Abstract
Purpose
Estrogen deficiency is the main reason of postmenopausal osteoporosis. Eldecalcitol (ED-71) is a new active vitamin D analogue clinically used in the treatment of postmenopausal osteoporosis. We aimed to investigate whether EphrinB2-EphB4 and RANKL/RANK/OPG signaling cooperate in mediating the process of osteoporosis by ED-71.
Methods
In vivo, the ovariectomized (OVX) rats were administered orally with 30 ng/kg ED-71 once a day for 8 weeks. HE staining, Masson staining and Immunofluorescence staining were used to evaluate bone mass, bone formation, osteoclastogenesis associated factors and the expression of EphrinB2, EphB4, RANKL and OPG. In vitro, H2O2 stimulation was used to simulate the cell environment in osteoporosis. Immunofluorescence, quantitative real time PCR (qRT-PCR), enzyme-linked immunosorbent assay (ELISA) and Western Blot were applied to detect the expression of EphrinB2, EphB4, RANKL and OPG. In osteoblasts, EphB4 was knocked down by EphB4 small-interfering RNA (siRNA) transfection. LY294002 (PI3K inhibitor) or ARQ092 (AKT inhibitor) was used to block PI3K/AKT pathway. An indirect co-culture system of osteoblasts and osteoclasts was established. The mRNA and protein expression of osteoclastogenes is associated factors were tested by qRT-PCR and Western Blot.
Results
ED-71 increased bone mass and decreased the number of osteoclasts in OVX rats. Moreover, ED-71 promoted the expression of EphrinB2, EphB4, and decreased the RANKL/OPG ratio in osteoblasts. Osteoclastogenesis was restrained when osteoclasts were indirectly co-cultured with ED-71-treated osteoblasts. After silencing of EphB4 expression in osteoblasts, ED-71 inhibited the expression of P-PI3K and P-AKT and increased the ratio of RANKL/OPG. This reversed the inhibitory effect of ED-71 on osteoclastogenes. Therefore, in ED-71-inhibited osteoclastogenes, EphB4 is a key factor affecting the secretion of RANKL and OPG by osteoblasts. EphB4 suppressed the RANKL/OPG ratio through activating PI3K/AKT signaling in osteoblasts.
Conclusion
ED-71 inhibits osteoclastogenesis through EphrinB2-EphB4-RANKL/OPG axis, improving bone mass in ovariectomized rats. PI3K/AKT pathway is involved this process.
Introduction
Osteoporosis is a systemic bone disease that affects people of all races and the aged. It decreases bone mass, strength and disrupts microstructure.Citation1 Postmenopausal osteoporosis is a clinical disease with a morbidity of more than 30% in women over the age of 50.Citation2,Citation3 The main reason of bone loss in postmenopausal osteoporosis is estrogen deficiency.Citation4 Deficiency of estrogen gives rise to an imbalance in bone modulation,Citation5 which is evidenced by the increased osteoclast activity, enhanced bone resorption and increased fracture risk.Citation6,Citation7
Osteoblasts and osteoclasts co-regulate bone remodeling. In the past, many studies focused on the separate effects on Osteoblasts and osteoclasts, while ignoring the communication between them.Citation8,Citation9 During the stage of new bone formation, osteoblasts adjust osteoclast formation and activity through several pathways including paracrine and juxtacrine signaling.Citation10 In the paracrine pathway, RANK/RANKL/OPG system is an important pathway for osteoclast formation and differentiation.Citation11 RANKL released by osteoblast combines with RANK on the surface of osteoclast precursor cells, thereby triggering the formation of osteoclasts. Osteoprotegerin (OPG), also mainly produced and secreted by osteoblasts, competes against RANK for the combination with RANKL, which inhibits the formation of osteoclasts and avoiding excessive bone resorption.Citation12 Therefore, the RANKL/OPG ratio is a critical determinant of maintaining bone mass and bone homeostasis.
The primary method of juxtacrine signaling is direct cell-to-cell contact. EphrinB2-EphB4 has been reported to be an important pathway in bone modulation.Citation10 The interaction of EphrinB2 and EphB4 in osteoblasts also plays an important role in regulating the maturation of osteoblasts and inhibiting the differentiation of osteoclasts.Citation13 Paracrine and juxtacrine signaling exist simultaneously and may influence each other. Interestingly, blocking the EphrinB2-EphB4 signaling pathway on osteoblasts would stimulate the production of RANKL and other osteoclast factors, and promote osteoclast formation,Citation14 suggesting a possible communicative crosstalk between EphrinB2-EphB4 and RANK/RANKL/OPG. How does EphB4 affect the secretion of RANKL in osteoblasts remains unclear.
The phosphatidylinositol 3 kinase/protein kinase B (PI3K/AKT) signal is a classic pathway that regulates cell biological behavior. It is involved in the pathogenesis of various diseases, such as tumors,Citation15 diabetes and obesity.Citation16 Studies have shown that PI3K/AKT participate in the pathogenesis of osteoporosis by affecting the differentiation of osteoblasts and osteoclasts.Citation17 In addition, PI3K/AKT is also associated with cell proliferation and tumor progression activated by EphB4.Citation18 However, whether PI3K/AKT is involved in osteoporosis activated by EphB4 has not been elucidated.
