Abstract
The Coronavirus disease 2019 (COVID-19) pandemic is one of the most considerable health problems across the world. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the major causative agent of COVID-19. The severe symptoms of this deadly disease include shortness of breath, fever, cough, loss of smell, and a broad spectrum of other health issues such as diarrhea, pneumonia, bronchitis, septic shock, and multiple organ failure. Currently, there are no medications available for coronavirus patients, except symptom-relieving drugs. Therefore, SARS-CoV-2 requires the development of effective drugs and specific treatments. Heterocycles are important constituents of more than 85% of the physiologically active pharmaceutical drugs on the market now. Several FDA-approved drugs have been reported including molnupiravir, remdesivir, ritonavir, oseltamivir, favipiravir, chloroquine, and hydroxychloroquine for the cure of COVID-19. In this study, we discuss potent anti-SARS-CoV-2 heterocyclic compounds that have been synthesized over the past few years. These compounds included; indole, piperidine, pyrazine, pyrimidine, pyrrole, piperazine, quinazoline, oxazole, quinoline, isoxazole, thiazole, quinoxaline, pyrazole, azafluorene, imidazole, thiadiazole, triazole, coumarin, chromene, and benzodioxole. Both in vitro and in silico studies were performed to determine the potential of these heterocyclic compounds in the fight against various SARS-CoV-2 proteins.
Introduction
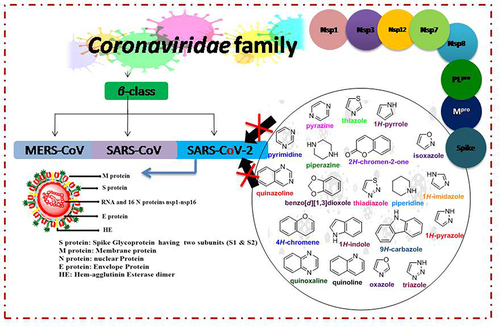
Coronavirus disease 2019 (COVID-19 or 2019-nCoV), a fatal respiratory illness, was first identified in late December 2019 in Wuhan, China, and spread rapidly across 200 countries around the world.Citation1 On March 11, 2020, the World Health Organization (WHO) declared COVID-19 as a pandemic.Citation2 The WHO renamed the virus as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on February 20, 2020.Citation3 The Beta, Delta, and Omicron variants started to emerge in Africa in May 2020, in India by October 2020, and in various other regions in November 2021, causing many concerns worldwide.Citation4 The coronavirus, is highly contagious in the air, can transmit from one person to another through respiratory aerosols and droplets.Citation5,Citation6 The breathing problems caused by COVID-19 can be fatal in some cases, resulting in a global public health emergency.Citation7 Common symptoms of the disease include; shortness of breath (19%), fever (88%), loss of smell (15–30%), and cough (68%), as well as diarrhea, pneumonia, bronchitis, septic shock, multiple organ failure, and acute respiratory distress syndrome.Citation8,Citation9
The causative agent of COVID-19 is a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).Citation10 The SARS-CoV-2 is an enveloped, crown-shaped, single-stranded positive RNA virus that belongs to the genus β-coronavirus, subgenus Sarbecovirus, and family Coronaviridae.Citation11 The SARS-CoV-2 is the seventh type of human coronavirus (HCoVs) after SARS-CoV, HCoV-229E, HCoV-HKU1, HCoV-NL63, HCoV-OC43, and middle east respiratory syndrome (MERS-CoV).Citation12 The SARS-CoV-2, MERS-CoV, and SARS-CoV viruses cause severe acute respiratory infections, whereas the HCoV-HKU1, HCoV-229E, HCoV-OC43, and HCoV-NL63 viruses affect with milder symptoms. The genome sequence of SARS-CoV-2 is 88% similarity to SARS-like bat coronaviruses (bat-SL-CoVZXC21 and bat-SL-CoVZC45), about 79% similar to SARS-CoV, and has about 50% similarity to MERS-CoV.Citation13 The SARS-CoV-2 genome contains approximately 30,000 nucleotides in the sequence of 5′-replicase-S-E-M-N-3′.Citation14 The SARS-CoV-2 genome has two major overlapping open reading frames (ORF1a/b) that encode four structural proteins (sps): spike (S), envelope (E), membrane (M), and nucleocapsid (N), and nonstructural proteins (nsps), as well as accessory factors.Citation15 The nsps1–nsps16 are encoded proteins that are responsible for viral transcription and replication, such as papain-like protease (PLpro; nsp3), RNA-dependent RNA polymerase (RdRp; nsp12), 3-chymotrypsin-like protease (3CLpro; nsp5), also called the main protease (Mpro), and RNA helicase (nsp13).Citation16–18
The spike protein is responsible for SARS-CoV-2 penetration into human ACE2-expressing cells. SARS-CoV-2 spike protein binds to angiotensin-converting enzyme 2 (ACE2) on the surface of human lung epithelial cells, allowing the virus to enter the host cells via the ACE2 receptor.Citation19 The cells with greater levels of ACE2 expression may be considered as potent SARS-CoV-2 infection sites.Citation20 The increased ACE2 expression causes a rise in SARS-CoV-2 viral infection.Citation21 COVID-19 disease may be treated effectively by targeting ACE2 expression.Citation22 Preventing SARS-CoV-2 entry into the host cell via ACE2 receptor sites may be an effective method of combating COVID-19.Citation23
RNA-dependent RNA polymerase (RdRp) is a nonstructural protein that catalyzes the synthesis of the viral genome and thus plays a pivotal role in the replication and transcription of SARS-CoV-2.Citation24 Viral RNA synthesis also requires the presence of two additional proteins, nsp7 and nsp8, which are thought to have a primase or 3′-terminal adenylyl-transferase function.Citation25,Citation26
The chymotrypsin-like cysteine protease (3CLpro), also called Mpro, belongs to the 16 nonstructural proteins of SARS-CoV-2 and is an essential enzyme that plays a valuable role in viral RNA replication and transcription of coronaviruses (CoVs).Citation27 The enzyme encodes the translated polyproteins from coronaviruses (CoVs) RNA along with papain-like proteases (PLpro).Citation28 In structural identification, 3CLpro comprises three domains: the chymotrypsin-like domain (domain I, residues 8−101), picornavirus 3C protease-like domain (domain II, residues 102−184), and a globular cluster produced via five helices (domain III, residues 201−303). The 3CLpro active site (substrate-binding site), made up of four subsites (S1, S2, S3, and S4), is situated between domains I and II on β barrels.Citation29 Consequently, two distinct potent 3CLpro inhibitors, including both non-peptidic inhibitors and peptidomimetic inhibitors, have been reported to fight a rapid rise in the COVID-19 pandemic.Citation30–33
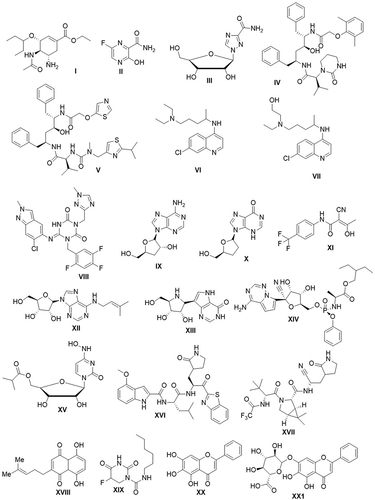
The effects of the SARS-CoV-2 epidemic were profound regarding the world’s economy as well as on public health. Consequently, the World Health Organization (WHO) declared a worldwide health emergency in order to coordinate scientific and medical efforts to develop a treatment for patients as soon as possible.Citation34 There is a dire need for the discovery of new anti-SARS-CoV-2 inhibitors to reduce the severity of illness in COVID-19 affectees and to target the various types of SARS-CoV-2 proteins because there are no effective medicines available for the treatment of the coronavirus disease. Several anti-malarial, and herbal medicines have been used as alternative drugs to cure COVID-19.Citation35 Computer-aided drug design methods have been successfully implemented in anti-COVID-19 analyses to find potential inhibitors rapidly, in repurposing drugs, and in explaining the action mechanism of the therapeutic agents against SARS-CoV-2 based on the crystal structures of SARS-CoV or SARS-CoV2 Mpro.Citation36 Drug repurposing is the quickest option to propose effective treatments for the ongoing COVID-19 pandemic.Citation37 Several FDA-approved anti-retroviral drugs are already in use, including, oseltamivir I,Citation38 favipiravir II/ribavirin III,Citation39 lopinavir IV/ritonavir V,Citation40 chloroquine VI, and hydroxychloroquine VII,Citation41 ensitrelvir VIII,Citation42 cordycepin IX,Citation43 didanosine X,Citation44 teriflunomide XI,Citation45 riboprine XII, and forodesine XIII,Citation46 as well as other similar nucleoside and nucleotide analogs, which showed effective antiviral activities against SARS-CoV-2. Thymohydroquinone, dithymoquinone, ginger, strychnine bush, and pineapple ingredients are used as natural enemies of SARS-CoV-2.Citation47–49
Remdesivir XIV is an adenosine analogue RNA-dependent RNA polymerase (RdRp) inhibitor used for the treatment of SARS-CoV-2-infected patients.Citation50 Remdesivir, which has been recommended by the FDA for emergency use, can be injected intravenously into hospitalized patients. It reduces recovery time but not mortality. It is highly efficient with rotavirus polymerases, therefore exhibiting a low potential to cause human toxicity.Citation51 Molnupiravir (EIDD-2801) XV, a prodrug of the nucleoside hybrid N4-hydroxycytidine (NHC), was approved by the FDA for the cure of COVID-19 in specific adults. It exhibits remarkable toxic effects in certain cell-based processes, such as induced mutagenesis in mammalian cells.Citation52–54 Molnupiravir has some limitations, including its use in patients over the age of 18 because of its side effects on the growth of cartilage and bone and in non-pregnant women because of the potential risk of fatal harm. The most widely used 3CLpro inhibitors are peptidomimetic inhibitors, such as YH-53 with benzothiazolyl ketone XVI,Citation55 and nirmatrelvir with nitrile XVII.Citation56 Nonpeptidic inhibitors include covalent and noncovalent inhibitors. Covalent inhibitors include shikonin XVIII and carmofur XIX.Citation57 Non-covalent inhibitors of flavonoid derivatives include baicalin XX and baicalein XXICitation58 ().
Some repurposing drugs are used for the treatment of COVID-19, however, their effectiveness and side effects are still unknown. Therefore, there is a tremendous need to synthesize novel heterocyclic moieties with improved potency and optimized pharmacological properties. The review aims to report in vitro and in silico studies of chemically synthesized heterocyclic compounds against the structural and non-structural proteins of SARS-CoV-2.
In vitro Studies Against COVID-19
Current Advances in Heterocycles as Anti-COVID-19 Agents
Indole Derivatives as Anti-SARS-CoV-2 Agents
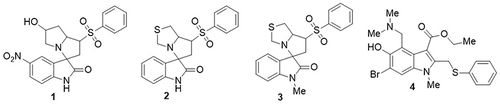
Barakat et al,Citation59 synthesized spirooxindole-based phenylsulfonyl hybrids as potent SARS-CoV-2 inhibitors and screened their anti-COVID-19 activity using a colorimetric crystal violet assay. Among them, the combination of compound 1 with compound 3 and the combination of compound 2 with compound 3 was found to be more potent and active. These compounds showed the most potent activity against SARS-CoV-2 in Vero E6 cells, with half-maximal inhibitory concentration (IC50) values of 3.657 and 3.275µM and with a selectivity index of 698.4 and 3612.8, respectively. Remdesivir was used as the standard drug with an IC50 value of 6.721µM. Tanaka et al,Citation60 prepared umifenovir analogs and tested their anti-SARS-CoV-2 activities using biological assays. From all the derivatives that were examined, compound 4 exhibited the maximum 20% inhibition of the SARS-CoV-2 spike protein and ACE2 receptor expression ().
