from: http://www.lung.org/assets/documents/research/copd-trend-report.pdfAccessed October 26, 2015
- Whoint [webpage on the Internet]COPD: DefinitionGenevaWorld Health Organization Available from: http://www.who.int/respiratory/copd/burden/en/Accessed October 26, 2015
- ManninoDMBuistASGlobal burden of COPD: risk factors, prevalence, and future trendsLancet2007370958976577317765526
- BuistASMcBurnieMAVollmerWMBOLD Collaborative Research GroupInternational variation in the prevalence of COPD (the BOLD Study): a population-based prevalence studyLancet2007370958974175017765523 ErratumLancet2012380984480622939685
- McCarthyJwebpage on the InternetUS, Smoking Rate Lowest in Utah, Highest in KentuckyWashington, D.CGallup Available from: http://www.gallup.com/poll/167771/smoking-rate-lowest-utah-highest-kentucky.aspxAccessed October 26, 2015
- MenezesAMPerez-PadillaRJardimJRPLATINO TeamChronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence studyLancet20053661875188116310554
- AntóJMVermeirePVestboJSunyerJEpidemiology of chronic obstructive pulmonary diseaseEur Respir J200117598299411488336
- MathersCFatDMBoermaJTWHOThe Global Burden of Disease: 2004 UpdateGenevaWHO20081146
- MarshSAldingtonSShirtcliffePWeatherallMBeasleyRSmoking and COPD: what really are the risks?Eur Respir J200628488388417012635
- LundbackBLindbergALindstromMObstructive Lung Disease in Northern Sweden StudiesNot 15 but 50% of smokers develop COPD? Report from the obstructive lung disease in Northern Sweden studiesRespir Med20039711512212587960
- LindbergABjergARönmarkELarssonLGLundbäckBPrevalence and underdiagnosis of COPD by disease severity and the attributable fraction of smoking report from the obstructive lung disease in Northern Sweden StudiesRespir Med2006100226427215975774
- ViegiGScognamiglioABaldacciSPistelliFCarrozziLEpidemiology of chronic obstructive pulmonary disease (COPD)Respiration200168141911223724
- Diaz-GuzmanEManninoDMEpidemiology and prevalence of chronic obstructive pulmonary diseaseClin Chest Med201435171624507833
- IssacMSAshurWMousaHGenetic polymorphisms of surfactant protein D rs2243639, Interleukin (IL)-1β rs16944 and IL-1RN rs2234663 in chronic obstructive pulmonary disease, healthy smokers, and non-smokersMol Diagn Ther201418334335424504887
- BosséYUpdates on the COPD gene listInt J Chron Obstruct Pulmon Dis2012760763123055711
- SadeghnejadAOharJAZhengSLAdam33 polymorphisms are associated with COPD and lung function in long-term tobacco smokersRespir Res2009102119284602
- OharJAHamiltonRFJrZhengSCOPD is associated with a macrophage scavenger receptor-1 gene sequence variationChest201013751098110720081102
- YuanYHouXZhangJChenYFengYSuZGenetic variations in RORα are associated with chronic obstructive pulmonary diseaseJ Hum Genet201459843043624943193
- WangBZhouHYangJAssociation of HHIP polymorphisms with COPD and COPD-related phenotypes in a Chinese Han populationGene2013531110110523994291
- WangBLiangBYangJAssociation of FAM13A polymorphisms with COPD and COPD-related phenotypes in Han ChineseClin Biochem20134616–171683168823891779
- Rodriguez-GonzalezEFerrer-SanchoJOccupational exposure and COPDCurr Respir Med Rev20128436440
- BecklakeMRRelationship of acute obstructive airway change to chronic (fixed) obstructionThorax199550suppl 1S16S217570457
- ChristianiDCWangXRPanLDLongitudinal changes in pulmonary function and respiratory symptoms in cotton textile workers. A 15-yr follow-up studyAm J Respir Crit Care Med2001163484785311282755
- MoiraCYEnarsonDAKennedySMThe impact of grain dust on respiratory healthAm Rev Respir Dis19921452 pt 14764871736761