The current medical treatment to osteoporosis mainly includes bisphosphonates, selective estrogen receptor modulator, mixed steroid receptor agonist, monoclonal antibody against RANKL, parathyroid hormone analogue and monoclonal antibody against sclerostin.Citation19 Vitamin D has been recommended in the clinical treatment of osteoporosis.Citation20 Eldecalcitol (ED-71), developed in early 80s, is an active Vitamin D analog with a hydroxypropoxy group at the 2β position of 1.25 (OH)2D3.Citation21,Citation22 It has been shown that ED-71 inhibits the resorption of osteoclasts, increases bone density more effectively than Alfacalcidol, prevents fractures,Citation23 and reduces the risk of hypercalcemia.Citation24 However, the specific mechanism by which ED-71 exerts the beneficial effect on osteoclasts remains unclear.
In this study, we used the osteoporosis model to investigate the effect and mechanism of ED-71 on osteoclasts formation. We also explored whether the EphB4-EphrinB2 and RANK/RANKL/OPG pathway are synergistically involved in this process.
Materials and Methods
Establishment and Collection of Ovariectomized (Ovx) Rat Models
All the animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of the National Institutes of Health. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Hospital of Stomatology, Shandong University (Approval No. 20,230,502).
Eight-week-old female rats were selected and randomly divided into three groups (n=5), namely the sham group, the OVX group, and the OVX+ED-71 group. The required experimental animal size was calculated according to resource equation method.Citation25 Detail methodology can be found in the Supplementary Data 1. The animals were fed under standard laboratory conditions. The rats were anesthetized with 1% sodium pentobarbital. An incision was then made on the back. After the subcutaneous tissue and muscle was separated, the ovary and fallopian tube was exposed. The fallopian tube was ligated and ovarian tissue was removed to establish an OVX model. ED-71 at a concentration of 30ng/kg was administered orally daily for 8 weeks.
After 8 weeks of treatment, rats were anesthetized and fixed with 4% paraformaldehyde for cardiac perfusion. Tibias were isolated after fixation and decalcified in 4% EDTA-2Na. The samples were then dehydrated in graded ethanol. After being transparent in xylene, they were embedded in paraffin and cut into 5-μm thick slices continuously.
Hematoxylin and Eosin (HE) Staining
Paraffin sections were deparaffinized with xylene and then hydrated with graded ethanol. The sections were stained with hematoxylin for 10 minutes, and then washed thoroughly with distilled water. After which the samples were stained with eosin for 7 minutes, and then rinsed with distilled water. The sections were sealed and observed, and digital images were collected with an optical microscope (Olympus BX-53, Tokyo, Japan). The images were quantitatively analyzed using Image pro Plus 6.0 (IPP 6.0) software (Media Cybernetics, Silver Spring, MD, USA).
Masson Staining
Paraffin sections were deparaffinized with xylene, hydrated with graded ethanol, stained with hematoxylin for 10 minutes, and rinsed with distilled water. The sections were soaked in acid fuchsin solution for 7 minutes and rinsed with glacial acetic acid in water. After which, they were differentiated in a phosphomolybdic acid solution for 4 minutes, and then directly immersed in aniline blue solution for 1 minute without washing. They were immersed in glacial acetic acid aqueous solution. After mounted, the stained sections were observed with an optical microscope (Olympus BX-53, Tokyo, Japan), and the digital images were obtained.
Immunofluorescence Staining in vivo
Paraffin sections were deparaffinized with xylene and hydrated with graded ethanol. Sections were treated with 1% bovine serum albumin (BSA) in PBS solution for 20 minutes to block non-specific staining. These sections were incubated with rabbit anti-EphB4 (1:50, 20,883-1-AP), rabbit anti-EphrinB2 (1:50, DF7450), rabbit anti-RANKL (1:50, BC074890), rabbit anti-OPG (1:50, ab73400) and rabbit anti-cathepsin K (Ctsk) (1:50, ab19027) overnight at 4 °C, respectively. After being washed with PBS, these sections were incubated with the fluorescein (FITC)-conjugated affinity pure goat anti-rabbit IgG (H + L) (1:200, SA00003-2) at room temperature for 1 hour in the dark, and incubated with DAPI for 5 minutes. Slides were observed with a fluorescence microscope (DMi8 automation, Leica Microsystems CMS GmbH, Germany). The fluorescence intensity was analyzed with Image J.
Cell Treatment
MC3T3-E1 cells (Shanghai Cell Center, Shanghai, China) were cultured in 10% fetal bovine serum and 1% penicillin streptomycin modified α-modified Eagle’s medium (α-MEM). Culturing dishes or flasks were placed in a 37°C, 5% CO2 incubator. According to our previous study,Citation26 the cells were stimulated with H2O2 to imitate the cell environment of osteoporosis in vitro. MC3T3-E1 cells were pre-stimulated with 100 μM H2O2 for 2 hours. Then they were cultured by 10−8 M dexamethasone, 50 mg/L ascorbic acid, and 10 mM β-glycerol phosphate sodium for 0, 3, 5, 7 days. In ED-71 group, 1 μm ED-71was added.Citation27 In some experiments, in MC3T3-E1 cells, 50 nM siEphB4-transfected mixture was used to knock down EphB4 and 10 μM LY294002 (HY-10108, MedChemExpress, USA) or 1 μM ARQ092 (HY19719, MedChemExpress, USA)Citation28 was used to block PI3K/AKT pathway.