Piperidine Derivatives as Anti-SARS-CoV-2 Agents
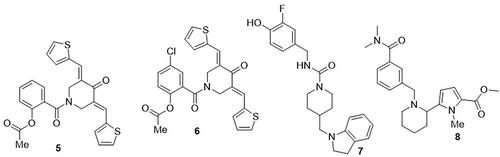
A novel series of aspirin-curcumin mimic conjugates was synthesized by Srour et al,Citation61 and their anti-SARS-CoV-2 properties were evaluated using the MTT-bio assay. The compounds 5 and 6 bearing a thienylidene ring system showed excellent inhibitory activity against SARS-CoV-2 with IC50 values of 8.828 and 3.316µM, CC50 values of 206.2 and 416.5µM, and selective index values of 233.6 and 125.6, respectively, as compared to the standard drugs hydroxychloroquine and chloroquine (IC50= 36.92 and 24.98µM, CC50= 356.4 and 377.7µM and SI= 9.7 and 15.1). Yang et al,Citation36 reported a number of non-peptide inhibitors with anti-SARS-CoV-2 potential. The results of the surface plasmon resonance experiment showed that, among the 49 tested molecules, compound 6 was bound to SARS-CoV-2 Mpro. Furthermore, fluorescence resonance energy transfer analysis was carried out and obtained IC50 values ranging from 0.68 to 2.05µM. Among them, compounds 7 and 8 showed the best potent activity against SARS-CoV-2 Mpro with IC50 values of 0.73 and 0.69µM, respectively. These compounds reduced viral replication in Vero E6 cells with half maximal effective concentration (EC50) values of 4.98 and 8.52µM, respectively ().
Pyrazine Derivatives as Anti-SARS-CoV-2 Agents
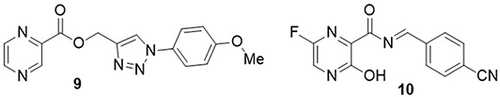
Pyrazine conjugates were synthesized and evaluated for their anti-SARS-CoV-2 activity using the MTT assay by Seliem et al.Citation62 Among these prepared derivatives, compound 9 exhibited significant activity towards coronavirus (IC50= 0.120mM, CC50= 0.378 m, SI= 3.150) in comparison to the control drug favipiravir (IC50= 0.1382mM, CC50= 5.262 mM, SI= 3.808). Rabie,Citation63 synthesized a novel favipiravir analog (cyanorona-20) 10 and found it was the most potent anti-SARS-CoV-2 agent. This derivative showed excellent inhibitory activity against RNA-dependent RNA polymerase (RdRp) of SARS-CoV-2, with an EC50 value of 450nM ().
Pyridine Derivatives as Anti-SARS-CoV-2 Agents
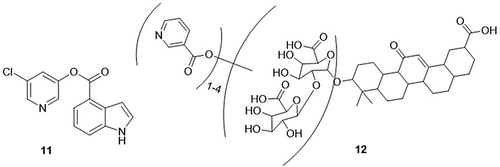
Ghosh et al,Citation64 synthesized 5-chloropyridinyl indole carboxylate hybrids and found that compound 11 was the most effective anti-COVID-19 agent targeting SARS-CoV-2 3CLpro with an IC50 value of 250nM. The MTT assay was performed for the in vitro analysis of the synthesized compounds. Compound 11 significantly reduced the cytopathic effect and replication of SARS-CoV-2 in Vero E6 cells (EC50= 15μM) as compared with the standard drug (EC50= 1.2μM,). This compound also showed a half-maximal cytotoxic concentration (CC50> 100μM) against coronavirus 2. A derivative of glycyrrhizin nicotinate, “glycyvir”, was prepared by Fomenko et al,Citation65 and, its anti-SARS-CoV-2 activity was determined through in vitro analysis. Glycyvir 12 exhibited significant antiviral activity to inhibit the replication of SARS-CoV-2 in Vero E6 cells, with an IC50 value in the range of 2–8µM, as compared to the reference drug remdesivir (IC50= 1.5–3.3µM) ().
Pyrimidine Derivatives as Anti-SARS-CoV-2 Agents
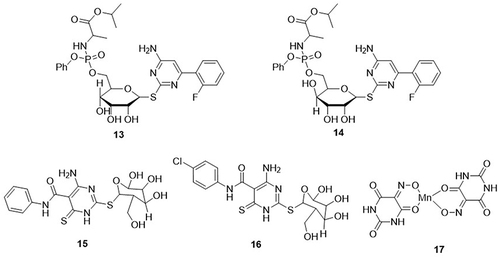
Abu-Zaied et al,Citation12 prepared pyrimidine derivatives and their corresponding phosphoramidates, and tested their anti-SARS-CoV-2 activity using MTT and plaque reduction assays. The most active derivatives reported in this study were 13 and 14 against SARS-CoV-2, with IC50 values of 14.91 and 12.16µM and CC50 values of 420.9 and 327.1µM, respectively. Cytosine thioglycoside phosphoramidates 13 and 14 was proven the most effective profiles in Vero E6 cells, with 83% and 86% inhibition at 0.25µM concentrations respectively. The selective index values of these compounds were 28.2 and 26.9. Pyrimidine thioglycoside derivatives were prepared and evaluated for their anti-SARS-CoV-2 properties through in vitro analysis using MTT assay by the same authors.Citation66 Among all the derivatives, compounds 15 and 16 showed the best potent activity against SARS-CoV-2, with IC50 values of 18.47 and 15.41μmol and selectivity index (SI) values of 25.33 and 23.40, respectively. These compounds exhibited cytotoxicity in Vero E6 cells with half maximal cytotoxic concentrations (CC50) values of 467.9 and 360.9μmol. Harbi et al,Citation67 prepared novel nanocrystallites of violurate-based Mn(II) and Cu(II) complexes and screened their anti-SARS-CoV-2 activities using plaque reduction and MTT assays. Violuric acid and its Mn(II) and Cu(II) complexes displayed good anti-SARS-CoV-2 properties with CC50 values of 43.87, 93.45 and 88.38µM, respectively. These compounds inhibited the replication of SARS-CoV-2 in Vero-E6 cells with IC50 values of 84.01, 39.58, and 44.86µM, respectively. These compounds suppressed SARS-CoV-2 replication by 20%, 49%, and 72%, respectively. Of these, Mn(II) complex 17 was the most effective against SARS-CoV-2, with an IC50 value of 39.58μM, whereas the control drug remdesivir had an IC50 value of 0.2μM ().
Pyrrole Derivative as Anti-SARS-CoV-2 Agent
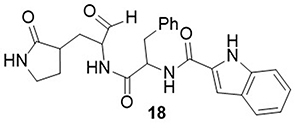
A series of peptidomimetic aldehydes was designed and tested through in vitro analysis for their anti-SARS-CoV-2 activity using a fluorescence resonance energy transfer (FRET) protease assay.Citation27 Among them, compound 18 displayed significant inhibitory activity towards the main protease (3CLpro) of SARS-CoV-2, with an IC50 value of 0.034μM. This compound reduced the replication of SARS-CoV-2 in Vero E6 cells with an EC50 value of 0.29μM. The half maximal cytotoxic concentration (CC50), and selectivity index (SI) values of these compounds were 808.7, and 2786 respectively ().
Piperazine Derivatives as Anti-SARS-CoV-2 Agent
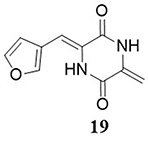
Nishiuchi et al,Citation68 synthesized neoechinulin B and its derivatives and evaluated their anti-SARS-CoV-2 activities in vitro. Most of the synthesized derivatives exhibited the best anti-SARS-CoV-2 properties. Among the studied analogs, compound 19 demonstrated the highest activity against SARS-CoV-2, with an IC50 value of 9.3μM. This compound exhibited low cytotoxicity against Vero E6 cells (CC50 >80 μM) ().
Oxazole Derivatives as Anti-SARS-CoV-2 Agents
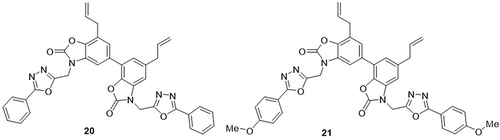
Guo et al,Citation69 prepared honokiol hybrids containing 3-((5-phenyl-1,3,4-oxadiazol-2-yl)methyl)oxazol-2(3H)-ones and tested their anti-SARS-CoV-2 activity using an MTT bioassay. Of these, compounds 20 and 21 showed remarkable inhibition of SARS-CoV-2 pseudovirus entry into HEK-293T-ACE2h cells, with IC50 values of 29.23 and 9.82µM, respectively, compared to the parental honokiol with IC50 values of >50µM. The positive control inhibitor used in this assay suppressed pseudovirus entry into host cells, with an IC50 value of 0.035µM. Compound 21 with a TC50> 100 µM showed a biologically safe profile for HEK-293T-ACE2h cells compared to parental honokiol (TC50> 48.23µM). From all honokiol derivatives, compound 21 was found to be the most potent against a pseudovirus, with a selective index (SI) value exceeding 10.18. A competitive enzyme-linked immunosorbent assay (ELISA) was employed to investigate the interactions of the SARS-CoV-2 spike RBD with the ACE2 protein. A SARS-CoV-2 inhibitor with an IC50 value of 2.59nM was utilized as the positive control in the ELISA assay. Compound 21 suppressed the binding of the SARS-CoV-2 spike RBD and ACE2 by 26.08%, 37.16%, and 50.41% at 12.5, 25, and 50µM concentrations. The inhibition rates of parental honokiol were 13.81% and 20.94% at 25 and 50µM concentrations ().
Quinazoline Derivatives as Anti-SARS-CoV-2 Agents
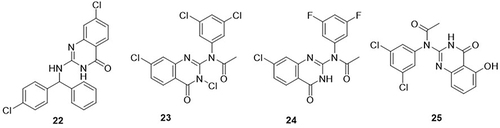
A series of 2-benzylaminoquinazolin-4(3H)-one analogues were prepared and evaluated for their anti-SARS-CoV-2 activity using human ether-a-go-go-related gene (hERG), cytotoxicity, cytochrome P450 (CYP) inhibition, microsomal stability, and the plasma protein binding (PPB) assay.Citation70 Among them, compound 22 demonstrated the most potent activity against SARS-CoV-2 with an IC50 value of 4.2μM, whereas the IC50 values of the standard drugs remdesivir, chloroquine, and lopinavir were 7.6, 9.4, and 16.6μM, respectively. This compound showed lower cytotoxicity in Vero E6 cells with CC50 and selective index (SI) values of 14.3µM and 3.4M, respectively, as compared to the reference drugs (CC50>25µM and SI >3.2, >2.6, and >1.5µM). A novel series of 2-aminoquinazolin-4-(3H)-one hybrids was prepared by Shin et al,Citation71 and screened for anti-SARS-CoV-2 activity through in vitro studies. An immunofluorescence assay was used to determine the half-maximal inhibitory concentration (IC50) of these derivatives in Vero E6 cells at a 10µM concentration. The cytotoxicity (CC50) was also examined in uninfected Vero E6 cells. Among the reported derivatives, acetylated compounds 23, 24, and 25 showed the best inhibitory activity against SARS-CoV-2 with IC50 values of 0.29, 0.11, and 0.33µM, respectively, as compared to the reference drug remdesivir (IC50 = 3.47µM) ().