- NoertjojoHKDimich-WardHPeelenSDittrickMKennedySMChan-YeungMWestern red cedar dust exposure and lung function: a dose-response relationshipAm J Respir Crit Care Med19961544 pt 19689738887593
- ChristianiDCOrganic dust exposure and chronic airway diseaseAm J Respir Crit Care Med19961548338348887570
- CoggonDNewman TaylorACoal mining and chronic obstructive pulmonary disease: a review of the evidenceThorax19985353984079708233
- Al-NeaimiYIGomesJLloydOLRespiratory illnesses and ventilator function among workers at a cement factory in a rapidly developing countryOccup Med (Lond)200151636737311584114
- FishwickDBradshawLMD’SouzaWChronic bronchitis, shortness of breath, and airway obstruction by occupation in New ZealandAm J Respir Crit Care Med19971565144014469372658
- DavisonAGFayersPMTaylorAJCadmium fume inhalation and emphysemaLancet1988185876636672895211
- SferlazzaSJThe respiratory health of weldersAm Rev Respir Dis19911435 pt 1113411482024826
- BalmesJBecklakeMBlancPEnvironmental and Occupational Health AssemblyAmerican Thoracic SocietyEnvironmental and Occupational Health Assembly, American Thoracic Society. American Thoracic Society Statement: occupational contribution to the burden of airway diseaseAm J Respir Crit Care Med2003167578779712598220
- KampaMCastanasEHuman health effects of air pollutionEnviron Pollut2008151236236717646040
- SametJMAir pollution and epidemiology: “déjà vu all over again?”Epidemiology200213211811911880748
- MacNeeWDonaldsonKExacerbations of COPD: environmental mechanismsChest20001175 suppl 2390S397S10843983
- SunyerJUrban air pollution and chronic obstructive pulmonary disease: a reviewEur Respir J2001171024103311488305
- DockeryDWHealth effects of particulate air pollutionAnn Epidemiol200919425726319344865
- BellMLDavidDLReassessment of the lethal London fog of 1952: novel indicators of acute and chronic consequences of acute exposure to air pollutionEnviron Health Perspect2001109suppl 338939411427388
- SchwartzJMarcusAMortality and air pollution in London: a time series analysisAm J Epidemiol199013111851942403468
- AndersonHRLimbESBlandJMPonce de LeonAStrachanDPBowerJSHealth effects of an air pollution episode in London, December 1991Thorax19955011118811938553276
- NemeryBHoetPHNemmarAThe Meuse Valley fog of 1930: an air pollution disasterLancet2001357925770470811247570
- EPA.gov [webpage on the Internet]Then, Now and FutureWashington, DCEPA’s Air, Climate, and Energy Research Available from: www2.epa.gov/air-research/history-air-pollutionAccessed October 26, 2015
- DockeryDWPopeCA3rdXuXAn association between air pollution and mortality in six U.S. citiesN Engl J Med1993329175317598179653
- WareJHFerrisBGJrDockeryDWSpenglerJDStramDOSpeizerFEEffects of ambient sulfur oxides and suspended particles on respiratory health of preadolescent childrenAm Rev Respir Dis198613358348423706894
- DassenWBrunekreefBHoekGDecline in children’s pulmonary function during an air pollution episodeJ Air Pollut Control Assoc198636122312273794084
- DockeryDWWareJHFerrisBGJrSpeizerFECookNRHermanSMChange in pulmonary function in children associated with air pollution episodesJ Air Pollut Control Assoc1982329379427130539
- GreenbaumDSBachmannJDKrewskiDSametJMWhiteRWyzgaREParticulate air pollution standards and morbidity and mortality: case studyAm J Epidemiol200115412 supplS78S9011744533
- KatsouyanniKTouloumiGSpixCShort-term effects of ambient sulphur dioxide and particulate matter on mortality in 12 European cities: results from time series data from the APHEA project. Air pollution and health: a European approachBMJ1997314165816639180068
- KatsouyanniKTouloumiGSamoliEConfounding and effect modification in the short-term effects of ambient particles on total mortality: results from 29 European cities within the APHEA2 projectEpidemiology20011252153111505171