RAW264.3 cells (Shanghai Cell Center, Shanghai, China) were cultured in 10% fetal bovine serum and 1% penicillin streptomycin modified D-modified Eagle’s medium (DMEM) in a 37°C, 5% CO2 incubator. The culture supernatant of MC3T3-E1 cells was collected separately, and then centrifuged to remove cell debris. The supernatant was filtered through a bacteria filter, and stored at −80°C.Citation29 As the conditioned medium (CM) for indirect co-cultivation of RAW264.3, the supernatant was mixed with D-modified Eagle’s medium (DMEM) with 1:1.Citation30
Cell Viability Assay
MC3T3-E1 cells were seeded into 96-well plates and pretreated with H2O2 at different concentrations (0 μM, 50 μM, 100 μM, 200 μM and 500 μM) for 2 h. After 24 h, 48 h and 72 h of culture, the cells were incubated with 10 μg CCK-8 solution for 2 h, the absorbance at 450 nm was measured by (iMark, Bio-Rad, US).
Immunofluorescence in vitro
Osteoblasts were fixed with 4% paraformaldehyde, and then infiltrated with 0.5% Triton X-100. Cells were rinsed with PBS, blocked with 5% BSA in PBS, and incubated with rabbit anti-EphB4 (1:200,20,883-1-AP), rabbit Anti-EphrinB2 (1:200, DF7450), rabbit anti-P-PI3K (1:200, ab278545), rabbit anti-P-AKT (1:200, ab192623) overnight at 4°C, respectively. After 24 h incubation, the cells were treated with fluorescein (FITC)-conjugated affinity pure goat anti-rabbit IgG (H + L) (1:500, SA00003-2) at room temperature for 1 hour in the dark. Nucleus were shown by incubation with DAPI for 5 minutes. Cells were observed under a fluorescence microscope (DMi8 automation, Leica Microsystems CMS GmbH, Germany). The relative fluorescence intensity was measured with Image J.
Enzyme-Linked Immunosorbent Assay (ELISA)
The culture supernatant of the osteoblasts in different group was collected. The secretion of RANKL in the culture supernatants was detected using a cytokine-specific ELISA kit (Abclonal, China) according to manufacturer’s instructions.
RNA Extraction and Quantitative Real‑time PCR (qRT-PCR)
RNA was extracted from MC3T3-E1 cells and RAW264.7 cells in different groups. Firstly, the culture supernatant was discarded, the cells were washed with PBS for three times. The RNA was extracted from the cells using Trizol reagent. cDNA was obtained with the Evo M-MLV Reverse Transcription Kit (Accurate Biology, Hunan, China) according to the manufacturer’s instructions. Real-time fluorescent quantitative PCR was performed using SYBR Green PCR kit (Accurate Biology, Hunan, China). Relative expression level of EphrinB2, EphB4, RANKL and OPG was quantified using the 2−ΔΔCT method and normalized with GAPDH levels. The primer sequences are listed in . qRT-PCR was performed in a real-time fluorescent quantitative PCR system (LightCycler®96 SW 1.1, Roche Ltd, Switzerland). All samples were performed in triplicate, and each experiment was repeated at least for three times.
Table 1 The Primer Sequences
Western Blot
According to the instructions, the protein in the cells were extracted by using RIPA cleavage buffer (Cwbio, China) mixed with protease inhibitors and phosphatase inhibitors under the ratio of 98:1:1. Protein concentration was determined using the BCA protein assay (Beyotime, China). The proteins were diluted in 1/4 volume of 5×SDS loading buffer and heated at 97°C for 5 minutes. Equal amounts of proteins were separated by 10% SDS-PAGE gel electrophoresis and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% BSA in TBST for 1 hour, and incubated with rabbit anti-EphB4 (1:1000,20,883-1-AP), rabbit anti-EphrinB2 (1:1000, DF7450), rabbit anti-RANKL (1:1000, BC074890), rabbit anti-OPG (1:1000, ab73400), rabbit anti-P-PI3K (1:1000, ab278545), rabbit anti-P-AKT (1:1000, ab192623), rabbit anti-metalloproteinase 9 (1:1000, ab283575) and rabbit anti-cathepsin K (Ctsk) (1:1000, ab184647) overnight at 4°C, respectively. The samples were incubated with horseradish peroxidase-labeled goat anti-rabbit IgG (1:2000, ab6721) at room temperature for 1 hour; Synergistic chemiluminescence reagent (B500024, Proteintech Group, United States). ECL detection system (Amersham Imager 600, General Electric Company, USA) was used to show the immunoreactive bands. Each experiment was repeated at least for three times.