Quinoline Derivatives as Anti-SARS-CoV-2 Agents
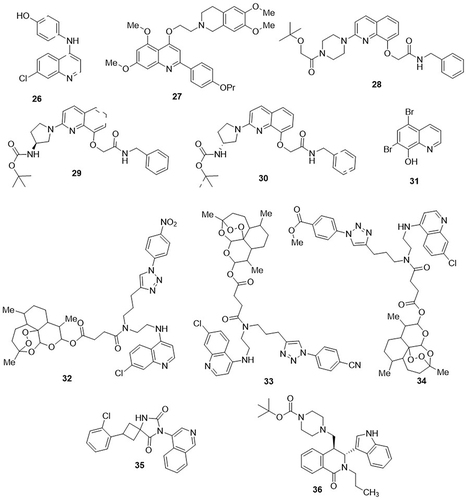
Guevara-Pulido et al,Citation72 designed and synthesized a series of chloroquine analogs and evaluated their anti-SARS-CoV-2 activity using in vitro drug-release assay. From these, 4-(7-chloroquinolin-4-yl)amino)phenol hybrid 26 was less toxic and exhibited a significant therapeutic effect against SARS-CoV-2 main protease (Mpro) with an IC50 value of 12nM, whereas the parent compound chloroquine had an IC50 value of 27nM. Nizi et al,Citation10 reported two phenylquinoline derivatives as potential inhibitors of SARS-CoV-2 replication, using a colorimetric formazan-based MTS assay. Amongst the studied compounds, Compound 27 bearing two methoxy groups at the C-4 position of the 2-phenylquinoline core, showed significant inhibitory activity against SARS-CoV-2 helicase (nsp13) with an IC50 value of 0.42µM. This compound reduced viral replication in Vero E6 cells with an EC50 value of 8.8µM as compared with the control drug chloroquine, with an EC50 value of 10.9µM. This compound demonstrated cytotoxicity in Vero E6 cells, with a CC50 value greater than 100µM. Zhao et al,Citation73 tested 101 quinoline hybrids against SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) using cell-based assays. Compounds 28, 29, and 30 inhibited RNA synthesis by the RdRp of SARS-CoV-2, with EC50 values of 1.08, 2.08, and 3.92µM, respectively. Remdesivir was used as the standard drug with an EC50 value of 1.39µM. These compounds showed cytotoxic effects on Vero E6 cells, with CC50 values of 70.79, 72.44, and 79.43μM, respectively. The therapeutic indexes (TI) for these compounds were 65.55, 34.83, and 20.26μM, respectively. Chen et al,Citation74 investigated chloroxine and hybrids of tanshinone IIA sulfonate sodium (TSS) as potential inhibitors of SARS-CoV-2 PLpro using a fluorescence polarization assay (FPA) and cell-based assay. Amongst them, chloroxine 31 inhibited the binding of SARS-CoV-2 PLpro to ISG15 (which plays an important role in viral replication) with an IC50 value of 6.0μM. Compound 31 also demonstrated excellent potent activity against omicron, WT, and delta variants of SARS-CoV-2, with EC50 values of 2.98, 4.59, and 2.70μM, respectively. The cytotoxicity of this compound in Vero E6 cells was determined using the cell counting kit-8 (CCK-8) assay, and the results showed a CC50 value of more than 100μM. A series of artesunic acid and peroxide-based derivatives were synthesized and screened for their anti-SARS-CoV-2 activity using a cell viability assay by Herrmann et al.Citation75 The most potent derivatives reported in this series were artesunic acid-quinoline derivatives 32, 33, and 34 with EC50 values in the range of 13–19μM against SARS-CoV-2, as compared to the parent compound artesunic acid (EC50>50μM). These derivatives showed no cytotoxic effects on Vero E6 cells, with CC50 values in the range of 100–110μM. Isoquinoline derivative with strong anti-SARS-CoV-2 activity was discovered by Rabie et al.Citation76 Compound 35 demonstrated the best inhibitory activities against anti-SARS-CoV-1 (EC50= 0.39μM), anti-SARS-CoV-2 (EC50= 0.11μM), anti-MERS-CoV (EC50= 0.20μM), anti-ExoN (EC50= 0.27μM), anti-RdRp (EC50= 0.16μM), and anti-Mpro (EC50= 0. 077μM). It has remarkable potency towards anti-SARS-CoV-2 activities in vitro (EC50> 0.11μM, reaching as low as 0.077μM) in comparison to ensitrelvir (EC50< 0.29μM, reaching as high as 0.50μM).Wang et al,Citation77 synthesized a novel series of tetrahydroisoquinoline derivatives and tested them as SARS-CoV-2 inhibitors through in vitro studies. An immunofluorescence assay was used to determine the half-maximum effective concentration (EC50) in Vero E6 cells. Among the studied derivatives, compound 36 exhibited remarkable inhibition of SARS-CoV-2 replication in Vero E6 cells (EC50= 3.15 μM, SI> 63.49). The cytotoxicity of this compound in Vero E6 cells was determined using the CCK-8 assay, and the results showed a CC50 value of more than 200μM. Compound 36 also significantly inhibited the viral replication of SARS-CoV-2 in human lung cells (EC50= 2.78μM, SI> 71.94) as compared to the standard drug chloroquine (EC50= 44.90μM SI= 2.94). ().
Thiazole Derivatives as Anti-SARS-CoV-2 Agents
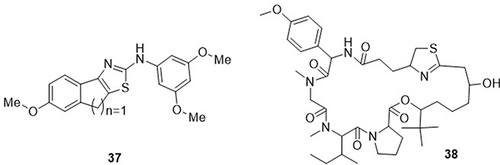
Wu et al,Citation78 synthesized 8H-Indeno[1,2-d]thiazole hybrids and evaluated their anti-SARS-CoV-2 (3CLpro) activities. Amongst them, compound 37 demonstrated the best inhibitory activity against SARS-CoV-2 3CLpro with an IC50 value of 1.28μM as compared to the standard drug nirmatrelvir, with an IC50 value of 0.012μM. This compound showed the highest potency with a 72.5–6.1% inhibition rate at a 20μM concentration. Pohl et al,Citation79 2022 tested apratoxin S4 (a Sec61 inhibitor) as an anti-SARS-CoV-2 agent using an MTT assay. Apratoxin S4 38 was the most potent activity against SARS-CoV-2. It reduced viral replication in HeLa-hACE2 cells (IC50= 0.71nM) and Vero E6 cells (IC50=0.17μM). Remdesivir was used as the reference drug with an IC50 value of 35nM ().
Quinoxaline Derivative as Anti-SARS-CoV-2 Agent
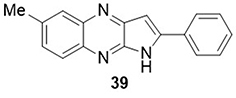
Chemboli et al,Citation80 synthesized pyrrolo[2,3-b]quinoxaline hybrids and tested their ability to inhibit cytokine storms in SARS-CoV-2 infection. All synthesized compounds were tested by in vitro studies for tumor necrosis factor-alpha (TNF-α) inhibition. Among them, Compound 39 demonstrated good inhibitory activity against TNF-α with an IC50 value of 5.14µM. This compound was not found to be better than the reference drug rolipram IC50= 0.91µM, but it was better than another known inhibitor: thalidomide(IC50=198.91µM) ().
Pyrazole Derivative as Anti-SARS-CoV-2 Agent
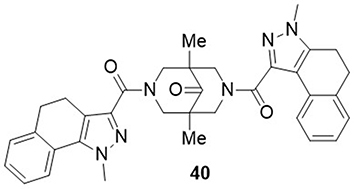
Shcherbakov et al,Citation81 investigated pyrazole derivatives as potential inhibitors of SARS-CoV-2 3CLpro using an enzymatic assay. The most potent compound reported in this study was compound 40 which showed an IC50 value of 0.75µM against the non-structural protein (3CLpro) of SARS-CoV-2, whereas control drugs disulfiram and ebselen had IC50 values of 6.1µM and 1.7µM, respectively ().
Benzodioxole Derivatives as Anti-SARS-CoV-2 Agents
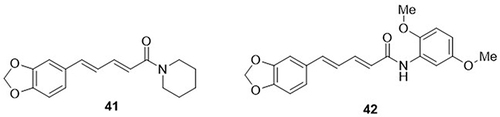
A novel series of N-aryl amide derivatives were designed and tested for their anti-COVID-19 activity by Wansri et al.Citation82 All derivatives were evaluated through in vitro analysis at a 100µM concentration. Compounds 41 and 42 reduced the activity of SARS-CoV-2 (3CLpro) by nearly 70% at 100µM concentration. From these, compound 42 demonstrated remarkable activity towards SARS-CoV-2 3CLpro with an IC50 value of 106.9µM as compared to the control drug rutin, with an IC50 value of 325.6µM ().
Coumarin Derivatives as Anti-SARS-CoV-2 Agents
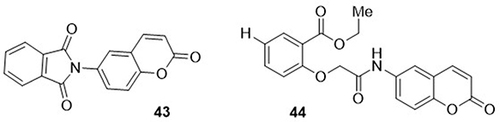
Mohamed and Eltelbany,Citation83 prepared 16 coumarin hybrids and evaluated them as potential inhibitors of the SARS-CoV-2 Mpro. At 100µM concentration, seven compounds showed approximately 50% inhibition of the main protease (Mpro) of SARS-CoV-2. Of these, cyclic amide 43 showed excellent potent activity against SARS-CoV-2 Mpro with an IC50 value of 15.0μg/mL, whereas the reference drug chloroquine had an IC50 of 13.1μg/mL. Compound 44 inhibited viral replication by 63.9%, with an IC50 value of 25.8μg/mL at 100μM concentration ().
Thiadiazole Derivative as Anti-SARS-CoV-2 Agents
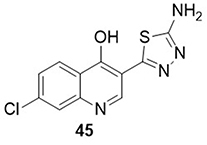
Rabie and Eltayb.Citation84 screened a novel series of aminothiadiazoles as inhibitors of COVID-19 employing in vitro anti-SARS-CoV-2 and anti-RdRp/ExoN bioassays. In Vero E6 cells, it was found that all the derivatives effectively inhibited and impaired SARS-CoV-2 replication and transcription. Amongst the studied derivatives, the compound 45 showed significant activity against the SARS-CoV-2 Omicron variant (EC50= 0.41µM), which was found to be 6.4 times more potent than the standard drug molnupiravir (EC50= 2.61μM). Compound 45 also displayed excellent anti-RdRp with an EC50 value of 0.17 μM as compared to the reference drug molnupiravir (EC50= 0.24 μM). The cytotoxicity assay showed that the CC50 value of compound 45 is remarkably greater than 100μM, and is expected to have a higher selectivity index (SI> 243.9) in comparison to the reference molnupiravir (SI> 38.3) ().