- SametJMDominiciFZegerSLSchwartzJDockeryDWThe national morbidity, mortality, and air pollution study. Part I: methods and methodologic issuesRes Rep Health Eff Inst200094pt 1514
- SametJMZegerSLDominiciFThe national morbidity, mortality, and air pollution study. Part II: morbidity and mortality from air pollution in the United StatesRes Rep Health Eff Inst200094pt 2570
- PopeCA3rdBurnettRTThunMJLung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollutionJAMA20022871132114111879110
- SchwartzJDockeryDWIncreased mortality in Philadelphia associated with daily air pollution concentrationsAm Rev Respir Dis199214536006041546841
- XuXGaoJDockeryDWChenYAir pollution and daily mortality in residential areas of Beijing, ChinaArch Environ Health19944942162228031176
- RossiGVigottiMAZanobettiARepettoFGianelleVSchwartzAir pollution and cause-specific mortality in Milan, Italy, 1980–1989Arch Environ Health199954315816410444036
- XuZYuDJingLXuXAir pollution and daily mortality in Shenyang, ChinaArch Environ Health200055211512010821512
- Tellez-RojoMMRomieuIRuiz-VelascoSLezanaMAHernandez-AvilaMMDaily respiratory mortality and PM10 pollution in Mexico City: importance of considering place of deathEur Respir J20001639139611028649
- WongTWTamWSYuTSWongAHAssociations between daily mortalities from respiratory and cardiovascular diseases and air pollution in Hong Kong, ChinaOccup Environ Med2002591303511836466
- KanHChenBAir pollution and daily mortality in Shanghai: a time-series studyArch Environ Health20035836036714992311
- ZekaAZanobettiASchwartzJShort term effects of particulate matter on cause specific mortality: effects of lags and modification by city characteristicsOccup Environ Med20056271872516169918
- NaessØNafstadPAamodtGClaussenBRoslandPRelation between concentration of air pollution and cause-specific mortality: four-year exposures to nitrogen dioxide and particulate matter pollutants in 470 neighborhoods in Oslo, NorwayAm J Epidemiol2007165443544317135427
- MengXWangCCaoDWongCKanHShort-term effect of ambient air pollution on COPD mortality in four Chinese citiesAtmos Environ201377149154
- SamoliEStafoggiaMRodopoulouSMED-PARTICLES Study GroupWhich specific causes of death are associated with short term exposure to fine and coarse particles in southern Europe? Results from the MED-PARTICLES projectEnvironment Int2014675461
- RhodenCRWelleniusGAGhelfiELawrenceJGonzalez-FlechaBPM-induced cardiac oxidative stress and dysfunction are mediated by autonomic stimulationBiochim Biophys Acta2005172530531316005153
- RobertsESRichardsJHJaskotRDreherKLOxidative stress mediates air pollution particle-induced acute lung injury and molecular pathologyInhal Toxicol2003151327134614569496
- RablAAir pollution mortality: harvesting and loss of life expectancyJ Toxicol Environ Health A2005681175118016024496
- BatesDVHealth indices of the adverse effects of air pollution: the question of coherenceEnviron Res19925923363491464287
- SunyerJAntóJMMurilloCSaezMEffects of urban air pollution on emergency room admissions for chronic obstructive pulmonary diseaseAm J Epidemiol19911343277286 discussion 287–2891877586
- SunyerJSáezMMurilloCCastellsagueJMartínezFAntóJMAir pollution and emergency room admissions for chronic obstructive pulmonary disease: a 5-year studyAm J Epidemiol199313777017058484361
- PönkäAVirtanenMChronic bronchitis, emphysema, and low-level air pollution in Helsinki, 1987–1989Environ Res19946522072178187737
- SchwartzJPM10, ozone, and hospital admissions for the elderly in Minneapolis-St. Paul, MinnesotaArch Environ Health1994493663747944569