Statistical Analysis
Data was presented as mean ± standard deviation. Statistical analysis was performed using GraphPad Prism 6.0 (GraphPad software, Inc., USA). All analyses were based on more than three separate experiments. Due to the small sample size per group, bootstrap was employed. Detail methodology can be found in the supplementary data 2-6. Differences among three or more groups were tested by one-way analysis of variance (ANOVA), with Fisher’s least significant difference (LSD) test for multiple comparisons. P< 0.05 was considered as significant difference.
Results
ED-71 Increased Bone Mass of Ovariectomized (Ovx) Rats and Decreased the Number of Osteoclasts in vivo
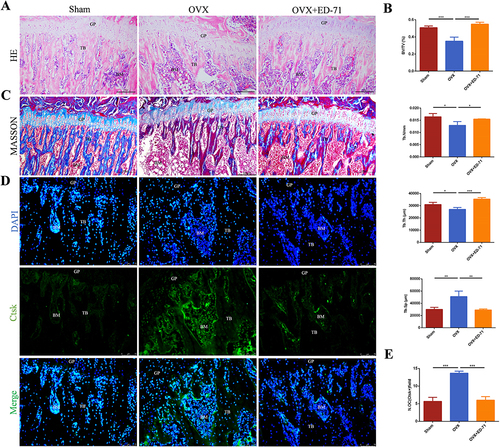
The OVX rat model was established. HE staining showed that compared with the sham group, the bone mass and bone tissue area of the OVX rats were significantly reduced. The bone trabeculae became lesser, scattered, narrower and thinner. However, the tibia bone mass, the number and the thickness of trabecular bone increased after ED-71 treatment ( and ). Masson staining indicated that the amount of new bone was reduced in the OVX group, while the new bone formation was increased in the OVX+ED-71 group, as shown in . Immunofluorescence showed that compared with the Sham group, the number of Ctsk-positive osteoclasts increased in the OVX group, while decreased in the OVX+ED-71 group ( and ). These results demonstrated that ED-71 prevented estrogen deficiency-induced osteoporosis by increasing bone mass and reducing the number of osteoclasts.
Figure 1 ED-71 increases bone mass and decreases the number of osteoclasts in vivo. (A) HE staining of rat femur in Sham, OVX, OVX+ED-71 groups at 8 weeks. Bar, 200μm. (B) The statistical analysis for the BV/TV, Tb.N, Tb.Th and Tb.Sp in the HE staining. (C) Masson staining of rat femur at 8 weeks. Bar, 200μm. (D) The immunofluorescence staining of Ctsk in rat femur at 8 weeks. Bar, 75μm. (E) The statistical analysis of the number of Ctsk-positive osteoclasts. Error bars stand for mean ± SD (n=5). *P < 0.05. **P < 0.01. ***P < 0.001. OVX, ovariectomy; OVX+ED-71, ovariectomy + eldecalcitol; GP, group plate; TB, trabeculae bone; BM, bone marrow; Ctsk, cathepsin K; BV/TV, bone volume/tissue volume; Tb.N, trabecular bone number; Tb.Th, trabecular bone thickness; Tb.Sp, trabeculae bone space; N.Oc, number of Ctsk-positive osteoclasts.
ED-71 Activated EphrinB2-EphB4 Signaling and Decreased RANKL/OPG Ratio in vivo
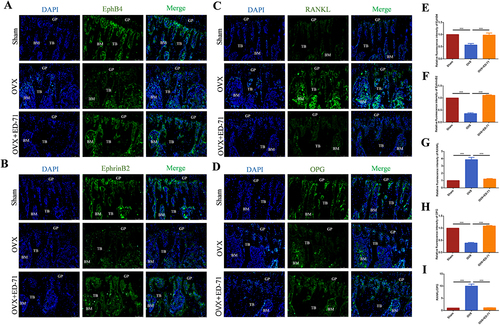
Immunofluorescence showed that the expression of EphrinB2 and EphB4 in the OVX group was reduced compared with the Sham group, while increased in the OVX+ED-71 group ( and ). Expression of RANKL was significantly higher in the OVX group than in the Sham group and OVX+ED-71 group. OPG immunofluorescence showed the opposite tendency ( and ). As shown by , the RANKL/OPG ratio was significantly increased in the OVX group compared with the Sham group, but decreased in the OVX+ED-71 group. These data showed that EphrinB2-EphB4 signaling is inhibited in OVX rats, but ED-71 treatment activates this signaling pathway. The suppressive effect of ED-71 on RANKL/OPG ratio is related to EphrinB2-EphB4 signaling.
Figure 2 ED-71 activates EphrinB2-EphB4 signaling and decreases RANKL/OPG ratio. (A-D) The immunofluorescence staining of EphB4, EphrinB2, RANKL, OPG in rat femur in Sham, OVX, OVX+ED-71 groups at 8 weeks. Bar, 75μm. (E-H) The statistical analysis of fluorescence intensity of EphB4, EphrinB2, RANKL, OPG. (I) Relative ratio of RANKL to OPG in rat femur at 8 weeks. Error bars stand for mean ± SD (n=5). ***P < 0.001.