In silico Studies of COVID-19
Current Advances in Heterocycles as Anti-COVID-19 Agents
Azafluorene Derivative as Anti-SARS-CoV-2 Agents
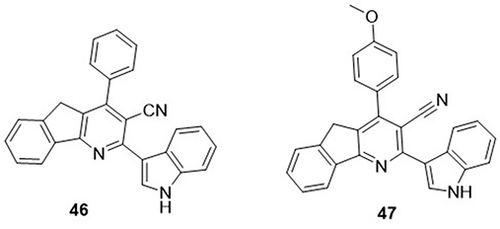
Azafluorene hybrids were synthesized and screened as potential inhibitors of SARS CoV-2 RdRp by Venkateshan et al.Citation85 The pharmacological model of the synthesized compounds was predicted using ADMET analysis. AutoDock Vina and MM-PB(GB)SA analyses were used to determine the binding and binding free energies. The compounds 46 and 47 showed good binding affinity against non-structural protein RdRp (nsp12) of SARS CoV-2 with binding energy values (−8.3 and −7.8kcal/mol) and binding free energy values (−11.04 and −39.42 kcal/mol), whereas, control dugs favipiravir, hydroxychloroquine, and Galidesivir had binding energy values of −5.8, −6.3, and −7.6kcal/mol. Compound 46 exhibited H-bonding interactions with amino acid residues ASP-502, LYS-682, and TYR-503, and compound 47 displayed H-bonding interactions with amino acid residue ASP-507 ().
Imidazole Derivatives as Anti-SARS-CoV-2 Agents
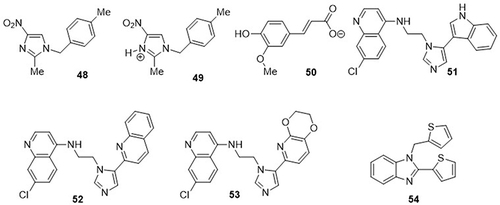
Satheesh et al,Citation1 investigated imidazolium salts as inhibitors of the non-structural RNA-binding protein (nsp9) of SARS CoV-2 (PDB code: 6W4B) through molecular docking analysis. Among the docked derivatives, compounds 48, 49, and 50 exhibited strong binding affinities against the non-structural RNA-binding protein (nsp9) of SARS CoV-2 with docking scores of −4.2, −4.0, and −3.5kcal/mol, respectively. Hydroxychloroquine and chloroquine were used as standard drugs with binding affinities of −3.6kcal/mol. Compounds 48 and 49 showed hydrogen bonding interactions with the amino acid residues SER-6A and LYS-37A and hydrophobic interactions with the amino acid residues THR-36 and PHE-41. Compound 50 interacted with amino residues PRO-7A and VAL-77B via hydrophobic interactions. Belhassan et al,Citation86 evaluated imidazole hybrids for the anti-SARS CoV-2 activity of imidazole hybrids using a computational approach. Among them, compounds 51, 52, and 53` showed good binding affinities against the main protease (Mpro) (Code PDB: 6LU7) of SARS CoV-2 with values of −8.5, −8.3, and −8.3kcal/mol, respectively, as compared to the reference drugs chloroquine (−5.9kcal/mol) and hydroxychloroquine (−6.6kcal/mol). Compound 51 exhibited H-bonding interactions with amino acid residues ARG-188A, HIS-164A, LEU-141A, and SER-144A and hydrophobic interactions with residues HIS-41A and MET-165A. Compound 52 displayed H-bonding interactions with the amino acid residue GLN-189A and hydrophobic interactions with the residues MET-165A and MET-49. Compound 53 demonstrated H-bonding interactions with the amino acid residues GLY-143A and hydrophobic interactions with MET-165A and CYS-145A. A series of substituted benzimidazole derivatives was synthesized by Mudi et al,Citation87 and their anti-SARS CoV-2 activities were tested using a molecular docking approach. Of these, thiophene-substituted compound 54 displayed the highest binding affinity for Mpro (PDB code: 6LU7), nsp2 (PDB code: 7JLT), and nsp7 (PDB code: 7EXM) of SARS CoV-2 with docking scores of −10.52, −5.07, and −6.63 kcal/mol. It showed H-bonding interactions with amino acid residues LYS-113 and GLU-110 of nsp2 and ASP-67 of nsp7. It displayed one H-bonding interaction with amino acid residue GLY-143 and hydrophobic interactions with amino acid residue HIS-41 of the CoV-2 main protease (Mpro). Compound 54 showed the lowest docking score (−10.52 kcal/mol) towards the main protease (Mpro) of SARS CoV-2 rather than the non-structural proteins nsp2 and nsp7 ().
Oxazole Derivative as Anti-SARS-CoV-2 Agent
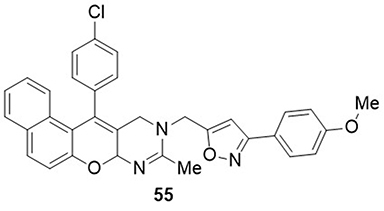
Algethami et al,Citation88 prepared isoxazole linked pyranopyrimidinone derivatives and screened them for inhibitors of the SARS CoV-2 main protease (Mpro) via molecular docking analysis. The most potent derivative in this study was compound 55 with the lowest binding energy score of −8.9 kcal/mol against the main protease (Mpro) of SARS CoV-2 as compared to the reference N3 inhibitor (−7.0kcal/mol). Compound 55 showed H-bonding interactions with the amino acid residue THR-26 and hydrophobic interactions with the amino acid residues PRO-168, MET-49, MET-165, and HIS-41 ().
Piperazine Derivative as Anti-SARS-CoV-2 Agent
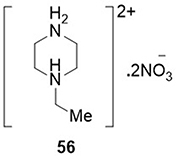
Gatfaoui et al,Citation89 synthesized 1-ethylpiperazine-1,4-diium bis(nitrate) and tested their anti-SARS-CoV-2 (PDB code: 6W4B) activity using docking studies. Compound 56 showed the highest binding affinity for SARS-CoV-2, with a binding energy of −68.15kcal/mol. It displayed H-bonding interactions with the amino acid residue SER-47B ().
Piperidine Derivative as Anti-SARS-CoV-2 Agent
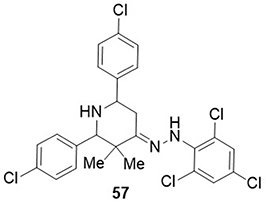
Anthony et al,Citation90 prepared a piperidine derivative and screened it for its inhibitory potential against COVID-19’s main protease (Mpro) through molecular docking and molecular dynamics simulation. Compound 57 demonstrated the highest binding affinity against COVID-19 (6WCF-6Y84) receptors with docking scores of −11.10 and −11.25kcal/mol, whereas the control drug hydroxychloroquine had docking score values of −8.96 and −8.68 kcal/mol. It formed H-bonds with the amino acid residues VAL-41 of the COVID-19 (6WCF) receptors and PRO-9 of the COVID-19 (6Y84) receptors ().
Pyrazole Derivatives as Anti-SARS-CoV-2 Agents
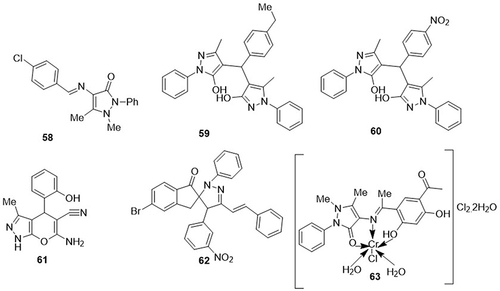
Alotaibi et al,Citation11 synthesized a series of novel pyrazole derivatives and examined their inhibitory potential against SARS-CoV-2 main protease (Mpro) (PDB ID: 6LU7) through molecular docking analysis. From the examined derivatives, compound 58 demonstrated an excellent binding affinity for the main protease (Mpro) of SARS-CoV-2 with a binding energy value of −8.7kcal/mol. Azithromycin, lopinavir, hydroxychloroquine, chloroquine, remdesivir, favipiravir, and baloxvir were used as standard drugs with binding energy values of −8.55, −9.26, −9.23, −8.95, −9.24, −9.23, and −9.18kcal/mol. It exhibited interactions with the amino acid residue of main protease (Mpro) through a variety of interactions, such as van der Waals, hydrogen bonding, π-alkyl, and π-sigma interactions. Gupta et al,Citation91 prepared a series of novel 4.4’-(arylmethylene)bis(1H-pyrazol-5-ols) compounds and tested them as potent inhibitors of COVID-19 main protease (3CLpro) (PDB ID: 6LU7) using molecular docking. The compounds 59 and 60 were found to exhibit significant binding affinity against COVID-19 Mpro with binding energy scores of −8.3 and −8.8Kcal/mol, respectively, as compared to the reference drugs oseltamivir (−4.7Kcal/mol), remdesivir (−6.5Kcal/mol), hydroxychloroquine (−5.3Kcal/mol), chloroquine (−5.1Kcal/mol), ribavirin (−5.6Kcal/mol), ritonavir (−7.3Kcal/mol) and favipiravir (−5.4Kcal/mol). Compound 59 showed H-bonding interactions with the amino acid residue GLU-288; hydrophobic interactions with residues LEU-286, LYS-137, and ALA-285; and pi-anion and pi-cation interactions with residues LYS-5 and GLU-288. Compound 60 displayed hydrophobic interactions with amino acid residues TRP-218 Immunofluorescence assay and LEU-220. Addoum et al,Citation92 prepared pyrano[2,3-c]pyrazole derivatives and investigated them as inhibitors of SARS-CoV-2 (3CLpro) (PDB ID: 6LU7) using molecular docking analysis. Among them, compound 61 exhibited a remarkable binding affinity against the main protease (3CLpro) of SARS-CoV-2 with a binding energy score of −6.2 kcal/mol, which was similar to the binding energy score of the reference drug chloroquine (−6.2Kcal/mol) and higher than both favipiravir (−4.2 kcal/mol) and hydroxychloroquine (−5.5Kcal/mol). It showed H-bonding interactions with amino acid residues CYST-145, SER-144, and GLYL-143. Masaret,Citation93 designed a series of new spiropyrazole hybrids and evaluated their anti-COVID-19 activity using a molecular docking approach. Amongst them, compound 62 showed the best binding affinity for the main protease (PDB code: 6LU7) of COVID-19, with the lowest docking score of −7.764kcal/mol. Compound 62 displayed H-bonding interactions with amino acid residues THR-26A and GLY-43A. Mohamed et al,Citation94 synthesized a tridentate Schiff base along with its complexes and screened them as inhibitors of the SARS-CoV-2 main protease UAW247 (6XBH) through in silico studies. Amongst all the complexes studied, Cr(III) complex 63 had the lowest binding energy for SARS-CoV-2 (−28.6kcal/mol). It demonstrated H-bonding interactions with amino acid residues MET-165 and GLN-166 and Pi-H interaction with residue GLU-189 ().
Pyridine Derivatives as Anti-SARS-CoV-2 Agents
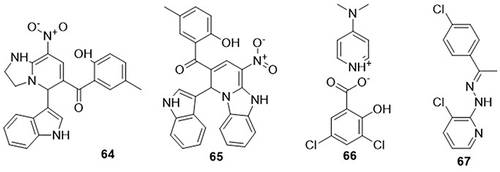
A series of 3-substituted indole-based 1.2-dihydropyridine hybrids were synthesized by Jayabal et al,Citation95 and their inhibitory potential against SARS-CoV-2 was tested using a molecular docking approach. Out of the 20 prepared derivatives, compound 64 showed good binding affinity against the main protease (Mpro) (PDB code: 6LU7) of SARS-CoV-2 with a binding energy score of −8.6kcal/mol, as compared to the control drug remdesivir with a binding energy value of −7.7kcal/mol. In addition, compound 65 exhibited the highest binding affinity towards spike glycoprotein (PDB code: 7NX7) of COVID-19 with a binding energy value of −7.3kcal/mol, whereby the standard drug remdesivir had the same binding energy value of −7.3kcal/mol. Compound 64 displayed H-bonding interactions with 6LU7 Mpro amino acid residues HIS-163 and GLY-143, and hydrophobic interactions with residues GLY-143 and GLU-166, while compound 65 showed H-bonding interactions with 7NX7 spike glycoprotein amino acid residues HIS-163 and PHE-140, and hydrophobic interactions with residues GLU-166, CYS-145, HIS-41, and MET-165. Tarika et al,Citation96 investigated 4-dimethylamino pyridinium 3.5-dichlorosalicylate (DADS) as a potential SARS-CoV-2 inhibitor in silico studies. DADS 66 demonstrated the best binding affinity against 6M2N, 7CHF, and 6M03 of the SARS-CoV-2 proteins, with binding energy of −2.66, −2.4, and −2.54 kcal/mol, respectively. It formed H-bonding interactions with 6M2N, 7CHF, and 6 M03 proteins. Topal et al,Citation97 prepared 3-chloro-2-{(2E)-2-[1-(4-chlorophenyl)ethylidene]hydra-zinyl}pyridine 67 and screened it for anti-COVID-19 activity using a molecular docking analysis. Compound 67 has been found to exhibit significant binding affinity (−6.4 kcal/mol) against 3CLpro (PDB code: 6LU7) of COVID-19. Chloroquine and hydroxychloroquine were used as control drugs, with binding energy values of −4.7 and −5.0kcal/mol. It showed H-bonding interactions with amino acid residues HIS-164A and HIS-41A, and hydrophobic interactions with residues HIS-41A and MET-165A ().