- SchwartzJAir pollution and hospital admissions for the elderly in Detroit, MichiganAm J Respir Crit Care Med19941506486558087333
- SchwartzJAir pollution and hospital admissions for the elderly in Birmingham, AlabamaAm J Epidemiol199413965895988172170
- BurnettRTDalesRERaizenneMEEffects of low ambient levels of ozone and sulfates on the frequency of respiratory admissions to Ontario hospitalsEnviron Res19946521721948187735
- SchoutenJPVonkJMde GraafAShort term effects of air pollution on emergency hospital admissions for respiratory disease: results of the APHEA project in two major cities in the Netherlands, 1977–89J Epidemiol Community Health199650suppl 1S22S298758220
- AndersonHRSpixCMedinaSAir pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the APHEA projectEur Respir J199710106410719163648
- MorganGCorbettSWlodarczykJAir pollution and hospital admissions in Sydney, Australia, 1990 to 1994Am J Public Health199888176117669842371
- ChenLYangWJennisonBLOmayeSTAir particulate pollution and hospital admissions for chronic obstructive pulmonary disease in Reno, NevadaInhal Toxicol20001228129810715629
- TolbertPEKleinMMetzgerKBInterim results of the study of particulates and health in Atlanta (SOPHIA)J Expo Anal Environ Epidemiol200010544646011051535
- FuscoDForastiereFMichelozziPAir pollution and hospital admissions for respiratory conditions in Rome, ItalyEur Respir J2001171143115011491157
- TeníasJMBallesterFPérez-HoyosSRiveraMLAir pollution and hospital emergency room admissions for chronic obstructive pulmonary disease in Valencia, SpainArch Environ Health2002571414712071359
- ChenYYangQKrewskiDShiYBurnettRTMcGrailKInfluence of relatively low level of particulate air pollution on hospitalization for COPD in elderly peopleInhal Toxicol2004161212514744661
- PeelJLTolbertPEKleinMAmbient air pollution and respiratory emergency department visitsEpidemiology200516216417415703530
- YangQChenYKrewskiDBurnettRTShiYMcGrailKMEffect of short-term exposure to low levels of gaseous pollutants on chronic obstructive pulmonary disease hospitalizationsEnviron Res2005999910516053934
- HinwoodALDe KlerkNRodriguezCThe relationship between changes in daily air pollution and hospitalizations in Perth, Australia 1992–1998: a case-crossover studyInt J Environ Health Res2006161274616507479
- DominiciFPengRDBellMLFine particulate air pollution and hospital admission for cardiovascular and respiratory diseasesJAMA2006295101127113416522832
- Medina-RamónMZanobettiASchwartzJThe effect of ozone and PM10 on hospital admissions for pneumonia and chronic obstructive pulmonary disease: a national multicity studyAm J Epidemiol2006163657958816443803
- SauerzapfVJonesAPCrossJEnvironmental factors and hospitalisation for chronic obstructive pulmonary disease in a rural county of EnglandJ Epidemiol Community Health200963432432819208692
- BelleudiVFaustiniAStafoggiaMImpact of fine and ultrafine particles on emergency hospital admissions for cardiac and respiratory diseasesEpidemiology201021341442320386174
- CireraLGarcía-MarcosLGiménezJDaily effects of air pollutants and pollen types on asthma and COPD hospital emergency visits in the industrial and Mediterranean Spanish city of CartagenaAllergol Immunopathol (Madr)201240423123721890258
- LiuXLessnerLCarpenterDOAssociation between residential proximity to fuel-fired power plants and hospitalization rate for respiratory diseasesEnviron Health Perspect2012120680781022370087
- FaustiniAStafoggiaMColaisPEpiAir Collaborative GroupAir pollution and multiple acute respiratory outcomesEur Respir J201342230431323314899
- KloogINordioFZanobettiACoullBAKoutrakisPSchwartzJDShort term effects of particle exposure on hospital admissions in the Mid-Atlantic States: a population estimatePLoS One201492e8857824516670