ED-71 Decreases RANKL/OPG Ratio in Osteoblasts Through EphrinB2-EphB4 Signaling
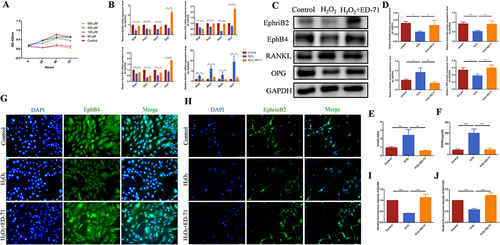
In order to further explore the mechanism of the ED-71 on the ratio of RANKL/OPG, H2O2 stimulation was used in vitro to simulate the cell environment in osteoporosis,Citation27 according to our previous research. CCK8 experiment showed that pretreatment of H2O2 at concentration of 100 μM significantly inhibited the cell proliferation ability, with 50% decrease compared with the control group. 100 μM was therefore selected as the working concentration of H2O2 stimulating (). RT-PCR showed that on the 3rd, 5th, and 7th day, the mRNA expression of EphrinB2, EphB4, and OPG in MC3T3-E1 cells decreased with H2O2 stimulation, compared with in control group. However, these expressions increased significantly with ED-71 treatment. On the contrary, the mRNA expression level of RANKL increased significantly in the H2O2 group, while decreased in the H2O2+ED-71 group (). The same trend was also found with the protein examination ( and ). Similarly, cell immunofluorescence examination showed that in MC3T3-E1 cells, the expression of EphrinB2 and EphB4 in the H2O2 group was reduced relative to the control group, while ED-71 increased their expression ( and ). On day 7 of the osteogenic induction, ED-71 decreased the ratio of RANKL/OPG in protein expression after H2O2 stimulation (). ELISA was used to detect the concentration of RANKL in the culture supernatant of osteoblasts. The results from ELISA assay showed that H2O2 stimulating increased the secretion of RANKL in the osteoblasts, evidenced by the higher concentration of RANKL in the supernatant than that in the control group. However, ED-71 treatment abrogated the effect of H2O2 on the secretion of RANKL in osteoblasts ().
Figure 3 ED-71 promotes the expression of EphrinB2, EphB4 and OPG, and inhibits the expression of RANKL in osteoblasts. (A) Proliferation of MC3T3-E1 cells after 50 μM, 100 μM, 200 μM, 500 μM H2O2 stimulating by CCK-8 assay. (B) The mRNA expression of EphrinB2, EphB4, RANKL and OPG in MC3T3-E1 cells were detected by RT-PCR in Control, H2O2 and H2O2 + ED-71 groups after 3, 5, 7 days of induce. (C) The protein level of EphrinB2, EphB4, RANKL and OPG in MC3T3-E1 cells was detected by Western blot on the day 7 of osteogenic induction. (D) The statistical analysis of Western blot results. (E) Relative ratio of RANKL to OPG in the protein level. (F) The secretion of RANKL in MC3T3-E1 cells was detected by ELISA after 7 days of induce. (G) The immunofluorescence stanning of EphB4 in MC3T3-E1 cells on the day 7 of osteogenic induction. Bar, 50 μm. (H) The immunofluorescence stanning of EphrinB2 in MC3T3-E1 cells on the day 7 of osteogenic induction. Bar, 75 μm. (I and J) The statistical analysis of fluorescence intensity. All experiences were carried out by at least 3 times and data were expressed as mean ± SD. *P<0.05. **P<0.01. ***P<0.001. ns, no significance. H2O2 + ED-71, H2O2 + eldecalcitol.
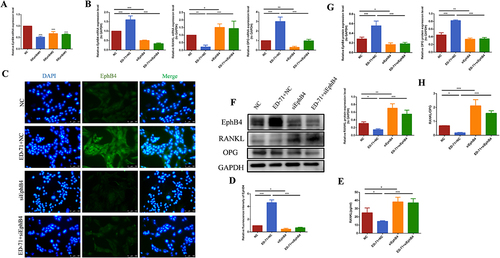
To explore the crosstalk between EphrinB2-EphB4 signaling and RANK/RANKL/OPG, we used EphB4-knockdown cells. Results from RT-PCR showed that cells transfected with siEphB4#1 have the lowest expression level of EphB4 (). At the same time, the cell fluorescence results showed that in the siEphB4-transfected cells, the fluorescence expression of EphB4 was significantly reduced ( and ), also indicating that the use of siRNA has been successful. All groups were stimulated by H2O2, after 7 days of osteogenic induction, results of RT-PCR and Western blot showed that the expression of EphB4 and OPG decreased in siEphB4-transfected cells. Moreover, siEphB4 transfection abrogated the promotive effect of ED-71 on OPG expression and the inhibitory effect on RANKL, as shown by and , which gives rise to an increased RANKL/OPG ratio (). ELISA assay on the expression of RANKL in MC3T3-E1 cells in culture supernatants showed ED-71 inhibited the secretion of RANKL. Nevertheless, the inhibitory effect of ED-71 was reversed in cells transfected with siEphB4, as shown in , which demonstrated that the regulatory effect of ED-71 on RANKL/OPG ratio might be mediated through EphrinB2-EphB4 signal pathway.