Pyrimidine Derivatives as Anti-SARS-CoV-2 Agents
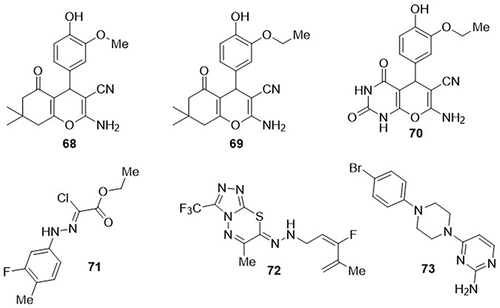
Nesaragi et al,Citation98 synthesized a series of pyrano[2,3-d]pyrimidinone and tetrahydrobenzo[b]pyran derivatives and tested them as SARS-CoV-2 inhibitors using a molecular docking approach. From the synthesized derivatives, compounds 68, 69, and 70 exhibited the highest binding affinities against the nucleoplasmid CoV-N-protein (PDB code: 6M3M) of SARS-CoV-2 with docking scores of −87.722, −87.274, and −75.076kcal/mol, respectively, whereas (6-chloro-7-((2-morpholinoethyl)amino)quinoline-5,8-dione) was used as a reference compound with a docking score of −77.045kcal/mol. These compounds showed different types of interactions with the amino acid residue ARG-89 and preferentially bound to the nucleoplasmid CoV-N-protein (PDB code: 6M3M) compared to the reference compound. Muhammad et al,Citation99 prepared azoloazines and tested them as inhibitors of the SARS-CoV-2 3CLpro. Among these derivatives, compounds 71 and 72 showed the highest docking scores against SARS-CoV-2 3C-Like proteinase (3CLpro) (PDB code: LU7), with values of −7.529 and −7.517kcal/mol, respectively. Compound 71 exhibited H-bonding interactions with the amino acid residues ASN-142, CYS-145, and HIS-164, while compound 72 displayed H-bonding interactions with the residues CYS-145, HIS-163, and HIS-164. A novel series of 2-amino-4-chloro-pyrimidine hybrids was synthesized by Qureshi et al,Citation100 and screened for their anti-SARS-CoV-2 activity using a computational approach. Out of these, compound 73 demonstrated good affinity against 3CLpro (PDB ID: 6LU7) of SARS-CoV-2 with a binding free energy score of −8.12kcal/mol as compared to the standard drug remdesivir with a binding free energy score of −6.41kcal/mol. It exhibited H-bonding interactions with amino acid residues ASN-142 and PHE-140, and hydrophobic interactions with amino acid residues HIS-41, HIS-172, MET-165, MET-49, LEU-141, TYR-54, HIS-163, HIS-164, ASP-187, and ARG-188 ().
Quinoline Derivatives as Anti-SARS-CoV-2 Agents
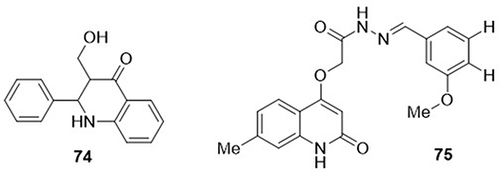
Nepolraj et al,Citation7 synthesized 3-(hydroxymethyl)-2-phenyl-2,3-dihydroquinolin-4(1H)-one and tested it as an inhibitor of SARS-CoV-2 main protease (Mpro) (PDB code: 6LU7) through in silico studies. Compound 74 showed the highest binding affinity against SARS-CoV-2 main protease (−6.70kcal/mol by AutoDock) and (−7.52kcal/mol by AutoDock). It formed H-bonding interactions with amino acid residues GLY-143, SER-144, CYS-145, and LEU-141, and hydrophilic interactions with CYS-145. Drug scoring, drug likeness, and toxicity were determined through in silico studies using ADME analysis. Alshammari et al,Citation101 designed a series of N-substituted-2-quinolonylacetohydrazides and evaluated their anti-COVID-19 activity using in silico analysis. Among them, compound 75 showed the strongest binding affinity against main protease (Mpro) (PDB code: 6LU7) and RNA-dependent RNA polymerase (RdRp) (PDB code: 6M71) of SARS-CoV-2 with docking scores of −9.7, and −7.7 kcal/mol, respectively. Remdesivir was used as a standard drug and exhibited binding affinity towards RdRP(−5.6kcal/mol) and Mpro(−8.5kcal/mol). Compound 75 preferentially binds Mpro to RdRp. It displayed H-bonding interactions with amino acid residues SER-144, GLU-166, LEU-141, and HIS-163 of Mpro and ASP-623, TYR-619, and GLU-811 of RdRp ().
Quinoxaline Derivative as Anti-SARS-CoV-2 Agent
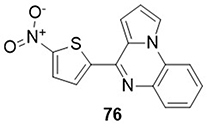
The quinoxaline derivative 4-(5-nitro-thiophen-2-yl)-pyrrolo[1,2-a]quinoxaline 5NO2TAAPP) 76 was prepared by Divya et al,Citation102 and tested its anti-SARS-CoV-2 main protease 3CLpro (PDB code: 6LU7) activity using molecular docking studies. The compound (5NO2TAAPP) 76 showed a lower binding energy towards SARS-CoV-2 main protease 3CLpro (−7.95Kcal mol−1) as compared to the reference drugs remdesivir (−4.96Kcal mol−1) and hydroxychloroquine (−6.06Kcal mol−1). It exhibited H-bonding interactions with the amino acid residues GLY-143A and CYS-145A, and hydrophobic interactions with residues MET-49A and MET-165A ().
Thiadiazole Derivatives as Anti-SARS-CoV-2 Agents
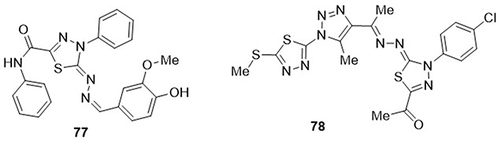
A series of novel 1,3,4-thiadiazole hybrids was prepared by Rashdan and Abdel monsef,Citation103 and tested them as anti-Covid-19 agents through in silico studies. Among the docked hybrids, compound 77 showed the highest binding affinity against receptor-binding domain (RBD), main protease (Mpro), RNA-dependent RNA polymerase (RdRp), and papain-like protease (PLpro) of SARS-CoV-2 with docking scores of −6.8, −11.4, −8.2, and −9.4Kcal mol−1, respectively. Compound 77 showed the best bonding interactions with main protease (Mpro) (−11.4Kcal mol−1) as compared to the standard drug darunavir (−7.5Kcal mol−1). It showed hydrogen bond interactions with amino acid residues THR-24 and SER-144 of the targeted Mpro; pi-stacked interactions with residues LYS-218, TYR-251, and PHE-258 of the targeted PLpro; and ARG-116 and PHE-35 of the targeted RdRp. It also displayed H-bonding interactions with amino acid residues TYR-385A and ARG-393A of the targeted RBD. Rashdan and Abdel monsef,Citation104 also designed a thiadiazole-based derivative bearing 1,2,3-triazole ring and evaluated its inhibitory potential towards the main protease SARS-CoV-2 using in silico studies. Of these, compound 78 exhibited the lowest binding energy against COVID-19’s main protease (−8.3Kcal/mol). It formed H-bonding interactions with the amino acid residues LYS-5 and THR-199, as well as π-cation interactions with residues ARG-131 ().
Thiazole Derivatives as Anti-SARS-CoV-2 Agents
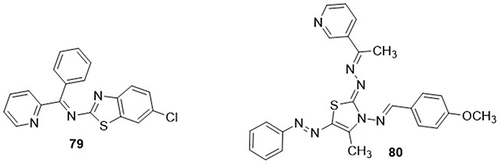
Al-janabi et al,Citation105 synthesized novel thiazole derivatives and screened them for SARS-CoV-2 3CLpro inhibitors using a molecular docking approach. Among these derivatives, N-(6-chlorobenzo[d]thiazol-2-yl)-1-phenyl-1-(pyridin-2-yl)methanimine 79 showed the best binding affinities for SARS-CoV-2 with the same values of −7.6Kcal/mol. It displayed van der Waals interactions with amino acid residues HIS-41, CYS-145, and GLU-166. Adel Alghamdi et al,Citation106 synthesized a new series of thiazole-clubbed pyridine hybrids and examined them as potential inhibitors of COVID-19 Mpro (PDB code: 6LU7) via in silico studies. From the studied hybrids, compound 80 displayed the highest binding affinity against the main protease of SARS-CoV-2 with a binding energy value of −8.6 kcal/mol as compared to the standard Inhibitor N3 (−8.0kcal/mol). It displayed H-bonding interactions with the amino acid residues GLY-71A, GLN-19A, and MET-17A and hydrophobic interactions with the amino acid residues GLU-14A, GLY-120A, ALA-70A, LYS-97A, VAL-18A, and TRP-31A. The molecular docking approaches, physical parameters, and toxicity assessment showed that the compound 80 was found to be non-toxic and could bind with the main protease of SARS-CoV-2 via different types of interactions. ().