- YorifujiTSuzukiEKashimaSHourly differences in air pollution and risk of respiratory disease in the elderly: a time-stratified case-crossover studyEnviron Health2014136725115710
- WongTWLauTSYuTSAir pollution and hospital admissions for respiratory and cardiovascular diseases in Hong KongOccup Environ Med1999561067968310658547
- BurrilloJMDíezFBPérez-HoyosSUse of different hospital data bases in the estimation of the relation between air pollution and chronic obstructive pulmonary diseaseEpidemiology200112228011246595
- PandeJNBhattaNBiswasDOutdoor air pollution and emergency room visits at a hospital in DelhiIndian J Chest Dis Allied Sci2002441131911845928
- GouveiaNde FreitasCUMartinsLCMarcilioIOHospitalizações por causas respiratórias e cardiovasculares associadas à contaminação atmosférica no Município de São Paulo, Brasil [Respiratory and cardiovascular hospitalizations associated with air pollution in the city of Sao Paulo, Brazil]Cad Saúde Pública20062226692677 Portuguese17096045
- YangCYChenCJAir pollution and hospital admissions for chronic obstructive pulmonary disease in a subtropical city: Taipei, TaiwanJ Toxicol Environ Health A200770141214121917573635
- LeeIMTsaiSSChangCCHoCKYangCYAir pollution and hospital admissions for chronic obstructive pulmonary disease in a tropical city: Kaohsiung, TaiwanInhal Toxicol200719539339817365044
- KoFWTamWWongTWTemporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong KongThorax200762978078517311838
- ArbexMAde Souza ConceiçãoGMCendonSPUrban air pollution and chronic obstructive pulmonary disease-related emergency department visitsJ Epidemiol Community Health2009631077778319468016
- MilutinovićSNikićDStosićLStankovićABogdanovićDShort-term association between air pollution and emergency room admissions for chronic obstructive pulmonarydisease in Nis, SerbiaCent Eur J Public Health200917181319418713
- QiuHYuITSWangXTianLTseLAWongTWSeason and humidity dependence of the effects of air pollution on COPD hospitalizations in Hong KongAtmos Environ2013767480
- TsaiSSChangCCYangCYFine particulate air pollution and hospital admissions for chronic obstructive pulmonary disease: a case-crossover study in TaipeiInt J Environ Res Public Health201310116015602624284359
- GhozikaliMGMosaferiMSafariGHJaafariJEffect of exposure to O3, NO2, and SO2 on chronic obstructive pulmonary disease hospitalizations in Tabriz, IranEnviron Sci Pollut Res Int20152242817282325217280
- TzonouAMaragoudakisGTrichopoulosDUrban living, tobacco smoking, and chronic obstructive pulmonary disease: a study in AthensEpidemiology19923157601554811
- TashkinDPDetelsRSimmonsMThe UCLA population studies of chronic obstructive respiratory disease: XI. Impact of air pollution and smoking on annual change in forced expiratory volume in one secondAm J Respir Crit Care Med1994149120912178173761
- Ackermann-LiebrichULeuenbergerPSchwartzJLung function and long term exposure to air pollutants in Switzerland. Study on air pollution and lung diseases in adults (SAPALDIA) TeamAm J Respir Crit Care Med19971551221299001300
- AvinoPDe LisioVGrassiMInfluence of air pollution on chronic obstructive respiratory diseases: comparison between city (Rome) and hillcountry environments and climatesAnn Chim2004949–1062963515506613
- SchikowskiTSugiriDRanftULong-term air pollution exposure and living close to busy roads are associated with COPD in womenRespir Res2005615216372913
- SunyerJJarvisDGotschiTChronic bronchitis and urban air pollution in an international studyOccup Environ Med2006631283684316847030
- CesaroniGBadaloniCPortaDForastiereFPerucciCAComparison between various indices of exposure to traffic-related air pollution and their impact on respiratory health in adultsOccup Environ Med2008651068369018203803