Figure 4 The inhibitory effect of ED-71 on RANKL/OPG is reversed by knocking down EphB4 in MC3T3-E1 cells. (A) The effective small interfering RNA was screened by RT-PCR. (B) The mRNA expression of EphB4, RANKL and OPG in MC3T3-E1 cells in NC, ED-71+NC, SiEphB4, and ED-71 + SiEphB4 groups was detected by RT-PCR after 7 days of induce. All groups were stimulated by H2O2. (C) The immunofluorescence staining of EphB4 in MC3T3-E1 cells after siEphB4 transfection. Bar, 100 μm. (D) The statistical analysis of fluorescence intensity. (E) The secretion of RANKL was detected by ELISA after siEphB4 transfection. (F) The protein level of EphB4, RANKL and OPG in MC3T3-E1 cells were detected by Western blot after 7 days of induce. (G) The statistical analysis of Western blot results. (H) Relative ratio of RANKL to OPG in the protein level after the siEphB4 transfection. All experiments were carried out by at least 3 times and data were expressed as mean ± SD. *P<0.05. **P<0.01. ***P<0.001. NC, negative control; ED-71+NC, eldecalcitol+ negative control; SiEphB4, small-interfering EphB4; and ED-71 + SiEphB4, eldecalcitol+ small-interfering EphB4.
ED-71 inhibited osteoclasts differentiation through the EphrinB2-EphB4-RANKL/OPG axis under indirect co-culture conditions
To further investigate the effect of ED-71 on osteoclasts differentiation, we used the culture supernatant of MC3T3-E1 cells as CM. RAW264.3 cells were respectively cultured in CM from the control group, the H2O2 group and H2O2+ED-71 group. Cells were induced differentiation to osteoclasts. On the 7th day, the RT-PCR and Western blot assays showed that both the mRNA and protein expression of CK and MMP9 significantly increased in cells cultured in the CM from the H2O2 group, while decreased in the cells cultured in the CM from the H2O2+ED-71 group ( and ). In order to further explore the mechanism, RAW264.3 cells were cultured in the CM from the NC group, NC+ED-71 group, siEphB4 group and siEphB4+ED-71 group, respectively. The inhibitory effect of ED-71 on Ctsk and MMP9 at mRNA and protein levels was changed in the cells cultured in the CM from the siEphB4+ED-71 group ( and ). Since there was higher concentration of RANKL in the CM from the siEphB4+ED-71 group than from the NC+ED-71 group, it is plausible to conclude that the inhibitory effect of ED-71 on osteoclasts differentiation might be through the EphrinB2-EphB4-RANKL/OPG axis.
Figure 5 ED-71 inhibits osteoclasts differentiation through EphrinB2-EphB4-RANKL axis under indirect coculture conditions. (A) RAW264.7 cells were cocultured indirectly with CM containing the culture supernatant of MC3T3-E1 cells in Control, H2O2 and H2O2 + ED-71 groups for 7 days. The mRNA expression of MMP9 and Ctsk was detected by RT-PCR. (B) RAW264.7cells were cocultured indirectly with CM containing the culture supernatant of MC3T3-E1 cells in Control, H2O2 and H2O2 + ED-71 groups for 7 days. The protein level of MMP9 and Ctsk was detected by Western blot. (C) The statistical analysis of Western blot results. (D) RAW264.7 cells were cocultured indirectly with CM containing the culture supernatant for MC3T3-E1 cells in NC, ED-71+NC, SiEphB4, and ED-71 + SiEphB4 groups for 7 days. The mRNA expression of MMP9 and Ctsk in RAW264.7 cells were detected by RT-PCR. (E) The protein level of MMP9 and Ctsk in RAW264.7 cells were detected by Western blot. (F) The statistical analysis of Western blot results. All experiences were carried out by at least 3 times and data were expressed as mean ± SD.*P<0.05. **P<0.01. ***P<0.001. CM, conditioned medium; MMP9, Matrix metalloproteinase-9.