Triazole Derivatives as Anti-SARS-CoV-2 Agents
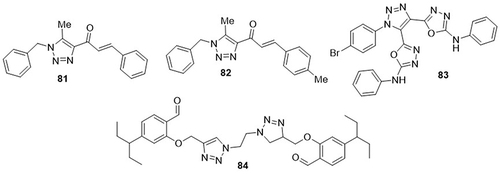
Murugavel et al,Citation4 prepared triazole derivatives and tested them as potent inhibitors of COVID-19 using in silico analyses. From these, compound 81 showed a remarkable binding affinity for the spike protein (PDB ID: 7DF4) of SARS-CoV-2 (−8.9Kcal/mol). The compounds 81 and 82 exhibited the lowest binding energies towards the ACE2 receptor protein (PDB ID: 7DF4) of SARS-CoV-2, with a similar docking score of −9.1Kcal/mol. BMTPP 81 exhibited H-bonding interactions with amino acid residues ARG-355, GLU-465, and ARG466, as well as hydrophobic interactions with residues GLU-465, PHE-464, and LYS-462 of the spike protein receptors. Compounds 81 and 82 displayed H-bonding interactions with the amino acid residues HIS-401, ASP-350, TRP-349, and ALA-348 and hydrophobic interactions with residues PHE-40, TRP-349, ARG-393, HIS-401, and PHE-390 of the SARS-Cov-2 ACE2 receptor protein. A novel series of 1,2,3-triazole hybrids were synthesized by Aouad et al,Citation107 and screened for their anti-COVID-19 activity using in silico studies. Amongst them, compound 83 showed the best binding affinity against the main protease (PDB code: 6LU7) of SARS-CoV-2 (−8.8Kcalmol−1). It displayed the highest binding affinity, higher than the standard drugs hederagenin (−6.9Kcalmol−1), cloperastine (−7.0Kcalmol−1), vigabatrin (−4.1Kcalmol−1), methotrexate (−8.2Kcalmol−1), furidiazine (−6.1Kcalmol−1), ursolic acid (−7.1Kcalmol−1), acetazolamide (−5.2Kcalmol−1), doravirine (−7.8Kcalmol−1), desaglybuzole (−6.5Kcalmol−1), and nortriptyline (−7.3Kcal mol−1), and a similar one to maraviroc (−8.8Kcalmol−1) but had a lower one as compared to raltegravir (−9.6Kcalmol−1). It showed either H-bonding or hydrophobic interactions with the amino acid residues GLN-189A, PHE-140A, and SER-144. Singh et al,Citation108 prepared a bis-triazolyl probe and evaluated its anti-COVID-19 activity using a molecular docking analysis. Compound 84 exhibited the best binding affinity against COVID-19 main protease (PDB code: 6LU7), with a value of −6.43 kcal/mol. It exhibited H-bonding interactions with amino acid residue ARG-298A and hydrophobic interactions with residues PHE-8A, PRO-9A, TYR-154A, and PHE-294A ().
Chloroquine Derivatives as Anti-SARS-CoV-2 Agents
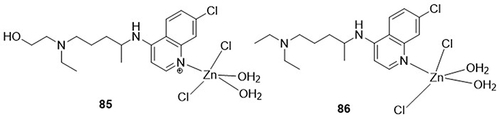
Hussein and Elkhair,Citation109 synthesized zinc complexes of hydroxychloroquine and chloroquine and evaluated them as potential inhibitors of COVID-19’s Mpro (PDB code: 6LU7) using in silico studies. Among the docked derivatives, complexes 85 and 86 showed the highest binding affinities against the main protease of COVID-19 with values of −7.54 and −7.7Kcal/mol, respectively, whereas parent compounds hydroxychloroquine and chloroquine had docking scores of −6.87Kcal/mol and −7.08Kcal/mol, respectively. Complex 85 formed H-bonding interactions with the main protease amino acid residues GLN-189A, HIS-164A, CYS-145A, GLU-166A, MET-165A, and ARG-188A, and complex 86 displayed H-bonding interactions with the main protease amino acid residues GLU-166A and ARG-188A ().
Chromene Derivatives as Anti-SARS-CoV-2 Agents
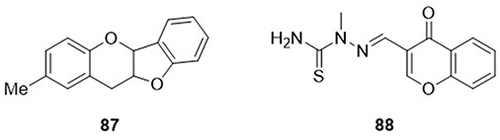
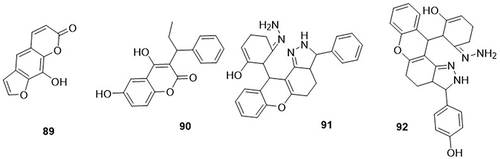
A series of dihydrobenzofuro[3,2-b]chromene hybrids were prepared by Gorai et al,Citation110 and screened as SARS-CoV-2 3CLpro (PDB code: 6lu7) inhibitors through molecular docking analysis. From these, compound 87 demonstrated lower binding energy towards the 3CLpro of SARS-CoV-2 (−7.6kcal/mol). It exhibited H-bonding interactions with amino acid residues CYS-145A; van der Waals interactions with residues TYR-54A, ASN-142A, SER-144A, HIS-164A, GLU-166A, ASP-187A, ARG-188A, GLN-189A, LEU-141A, and GLY-143A; hydrophobic interactions with PRO-52A, MET-49A, and HIS-41; and Pi-sulfur interactions with residue MET-165A. Mary et al,Citation111 synthesized N-methyl-2-[(4-oxo-4H-chromen-3-yl)methylidene]-hydrazinecarbothioamide (MCMH) and evaluated its anti-COVID-19 activity using in silico studies. Compound (MCMH) 88 exhibited the highest binding affinity towards the main protease (Mpro) (PDB code: 5r80) of SARS-CoV-2, with a docking score of −8.8kcal/mol. It displayed H-bonding interactions with amino acid residues GLY-143A, ASN-142A, HIS-163A, SER-144A, and THR-26A and hydrophobic interactions with amino acid residue MET-165A ().
Coumarin Derivatives as Anti-SARS-CoV-2 Agents
Mun et al,Citation112 synthesized coumarin derivatives and tested them as anti-COVID-19 agents using a molecular docking analysis. Among them, psoralen 89 showed the highest binding affinity against RdRp (PDB code: 7BV2), PLpro (PDB code: 6W9C), Mpro (PDB code: 6W63), and spike glycoprotein (PDB code: 6M0J) of SARS-CoV-2 with values of −6.5, −6.2, −6.6, and −6.4kcal/mol, respectively. Phenprocoumon 90 also exhibited the highest binding potential towards the main protease (Mpro) (PDB code: 6W63) of SARS-CoV-2 (−7.2 kcal/mol) in comparison to other target proteins of SARS-CoV-2. Remdesivir was used as a standard drug and had a binding affinity with RdRp (−6.1kcal/mol), PLpro (−7.4kcal/mol), Mpro (−7.8kcal/mol), and Spike protein (−6.9kcal/mol). Psoralen 89 displayed H-bonding interactions with the amino acid residues SER-795A and ASP-164A of RdRp; TYR-213A, GLU-214A, TYR-305A, and LYS-217Aof PLpro, HIS-164A and GLY-166A of Mpro; and HIS-493A, TYR-613A, and ARG-482A of SP. Phenprocoumon 90 formed H-bonding interaction with the amino acid residues GLU-166A and HIS-163A of the main protease (Mpro). Chidambaram et al,Citation113 designed a series of novel coumarin hybrids and screened them as inhibitors of SARS-CoV-2 main protease (PDB code: 5N5O) through in silico studies. Amongst the studied hybrids, compounds 91 and 92, both showed the highest binding affinities against the main protease of SARS-CoV-2 with a binding energy value of −7.9kcal/mol as compared to the standard drugs alpha-ketoamide (−6.6kcal/mol) and hydroxychloroquine (−5.8kcal/mol). Compound 91 demonstrated hydrophobic interactions with amino acid residues THR-25A, HIS-41A, CYS-145A, and GLY-143A. Compound 92 displayed H-bonding interactions with the main amino acid residue HIS-163A and hydrophobic interactions with amino acid residues MET-49A, LEU-141A, CYS-145A, ASN-142A, and CYS-143A ().
Conclusion
Heterocyclic compounds are important in medicinal chemistry owing to their physicochemical properties. The spectrum of heterocycle-containing molecules utilized in medications is growing by the day, and their various derivatives offer a valuable and pivotal pathway for the development of drugs with a broad range of biological applications. This study has revealed that chemically synthesized heterocycles are crucial and versatile compounds with anti-SARS-CoV-2 activities. In vitro studies summarized here state that different heterocycles act against the structural and non-structural proteins of SARs-CoV-2. The half-maximal inhibitory concentration (IC50), half-maximal effective concentration (EC50), 50% cytotoxic concentration (CC50), and selective index of potent compounds were determined. In silico analyses reported herein describe that different heterocycles demonstrate binding affinities towards RdRp (PDB code: 7BV2), PLpro (PDB code: 6W9C), Mpro (PDB code: 6W63), and spike glycoprotein (PDB code: 6M0J) of SARS-CoV-2. Various binding interactions with the amino acid residues of the target proteins have also been reported. ADME analysis was performed to predict the pharmacokinetic properties of the prepared molecules.
Nitrogen- and oxygen-containing heterocyclic compounds with significant levels of demonstrated efficient antiviral effects came to the top of the pharmaceutical perspective for COVID-19 therapy. The current detailed in vitro/in silico preclinical research has investigated the anti-COVID-19 potential of various heterocyclic compounds. Interestingly, preclinical research findings reveal that the heterocyclic compounds have excellent and balanced drug-like behavior and could be further processed in vivo/clinical stages of drug assessment and development.
However, knowing the importance of heterocyclic compounds as antiviral agents, particularly against coronaviruses, a greater knowledge of heterocyclic frameworks as anti-SARS CoV, anti-MERS CoV, and anti-SARS CoV-2 agents. This overview focuses on various heterocyclic compounds employed in coronaviruses inhibition via in vitro and in silico techniques, which may serve as leading structures for the design and development of SARS CoV-2 inhibitors.
Disclosure
The authors report no conflicts of interest in this work.
References
- Satheesh D, Rajendran A, Chithra K. Protein-ligand binding interactions of imidazolium salts with SARS CoV-2. Heliyon. 2020;6(11):e05544. doi:10.1016/j.heliyon.2020.e05544
- Astuti I. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metabolic Syndrome. 2020;14(4):407–412.
- Verma VA, Saundane AR, Meti RS, Vennapu DR. Synthesis of novel indolo [3,2-c]isoquinoline derivatives bearing pyrimidine, piperazine rings and their biological evaluation and docking studies against COVID-19 virus main protease. J Mol Structures. 2021;1229:129829.
- Murugavel S, Vasudevan P, Chandrasekaran R, Archana V, Ponnuswany A. Synthesis, crystal structure elucidation, DFT analysis, drug-likeness and ADMET evaluation and molecular docking studies of triazole derivatives: binary inhibition of spike protein and ACE2 receptor protein of COVID-19. J Chin Chem Soc. 2022;69(6):884–900.
- Rashedi J, Mahdavi Poor B, Asgharzadeh V, et al. Risk factors for COVID-19. Infez Med. 2020;28(4):469–474.
- Jayaweera M, Perera H, Gunawardana B, Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ. Res. 2020;188:109819.
- Nepolraj A, Shupeniuk VI, Sathiyaseelan M. Synthesis of new 3-(hydroxymethyl)-2-phenyl-2, 3 dihydroquinolinone and in-silico evaluation of COVID-19 main protease inhibitor. Vietnam J Chem. 2021;59(4):511–521.
- Holmes EC, Goldstein SA, Rasmussen AL, et al. The origins of SARS-CoV-2: a critical review. Cell. 2021;184(19):4848–4856.
- Triggle CR, Bansal D, Ding H, et al. A comprehensive review of the viral characteristics, transmission, pathophysiology, immune response, and management of SARS-CoV-2 and COVID-19 as a basis for controlling the pandemic. Front Immunol. 2021;12:631139.
- Nizi MG, Persoons L, Corona A, et al. Discovery of 2-phenylquinolines with broad-spectrum anti-coronavirus activity. ACS Med Chem Lett. 2022;13(5):855–864.
- Alotaibi SH, Amer HH, Touil N, Abdel-Moneim AS, Soliman MM, Zaki YH. Synthesis, characterization and molecular docking of new nucleosides and Schiff bases derived from ampyrone as antiviral agents to contain the COVID-19 virus. Polycyclic Aromatic Compounds. 2023;43(3):2418–2429.
- Abu-Zaied MA, Elgemeie GH, Mahmoud NM. Anti-covid-19 drug analogues: synthesis of novel pyrimidine thioglycosides as antiviral agents against SARS-COV-2 and avian influenza H5N1 viruses. ACS omega. 2021;6(26):16890–16904.