- LindgrenAStrohEMontnémeryPNihlénUJakobssonKAxmonATraffic-related air pollution associated with prevalence of asthma and COPD/chronic bronchitis. A cross-sectional study in Southern SwedenInt J Health Geogr20098219154599
- BentayebMHelmerCRaherisonCTessierJFAnnesi-MaesanoIBronchitis-like symptoms and proximity air pollution in French elderlyRespir Med2010104688088820129767
- NuvoloneDDella MaggioreRMaioSGeographical information system and environmental epidemiology: a cross-sectional spatial analysis of the effects of traffic-related air pollution on population respiratory healthEnviron Health2011101221362158
- AndersenZJHvidbergMJensenSSChronic obstructive pulmonary disease and long-term exposure to traffic-related air pollution: a cohort studyAm J Respir Crit Care Med2011183445546120870755
- SalamehPSalameJKhayatGExposure to outdoor air pollution and chronic bronchitis in adults: a case-control studyInt J Occup Environ Med20123416517723022867
- RiceMBLjungmanPLWilkerEHLong-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham heart studyAm J Respir Crit Care Med2015191665666425590631
- ToTZhuJVilleneuvePJChronic disease prevalence in women and air pollution – a 30-year longitudinal cohort studyEnviron Int201580263225863281
- AdamkiewiczŁGayerAMuchaDBadydaAJDąbrowieckiPGrabskiPRelative risk of lung obstruction in relation to PM10 concentration as assessed by pulmonary function testsAdv Exp Med Biol2015849839125523626
- LawtherPJWallerREHendersonMAir pollution and exacerbations of bronchitisThorax197055255395489175
- HarréESPricePDAyreyRBToopLJMartinIRTownGIRespiratory effects of air pollution in chronic obstructive pulmonary disease: a three month prospective studyThorax19975212104010449516896
- LinnWSGongHJrClarkKWAndersonKRDay-to-day particulate exposures and health changes in Los Angeles area residents with severe lung diseaseJ Air Waste Manag Assoc1999499 Spec10811511002833
- SunyerJSchwartzJTobiasAMacfarlaneDGarciaJAntoJMPatients with chronic obstructive pulmonary disease are at increased risk of death associated with urban particle air pollution: a case-crossover analysisAm J Epidemiol20001511505610625173
- SunyerJBasagañaXParticles, and not gases, are associated with the risk of death in patients with chronic obstructive pulmonary diseaseInt J Epidemiol20013051138114011689536
- DesqueyrouxHPujetJCProsperMLe MoullecYMomasIEffects of air pollution on adults with chronic obstructive pulmonary diseaseArch Environ Health200257655456012696653
- SilkoffPEZhangLDuttonSWinter air pollution and disease parameters in advanced chronic obstructive pulmonary disease panels residing in Denver, ColoradoJ Allergy Clin Immunol2005115233734415696092
- TrengaCASullivanJHSchildcroutJSEffect of particulate air pollution on lung function in adult and pediatric subjects in a Seattle panel studyChest200612961614162216778283
- LagorioSForastiereFPistelliRAir pollution and lung function among susceptible adult subjects: a panel studyEnviron Health200651116674831
- PeacockJLAndersonHRBremnerSAOutdoor air pollution and respiratory health in patients with COPDThorax201166759159621459856
- PopeCA3rdBatesDVRaizenneMEHealth effects of particulate air pollution: time for reassessment?Environ Health Perspect199510354724807656877
- EPAgov [webpage on the Internet]Indoor Air QualityWashington, DCEPA Available from: http://cfpub.epa.gov/roe/chapter/air/indoorair.cfmAccessed on October 26, 2015
- ZhangJSmithKRMaYGreenhouse gases and other airborne pollutants from household stoves in China: a database for emission factorsAtmos Environ20003445374549