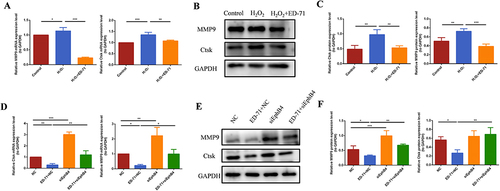
ED-71 Inhibited RANKL/OPG Ratio Through PI3K/AKT Pathway
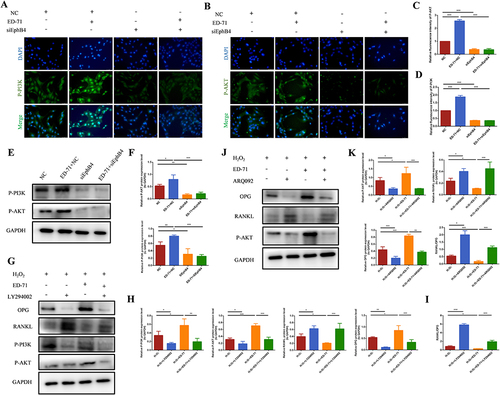
The Western blot results showed that after adding ED-71, the protein expression of phosphorylated PI3K and AKT was increased in osteoblasts ( and ). The results of cell fluorescence showed the same trend ( and ), suggesting the activation of PI3K/AKT pathway by ED-71. SiEphB4 transfected cells or PI3K/AKT inhibitors were then used to delineate the crosstalk between EphrinB2-EphB4 and PI3K/AKT signaling pathway. Cell immunofluorescence experiment showed that compared with the cells in NC+ED-71 group, the fluorescence expression of P-PI3K and P-AKT was significantly weakened in the cells from the siEphB4+ED-71 group ( and ), indicating that EphrinB2-EphB4 might activate the PI3K/AKT signaling pathway, and the activation of PI3K/AKT pathway by ED-71 was reversed when EphB4 was knocked down. The same changing profile was also seen in Western blot analysis ( and ). As shown by and 71 induced upregulation of OPG, P-PI3K and P-AKT and downregulation of RANKL in the presence of H2O2. However, the increased expression of OPG, P-PI3K and P-AKT was partially abrogated in the presence of PI3K inhibitor LY294002. Moreover, PI3K inhibitor increased the expression of RANKL, resulting in the upregulation of the ratio of RANKL/OPG. The same expression profile of OPG, P-AKT and RANKL was also found with AKT inhibitor ARQ092 treatment. As shown by and , ARQ092 abrogated the effect of ED-71 on the expression of RANKL, OPG and P-AKT. The data demonstrated that EphB4 inhibited the ratio of RANKL/OPG through the PI3K/AKT pathway, thereby mediating the process of osteoclastogenesis inhibited by ED-71.
Figure 6 ED-71 activates the PI3K/AKT pathway. (A) The protein level of P-PI3K and P-AKT in MC3T3-E1 cells in Control, H2O2 and H2O2 + ED-71 groups were detected by Western blot after osteogenic induction in 7 days. (B) The statistical analysis of Western blot results of P-PI3K and P-AKT. (C and D) The immunofluorescence staining of P-PI3K and P-AKT in MC3T3-E1 cells on the 7th day of osteogenic induction. Bar, 75 μm. (E and F) The statistical analysis of fluorescence intensity. All experiences were carried out by at least 3 times and data were expressed as mean ± SD. *P<0.05. **P<0.01. ***P<0.001.
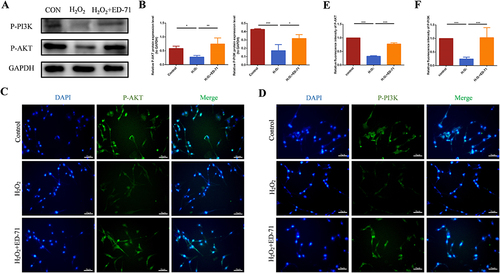
Figure 7 ED-71 decreases the RANKL/OPG ratio through the EphB4-PI3K/AKT axis. (A and B) The immunofluorescence staining of P-PI3K and P-AKT in MC3T3-E1 cells after SIEphB4 induction. Bar, 75 μm. (C and D) The statistical analysis of fluorescence intensity in MC3T3-E1 cells in NC, ED-71+NC, SiEphB4, and ED-71 + SiEphB4 groups. All groups were stimulated by H2O2 for 2 hours. (E) The protein level of P-PI3K and P-AKT in MC3T3-E1 cells were detected by Western blot. (F) The statistical analysis of Western blot results. (G) The protein level of P-PI3K, P-AKT, RANKL and OPG in MC3T3-E1 cells after being treated with LY294002(PI3K inhibitor) on the day 7 of osteogenic induction. (H) The statistical analysis of Western blot results in MC3T3-E1 cells in H2O2, H2O2+ LY294002, H2O2 + ED-71and H2O2 + ED-71+ LY294002 groups. (I) Relative ratio of RANKL to OPG in the protein level. (J) The protein level of P-AKT, RANKL and OPG in MC3T3-E1 cells after being treated with ARQ092 (AKT inhibitor) on the day 7 of osteogenic induction. (K) The statistical analysis of the P-AKT, RANKL and OPG Western blot results and relative ratio of RANKL to OPG in the protein level in MC3T3-E1 cells in H2O2, H2O2+ ARQ092, H2O2 + ED-71 and H2O2 + ED-71+ ARQ092 groups. All experiences were carried out by at least 3 times and data were expressed as mean ± SD. *P<0.05. **P<0.01. ***P<0.001. H2O2+ LY294002, H2O2+ PI3K inhibitor; H2O2 + ED-71+ LY294002, H2O2+ ED-71+PI3K inhibitor; H2O2+ ARQ092, H2O2+ AKT inhibitor; H2O2 + ED-71+ ARQ092, H2O2+ ED-71+ AKT inhibitor.