- Lu R, Zhao X, Li J, et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574.
- V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19(3):155–170.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus isolated from patients with pneumonia in China in 2019. N Engl J Med. 2020;382(8):727–733.
- Ziebuhr J, Snijder EJ, Gorbalenya AE. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J Gen Virol. 2000;81(4):853–879.
- Xu X, Liu Y, Weiss S, Arnold E, Sarafianos SG, Ding J. Molecular model of SARS coronavirus polymerase: implications for biochemical functions and drug design. Nucleic Acids Res. 2003;31:7117–7130.
- Eckerle LD, Becker MM, Halpin RA, et al. Infidelity of SARS-CoV Nsp14-exonuclease mutant virus replication is revealed by complete genome sequencing. PLoS Pathogens. 2010;6(5):e1000896.
- Hoffmann M, Arora P, Groß R, et al. SARS-CoV-2 variants B. 1.351 and B. 1.1. 248: escape from therapeutic antibodies and antibodies induced by infection and vaccination. BioRxiv. 2021;2021–2022.
- Wu C-Y, Lin Y-S, Yang YH, Shu LH, Cheng YC, Te Liu H. GB-2 inhibits ACE2 and TMPRSS2 expression: in vivo and in vitro studies. Biomed. Pharmacother. 2020;132:110816.
- Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11(1):1620.
- Swaminathan S, Dehghan M, Raj JM, et al. Associations of cereal grains intake with cardiovascular disease and mortality across 21 countries in Prospective Urban and Rural Epidemiology study: prospective cohort study. BMJ. 2021;372.
- Lu D, Chatterjee S, Xiao K, et al. MicroRNAs targeting the SARS-CoV-2 entry receptor ACE2 in cardiomyocytes. J Mol Cell Cardiol. 2020;148:46–49.
- Van de Leemput J, Han Z. Understanding individual SARS-CoV-2 proteins for targeted drug development against COVID-19. Mol Cell Biol. 2021.
- Gao Y, Yan L, Huang Y, et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368:6492.
- Tvarogová J, Madhugiri R, Bylapudi G, Ferguson LJ, Karl N, Ziebuhr J. Identification and characterization of a human coronavirus 229E nonstructural protein 8-associated RNA 3′-terminal adenylyltransferase activity. J Virol. 2019;93(12):10–1128.
- Dai W, Zhang B, Jiang XM, et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368(6497):1331–1335.
- Hilgenfeld R. From SARS to MERS: crystallographic studies of coronaviral proteases from SARS to MERS have enabled antiviral drug design. FEBS J. 2014;281(18):4085–4096.
- MacDonald EA, Frey G, Namchuk MN, Harrison SC, Hinshaw SM, Windsor IW. Recognition of divergent viral substrates by the SARS-CoV-2 main protease. ACS Infect. Dis. 2021;7(9):2591–2595.
- Verma N, Henderson JA, Shen J. Proton-coupled conformational activation of SARS coronavirus main proteases and opportunity for designing small-molecule broad-40 spectrum targeted covalent inhibitors. J Am Chem Soc. 2020;142(52):21883–21890.
- Frecer V, Miertus S. Antiviral agents against COVID-19: structure-based design of specific peptidomimetic inhibitors of SARS-CoV-2 main protease. RSC Adv. 2020;10(66):40244–40263.
- Zhang C-H, Spasov KA, Reilly RA, et al. Optimization of triarylpyridinone inhibitors of the main protease of SARS-CoV-2 to low -nanomolar antiviral potency. ACS Med Chem Lett. 2021;12(8):1325–1332.
- Thanigaimalai P, Konno S, Yamamoto T, et al. Development of potent dipeptide-type SARS-CoV 3CL protease inhibitors with novel P3 scaffolds: design, synthesis, biological evaluation, and docking studies. Eur. J. Med. Chem. 2013;68:372–384.
- Sohrabi C, Alsafi Z, O’Neill N, et al. Corrigendum to World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int J Surgery. 2020;77:217.
- Tobaiqy M, Qashqary M, Al-Dahery S. Therapeutic management of patients with COVID-19: a systematic review. Infection Prevention in Practice. 2020;2(3):100061.
- Yang J, Lin X, Xing N, Zhang H, Wu H, Xue W. Structure-based discovery of novel non-peptide inhibitors targeting SARS-CoV-2 Mpro. J Chem Inf Model. 2021;61(8):3917–3926.
- Gatti M, De Ponti F. Drug repurposing in the COVID-19 era: insights from case studies showing pharmaceutical peculiarities. Pharmaceutics. 2021;13(3):302.
- Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov. 2020;19(3):149–150.
- Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271.
- Lim J, Jeon S, Shin HY, et al. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. 2020;35:e79–e79.
- Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clinl Infect Dis. 2020;71(15):732–739.
- Eltayb WA, Abdalla M, Rabie AM. Novel Investigational Anti-SARS-CoV-2 Agent Ensitrelvir “S-217622”: a Very Promising Potential Universal Broad-Spectrum Antiviral at the Therapeutic Frontline of Coronavirus Species. ACS Omega. 2023;8(6):5234–5246.
- Rabie AM. Potent Inhibitory Activities of the Adenosine Analogue Cordycepin on SARS-CoV-2 Replication. ACS Omega. 2022;7(3):2960–2969.
- Rabie AM. Efficacious Preclinical Repurposing of the Nucleoside Analogue Didanosine against COVID-19 Polymerase and Exonuclease. ACS Omega. 2022;7(25):21385–21396.
- Rabie AM. Teriflunomide: a possible effective drug for the comprehensive treatment of COVID-19. Curr Res Pharmacol Drug Discovery. 2021;2:100055.
- Rabie AM, Abdalla M. Forodesine and Riboprine Exhibit Strong Anti-SARS-CoV-2 Repurposing Potential: in silico and In Vitro Studies. ACS Bio Med Chem. 2022;2(6):565–585.
- Rabie AM. New potential inhibitors of coronaviral main protease (CoV-Mpro): strychnine bush, pineapple, and ginger could be natural enemies of COVID-19. Int J New Chem. 2022;9(3):225–237.
- Rafiq A, Jabeen T, Aslam S, et al. A Comprehensive Update of Various Attempts by Medicinal Chemists to Combat COVID-19 through Natural Products. Molecules. 2023;28(12):4860.
- Esharkawy ER, Almalki F, Hadda TB. In vitro potential antiviral SARS-CoV-19-activity of natural product thymohydroquinone and dithymoquinone from Nigella sativa. Bioorg. Chem. 2022;120:105587.
- Dyer O. Covid-19: remdesivir Has Little or No Impact on Survival. WHO Trial Shows. 2020.
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med. 2020;383(19):1813–1836.
- Zhou S, Hill CS, Sarkar S, et al. β-D-N 4- hydroxycytidine inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to mammalian cells. J Infect Dis. 2021;224(3):415–419.
- Salganik RI, Vasjunina EA, Poslovina AS, Andreeva IS. Mutagenic action of N4-hydroxycytidine on Escherichia coli B cyt-. Mutat Res. 1973;20(1):1–5.
- Janion C, Glickman BW. N4-hydroxycytidine: a mutagen specific for AT to GC transitions. Mutat Res. 1980;72(1):43–47.
- Konno S, Kobayashi K, Senda M, et al. 3CL protease inhibitors with an electrophilic arylketone moiety as anti-SARS-CoV-2 agents. J Med Chem. 2021;65(4):2926–2939.
- Chia CB. Novel nitrile peptidomimetics for treating COVID-19 treatment. ACS Med Chem Lett. 2022;13(3):330–331.
- Jin Z, Du X, Xu Y, et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582(7811):289–293.
- Liu F, Han K, Blair R, et al. SARS-CoV-2 infects endothelial cells in vivo and in vitro. Front Cell Infect Microbiol. 2021;11:701278.
- Barakat A, Mostafa A, Ali M, et al. Design, synthesis and in vitro evaluation of the spirooxindole-based phenylsulfonyl moiety as a candidate anti-SAR-CoV-2 and MERS-CoV-2 agent with the implementation of combination studies. Int J Mol Sci. 2022;23(19):11861.
- Tanaka H, Miyagi S, Yoshida Y, et al. Synthesis and biological evaluation of umifenovir analogues as Anti-SARS-CoV-2 Agents. ChemistrySelect. 2022;7(30):e202202097.
- Srour AM, Panda SS, Mostafa A, et al. Synthesis of aspirin-curcumin mimic conjugates of potential antitumor and anti-SARS-CoV-2 properties. Bioorg. Chem. 2021;117:105466.
- Seliem IA, Girgis AS, Moatasim Y, et al. New Pyrazine Conjugates: synthesis, Computational Studies, and Antiviral Properties against SARS-CoV-2. ChemMedChem. 2021;16(22):3418–3427.
- Rabie AM. Improved synthesis of the anti-SARS-CoV-2 investigational agent (E)-N-(4-cyanobenzylidene)-6-fluoro-3-hydroxypyrazine-2-carboxamide (cyanorona-20). Rev Chim. 2022;73(4):69–75.
- Ghosh AK, Raghavaiah J, Shahabi D, et al. Indole chloropyridinyl ester-derived SARS-CoV-2 3CLpro inhibitors: enzyme inhibition, antiviral efficacy, structure–activity relationship, and X-ray structural studies. J Med Chem. 2021;64(19):14702–14714.
- Fomenko VV, Rudometova NB, Rogachev AD, Fando AA, Zaykovskaya AV, Salakhutdinov NF. Synthesis and in vitro study of antiviral activity of glycyrrhizin nicotinate derivatives against HIV-1 pseudoviruses and SARS-CoV-2 viruses. Molecules. 2022;27(1):295.
- Abu-Zaied MA, Elgemeie GH, Halaweish FT, Hammad SF. Synthesis of novel pyridine and pyrimidine thioglycoside phosphoramidates for the treatment of COVID-19 and influenza A viruses. Nucleosides Nucleotides Nucleic Acids. 2022;41(9):851–877.
- Al-Harbi SA. Synthesis and characterization of violurate-based Mn (II) and Cu (II) complexes nano-crystallites as DNA-binders and therapeutics agents against SARS-CoV-2 virus. J Saudi Chem Soc. 2022;26(5):101528.
- Nishiuchi K, Ohashi H, Nishioka K, et al. Synthesis and antiviral activities of neoechinulin B and its derivatives. J Natural Prod. 2021;85(1):284–291.
- Guo Y, Meng J-R, Liu J-Z, et al. Synthesis and biological evaluation of honokiol derivatives bearing 3-((5-phenyl-1, 3, 4-oxadiazol-2-yl) methyl)oxazol-2(3H)-ones as potential viral entry inhibitors against SARS-CoV-2. Pharmaceuticals. 2021;14(9):885.
- Lee JY, Shin YS, Jeon S, et al. Synthesis and biological evaluation of 2-benzylaminoquinazolin-4 (3 H)-one derivatives as a potential treatment for SARS-CoV-2 infection. Bull. Korean Chem. Soc. 2022;43(3):412–416.
- Shin YS, Lee JY, Jeon S, et al. Optimization of 2-Aminoquinazolin-4-(3H)-one Derivatives as Potent Inhibitors of SARS-CoV-2: improved Synthesis and Pharmacokinetic Properties. Pharmaceuticals. 2022;15(7):831.
- Guevara-Pulido J, Jiménez RA, Morantes SJ, Jaramillo DN, Acosta‐Guzmán P. Design, Synthesis, and Development of 4-[(7-Chloroquinoline-4-yl) amino] phenol as a Potential SARS-CoV-2 Mpro Inhibitor. ChemistrySelect. 2022;7(15):e202200125.