- ShresthaILShresthaSLIndoor air pollution from biomass fuels and respiratory health of the exposed population in Nepalese householdsInt J Occup Environ Health20051115016015875891
- NaeherLPBrauerMLipsettMWoodsmoke health effects: a reviewInhal Toxicol2007196710617127644
- SmithKRInaugural article: national burden of disease in India from indoor air pollutionProc Natl Acad Sci U S A200097132861329311087870
- SmithKRBiofuels, Air Pollution and Health: A Global ReviewNew York, NYPlenum Press1987
- WoolcockAJBlackburnCRChronic lung disease in the territory of Papula and New Guinea-an epidemiological studyAustralas Ann Med19671611196032024
- ClearyGJBlackburnRBAir pollution in native huts in the highlands of New GuineaArch Environ Health1968177857945698496
- PandeyMRDomestic smoke pollution and chronic bronchitis in a rural community of the Hill Region of NepalThorax19843953373396740536
- BeheraDJindalSKRespiratory symptoms in Indian women using domestic cooking fuelsChest199110023853881864111
- MenezesAMVictoraCGRigattoMPrevalence and risk factors for chronic bronchitis in Pelotas, RS, Brazil: a population-based studyThorax19944912121712217878555
- DossingMKhanJal-RabiahFRisk factors for chronic obstructive lung disease in Saudi ArabiaRespir Med1994885195227972976
- DennisRJMaldonadoDNormanSBaenaEMartinezGWoodsmoke exposure and risk for obstructive airways disease among womenChest19961091151198549171
- Perez-PadillaRRegaladoJVedalSExposure to biomass smoke and chronic airway disease in Mexican women. A case-control studyAm J Respir Crit Care Med19961547017068810608
- EllegårdACooking fuel smoke and respiratory symptoms among women in low-income areas in MaputoEnviron Health Perspect199610499809858899378
- AlbalakRFrisanchoARKeelerGJDomestic biomass fuel combustion and chronic bronchitis in two rural Bolivian villagesThorax199954111004100810525559
- GolshanMFaghihiMMarandiMMIndoor women jobs and pulmonary risks in rural areas of Isfahan, Iran, 2000Respir Med200296638238812117036
- KirazKKartLDemirRChronic pulmonary disease in rural women exposed to biomass fumesClin Invest Med20032624324814596485
- EkiciAEkiciMKurtipekEObstructive airway diseases in women exposed to biomass smokeEnviron Res200599939816053933
- PeabodyJWRiddellTJSmithKRIndoor air pollution in rural China: cooking fuels, stoves, and health statusArch Environ Occup Health200560869516983861
- ChapmanRSHeXBlairAELanQImprovement in household stoves and risk of chronic obstructive pulmonary disease in Xuanwei, China: retrospective cohort studyBMJ20053317524105016234255
- SezerHAkkurtIGulerNBerkSA case-control study on the effect of exposure to different substances on the development of COPDAnn Epidemiol2006161596215990336
- AkhtarTUllahZKhanMHNazliRChronic bronchitis in women using solid biomass fuel in rural Peshawar, PakistanChest200713251472147517646238
- LiuSZhouYWangXBiomass fuels are the probable risk factor for chronic obstructive pulmonary disease in rural South ChinaThorax20076288989717483137
- ZhongNWangCYaoWPrevalence of chronic obstructive pulmonary disease in China: a large, population-based surveyAm J Respir Crit Care Med2007176875376017575095 ErratumAm J Respir Crit Care Med200717611116918042661
- DesaluOOAdekoyaAOAmpitanBAIncreased risk of respiratory symptoms and chronic bronchitis in women using biomass fuels in NigeriaJ Bras Pneumol201036444144620835590
- JohnsonPBalakrishnanKRamaswamyPPrevalence of chronic obstructive pulmonary disease in rural women of Tamilnadu: implications for refining disease burden assessments attributable to household biomass combustionGlob Health Action20114722622065945
- da SilvaLFSaldivaSRSaldivaPHBandeira Científica ProjectImpaired lung function in individuals chronically exposed to biomass combustionEnviron Res201211211111722136759
- MaheshPAJayarajBSPrabhakarAKChayaSKVijaysimhaRIdentification of a threshold for biomass exposure index for chronic bronchitis in rural women of Mysore district, Karnataka, IndiaIndian J Med Res20131371879423481056
- DietteGBAccinelliRABalmesJRObstructive lung disease and exposure to burning biomass in the indoor environmentGlob Heart20127326527023139916
- EPANational ambient air quality standards for particulate matter; final ruleFed Regist1997621383865138701
- Whoint [webpage on the Internet]Air Quality Guidelines – Global Update 2005GenevaWorld Health Organization Available from: http://whqlibdoc.who.int/hq/2006/WHO_SDE_PHE_OEH_06.02_eng.pdf?ua=1Accessed October 26, 2015
- KrzyzanowskiMWHO air quality guidelines for EuropeJ Toxicol Environ Health A2008711475018080893
- KrzyzanowskiMCohenAUpdate of WHO air quality guidelinesAir Qual Atmos Health200811713
- FaustiniAStafoggiaMCappaiGForastiereFShort-term effects of air pollution in a cohort of patients with chronic obstructive pulmonary diseaseEpidemiology201223686187923018970
- GanWQFitzGeraldJMCarlstenCSadatsafaviMBrauerMAssociations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortalityAm J Respir Crit Care Med2013187772172723392442
- SulzbachMWhat’s driving Twin Cities air quality?Min Med20068953639
- BellMLMcDermottAZegerSLSametJMDominiciFOzone and short-term mortality in 95 US urban communities, 1987–2000JAMA2004292192372237815547165
- BellMLRogerDPDominiciFThe exposure-response curve for ozone and risk of mortality and the adequacy of current ozone regulationsEnviron Health Perspect200614453253616581541
- SchikowskiTAdamMMarconAAssociation of ambient air pollution with the prevalence and incidence of COPDEur Respir J201444361462624488569
- Pujades-RodríguezMMcKeeverTLewisSWhyattDBrittonJVennAEffect of traffic pollution on respiratory and allergic disease in adults: cross-sectional and longitudinal analysesBMC Pulm Med200994219703291
- SichletidisLSpyratosDTsiotsiosAExposure to PM10 as a risk factor for the development of nasal obstruction and chronic obstructive pulmonary diseaseInt J Occup Environ Health201420191524804336
- LadenFSchwartzJSpeizerFEDockeryDWReduction in fine particulate air pollution and mortality: extended follow-up of the Harvard Six Cities studyAm J Respir Crit Care Med2006173666767216424447
- PopeCA3rdEzzatiMDockeryDWFine-particulate air pollution and life expectancy in the United StatesN Engl J Med2009360437638619164188
- DockeryDRichDQGoodmanPGEffect of air pollution control on mortality and hospital admissions in IrelandRes Rep Health Eff Inst2013176310924024358
- BoogaardHJanssenNAFischerPHImpact of low emission zones and local traffic policies on ambient air pollution concentrationsSci Total Environ2012435–43613214022846773
- HenschelSAtkinsonRZekaAAir pollution interventions and their impact on public healthInt J Public Health201257575776822592907
- ZhangJJSmithKRHousehold air pollution from coal and biomass fuels in China: measurements, health impacts, and interventionsEnviron Health Perspect2007115684885517589590
- ZhouYZouYLiXLung function and incidence of chronic obstructive pulmonary disease after improved cooking fuels and kitchen ventilation: a 9-year prospective cohort studyPLoS Med2014113e100162124667834
- ZhouYHuGWangDCommunity based integrated intervention for prevention and management of chronic obstructive pulmonary disease (COPD) in Guangdong, China: cluster randomised controlled trialBMJ2010341c638721123342
- ShoferSChenTMGokhaleJKuschnerWGOutdoor air pollution: counseling and exposure risk reductionAm J Med Sci2007333425726017435421