- Zhao J, Zhang Y, Wang M, et al. Quinoline and quinazoline derivatives inhibit viral RNA synthesis by SARS-CoV-2 RdRp. ACS Infect. Dis. 2021;7(6):1535–1544.
- Chen X, Chen K, Zhang Z, et al. Investigating derivatives of tanshinone IIA sulfonate sodium and chloroxine for their inhibition activities against the SARS-CoV-2 papain-like protease. ACS omega. 2022;7(51):48416–48426.
- Herrmann L, Yaremenko IA, Çapcı A, et al. Synthesis and in vitro study of artemisinin/synthetic peroxide-based hybrid compounds against SARS-CoV-2 and cancer cells. ChemMedChem. 2022;17(9):e202200005.
- Rabie AM, Abdel-Dayem MA, Abdalla M. Promising Experimental Anti-SARS-CoV-2 Agent “SLL-0197800”: the Prospective Universal Inhibitory Properties against the Coming Versions of the Coronavirus. ACS Omega. 2023;8(39):35538–35554.
- Wang X, Burdzhiev NT, Hu H, et al. Novel Tetrahydroisoquinoline-Based Heterocyclic Compounds Efficiently Inhibit SARS-CoV-2 Infection In Vitro. Viruses. 2023;15(2):502.
- Wu J, Feng B, Gao L-X, et al. Synthesis and Biochemical Evaluation of eight H-indeno [1,2-d] thiazole Derivatives as Novel SARS-CoV-2 3CL Protease Inhibitors. Molecules. 2022;27(10):3359.
- Pohl MO, Martin-Sancho L, Ratnayake R, et al. Sec61 inhibitor apratoxin S4 potently inhibits SARS-CoV-2 and exhibits broad-spectrum antiviral activity. ACS Infect. Dis. 2022;8(7):1265–1279.
- Chemboli R, Kapavarapu R, Deepti K, et al. Pyrrolo[2,3-b] quinoxalines in attenuating cytokine storm in COVID-19: their sonochemical synthesis and in silico/in vitro assessment. J Mol Struct. 2021;1230:129868.
- Shcherbakov D, Baev D, Kalinin MM, et al. Design and evaluation of bispidine-based SARS-CoV-2 main protease inhibitors. ACS Med Chem Lett. 2021;13(1):140–147.
- Wansri R, Lin ACK, Pengon J, et al. Semi-synthesis of N-aryl amide analogs of piperine from Piper nigrum and evaluation of their antitrypanosomal, antimalarial, and anti-SARS-CoV-2 main protease activities. Molecules. 2022;27(9):2841.
- Mohamed NM, Eltelbany RF. Synthetic coumarin derivatives as SARS-CoV-2 major protease inhibitors: design, synthesis, bioevaluation and molecular docking. ChemistrySelect. 2021;6(47):13616–13626.
- Rabie AM, Eltayb WA. Potent Dual Polymerase/Exonuclease Inhibitory Activities of Antioxidant Aminothiadiazoles Against the COVID-19 Omicron Virus: a Promising In Silico/In vitro Repositioning Research Study. Mol Biotechnol. 2023;1–20.
- Venkateshan M, Muthu M, Suresh J, Kumar RR. Azafluorene derivatives as inhibitors of SARS CoV-2 RdRp: synthesis, physicochemical, quantum chemical, modeling and molecular docking analysis. J Mol Struct. 2020;1220:128741.
- Belhassan A, En-Nahli F, Zaki H, Lakhlifi T, Bouachrine M. Assessment of effective imidazole derivatives against SARS-CoV-2 main protease through computational approach. Life Sci. 2020;262:118469.
- Mudi PK, Mahato RK, Verma H, et al. In silico anti-SARS-CoV-2 activities of five-membered heterocycle-substituted benzimidazoles. J Mol Struct. 2022;1261:132869.
- Algethami FK, Cherif M, Jlizi S, et al. Design, microwave-assisted synthesis and in silico prediction study of novel isoxazole-linked pyranopyrimidinone conjugates as new targets for searching potential Anti-SARS-CoV-2 agents. Molecules. 2021;26(20):6103.
- Gatfaoui S, Sagaama A, Issaoui N, Roisnel T, Marouani H. Synthesis, experimental, theoretical study and molecular docking of 1-ethylpiperazine-1, 4-diium bis (nitrate). Solid State Sci. 2020;106:106326.
- Anthony LA, Rajaraman D, Sundararajan G, et al. Synthesis, crystal structure, Hirshfeld surface analysis, DFT, molecular docking and molecular dynamic simulation studies of (E)-2, 6-bis (4-chlorophenyl)-3-methyl-4-(2-(2, 4, 6-trichlorophenyl) hydrazono) piperidine derivatives. J Mol Struct. 2022;1266:133483.
- Gupta A, Iqbal S, Roohi MK, Zaheer MR, Shankar K. Visible light-promoted green and sustainable approach for one-pot Synthesis of 4, 4’-(arylmethylene) bis (1H-pyrazol-5-ols), In Vitro Anticancer Activity, and Molecular Docking with Covid-19 Mpro. ACS omega. 2022;7(38):34583–34598.
- Addoum B, El Khalfi B, Sakoui S, Derdak R, Elmakssoudi A, Soukri A. Synthesis and molecular docking studies of some pyrano [2,3-c] pyrazole as an Inhibitor of SARS-Coronavirus 3CL protease. Lett Appl NanoBioscience. 2022;11:3780.
- Masaret GS. Synthesis of new spiropyrazole derivatives under microwaves irradiation and docking study for inhibition the microbes and COVID-19. J Mol Struct. 2022;1269:133581.
- Mohamed GG, Omar MM, Ahmed YM. Metal complexes of Tridentate Schiff bases: synthesis, characterization, biological activity, and molecular docking studies with COVID-19 protein receptors. Z Anorg Allg Chem. 2021;647(23–24).
- Jayabal K, Elumalai D, Leelakrishnan S, et al. Green and regioselective approach for the synthesis of 3-substituted indole based 1, 2-dihydropyridine and azaxanthone derivatives as a potential lead for SARS-CoV-2 and delta plus mutant virus: DFT and docking studies. ACS omega. 2022;7(48):43856–43876.
- Tarika JD, Dexlin XD, Madhankumar S, Ayanthi DD, Beaula TJ. Tuning the computational evaluation of spectroscopic, ELF, LOL, and NCI analysis and molecular docking of the novel anti COVID-19 molecule 4-dimethylamino pyridinium 3, 5-dichlorosalicylate. Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy. 2021;259:119907.
- Topal T, Zorlu Y, Karapınar N. Synthesis, X-ray crystal structure, IR and Raman spectroscopic analysis, quantum chemical computational and molecular docking studies on hydrazone-pyridine compound: as an insight into the inhibitor capacity of main protease of SARS-CoV2. J Mol Struct. 2021;1239:130514.
- Nesaragi AR, Kamble RR, Hoolageri SR, Mavazzan A, Madar SF, Anand A. WELPSA: a natural catalyst of alkali and alkaline earth metals for the facile synthesis of tetrahydrobenzo [b] pyrans and pyrano [2,3-d] pyrimidinones as inhibitors of SARS-CoV-2. Appl. Organomet. Chem. 2022;36(1):e6469.
- Muhammad ZA, Farghaly TA, Althagafi I, Al‐Hussain SA, Zaki ME, Harras MF. Synthesis of antimicrobial azoloazines and molecular docking for inhibiting COVID-19. J Heterocycl Chem. 2021;58(6):1286–1301.
- Qureshi F, Nawaz M, Hisaindee S, et al. Microwave assisted synthesis of 2-amino-4-chloro-pyrimidine derivatives: anticancer and computational study on potential inhibitory action against COVID-19. Arabian J. Chem. 2022;15(12):104366.
- Alshammari MB, Ramadan M, Aly AA, et al. Synthesis of potentially new schiff bases of N-substituted-2-quinolonylacetohydrazides as anti-COVID-19 agents. J Mol Struct. 2021;1230:129649.
- Divya KM, Savitha DP, Krishna GA, Dhanya TM, Mohanan PV. Crystal structure, DFT studies, Hirshfeld surface and energy framework analysis of 4-(5-nitro-thiophen-2-yl)-pyrrolo[1,2-a] quinoxaline: a potential SARS-CoV-2 main protease inhibitor. J Mol Struct. 2022;1251:131932.
- Rashdan HR, Abdelmonsef AH, Abou-Krisha MM, Yousef TA. Synthesis and identification of novel potential thiadiazole based molecules containing 1, 2, 3-triazole moiety against COVID-19 main protease through structure-guided virtual screening approach. Appl Biochem Biotechnol. 2021;193:3602–3623.
- Rashdan HR, Abdelmonsef AH. In silico study to identify novel potential thiadiazole-based molecules as anti-Covid-19 candidates by hierarchical virtual screening and molecular dynamics simulations. Struct Chem. 2022;33(5):1727–1739.
- Al-Janabi AS, Elzupir AO, Yousef TA. Synthesis, anti-bacterial evaluation, DFT study and molecular docking as a potential 3-chymotrypsin-like protease (3CLpro) of SARS-CoV-2 inhibitors of a novel Schiff bases. J Mol Struct. 2021;1228:129454.
- Alghamdi A, Abouzied AS, Alamri A, et al. Synthesis, molecular docking, and dynamic simulation targeting main protease (Mpro) of new, Thiazole clubbed pyridine scaffolds as potential COVID-19 inhibitors. Curr Issues Mol Biol. 2023;45(2):1422–1442.
- Aouad MR, Khan DJ, Said MA, et al. Novel 1, 2, 3-triazole derivatives as potential Inhibitors against Covid-19 Main Protease: synthesis, characterization, molecular docking and DFT studies. ChemistrySelect. 2021;6(14):3468–3486.
- Singh G, Saini A, Kaur A. Design of new bis-triazolyl structure for identification of inhibitory activity on COVID-19 main protease by molecular docking approach. J Mol Struct. 2022;1250:131858.
- Hussein RK, Elkhair HM. Molecular docking identification for the efficacy of some zinc complexes with chloroquine and hydroxychloroquine against main protease of COVID-19. J Mol Struct. 2021;1231:129979.
- Gorai S, Junghare V, Kundu K, et al. Synthesis of dihydrobenzofuro [3, 2-b]chromenes as potential 3CLpro inhibitors of SARS-CoV-2: a molecular docking and molecular dynamics study. ChemMedChem. 2022;17(8):e202100782.
- Mary SJ, Pradhan S, James C. Molecular structure, NBO analysis of the hydrogen-bonded interactions, spectroscopic (FT–IR, FT–Raman), drug likeness and molecular docking of the novel anti COVID-2 molecule (2E)-N-methyl-2-[(4-oxo-4H-chromen-3-yl) methylidene]-hydrazinecarbothioamide (dimer)-quantum chemical approach. Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy. 2021;251:119388.
- Mun CS, Hui LY, Sing LC, Karunakaran R, Ravichandran V. Multi-targeted molecular docking, pharmacokinetics, and drug-likeness evaluation of coumarin-based compounds targeting proteins involved in development of COVID-19. Saudi J Biol Sci. 2022;29(12):103458.
- Chidambaram S, El-Sheikh MA, Alfarhan AH, Radhakrishnan S, Akbar I. Synthesis of novel coumarin analogues: investigation of molecular docking interaction of SARS-CoV-2 proteins with natural and synthetic coumarin analogues and their pharmacokinetics studies. Saudi J Biol Sci. 2021;28(1):1100–1108.