Discussion
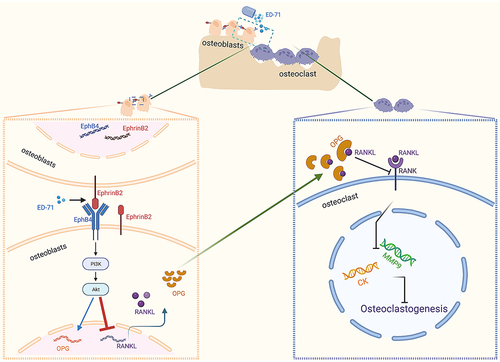
In this study, we investigated the effect of ED-71 on bone mass and the expression of osteoclastogenesis related factors in OVX rat. We also used H2O2 treated cells as in vitro model to explore the possible mechanism by which ED-71 exert the beneficial effects. The results of the study were as following: firstly, ED-71 inhibited osteoclasts differentiation and increased bone mass in OVX rats. In vivo study also showed that ED-71 application activated EphrinB2-EphB4 signaling and decreased RANKL/OPG ratio. Secondly, ED-71 decreased the ratio of RNAKL/OPG by activating EphrinB2-EphB4 signaling pathway in osteoblasts. Thirdly, in indirect culture system, ED-71 inhibited osteoclasts differentiation through the EphrinB2-EphB4-RANKL/OPG axis. Interestingly, as a downstream pathway of EphrinB2-EphB4, PI3K/AKT signaling was highly associated with the inhibitory effect of ED-71 on the ratio RANKL/OPG ().
Figure 8 Pattern Diagram: ED-71 activates the PI3K/AKT pathway through EphrinB2-EphB4 signal to inhibit the expression of RANKL. Created by BioRender.com.
The main cause of postmenopausal osteoporosis is that estrogen deficiency increases the activity of osteoclasts and bone resorption.Citation30 ED-71, as an active vitamin D analogue, has been widely used in the clinical treatment of postmenopausal osteoporosis due to the inhibition of bone resorption.Citation31 In order to explore the effect of ED-71 on osteoclasts, we established the postmenopausal osteoporosis model in rats based on our previous report.Citation26 Our results showed that the tibial bone mass and the new bone formation was reduced, indicating the successful establishment of the model. The postmenopausal osteoporosis is found to be in a state of oxidative stress.Citation32 We used H2O2 stimulated MC3T3-E1 cells as in vitro model, simulating those growing in osteoporosis environment.Citation26 The results showed that H2O2 increased osteoclast generation, as well as the protein and mRNA expression of CtsK and MMP9, both are related to the ability of osteoclast to undergo bone resorption.
Activation of EphrinB2-EphB4 during cell-cell contact may positively or negatively affect the activity of osteoblasts or osteoclasts.Citation33 Interestingly, our results have shown that ED-71 increased the expression of EphrinB2 and EphB4 in osteoblasts. ED-71 plays a potential role in regulating the forward signal generated by the expression of EphrinB2 and EphB4 in osteoblasts. In addition, ED-71 promoted H2O2-inhibited expression of OPG and suppressed the protein and mRNA expression of RANKL in osteoblasts. RANKLRANK/RANKL/OPG, as an important signaling pathway involved in osteoclast formation,Citation34 plays an important role in bone metabolism.Citation35 In the 12-week-old ovariectomized mice, daily administration of ED-71 increases bone mineral density and inhibits osteoclastic differentiation by suppressing RANKL expression.Citation36 After adding ED-71 in osteoblasts, the secretion of RANKL was increased. When indirectly RAW264.7 cells co-cultured with the supernatant of MC3T3-E1 cells, the formation of osteoclasts and the expression of MMP9 and cathepsin K was significantly inhibited. Osteoclastic differentiation is suppressed by decreasing accumulation of RANKL. Further studies are needed to investigate the role of EphB4 on RANKL expression in ED-71-treated osteoporosis.
Our results and some studies revealed the connection between EphB4 and RANKL. Studies have shown that inhibiting EphB4 in osteoblasts could partly increase the RANKL secretion and promote the differentiation of osteoclasts.Citation14,Citation29 In the model of aseptic loosening, the recombinant protein EphB4 regulated the ratio of RANKL/OPG.Citation37 Our results showed the inhibitory effect of ED-71 on the secretion of RANKL and osteoclastogenesis was abrogated by EphB4 siRNA transfection. Collectively, these results revealed the role of the interactions between EphB4 and RANKL during the process of ED-71 on suppressing osteoclastogenesis. ED-71 regulates the bone homeostasis by EphrinB2-EphB4-RANKL/OPG axis.
A variety of important pathways were involved in the pathogenesis of osteoporosis, among which PI3K/AKT has been reported to regulate the differentiation of osteoblasts and osteoclasts in osteoporosis.Citation17,Citation38 Interestedly, the expression of P-PI3K and P-AKT was increased after the application of ED-71. PI3K/AKT, as an important signaling pathway, is related to EphB4 or RANKL. Many studies have shown that EphB4 influences cell growth and proliferation by regulating PI3K/AKT.Citation18,Citation39 In the present study, we found siEphB4 transfection abrogated ED-71-induced augmentation of P-PI3K and P-AKT, suggesting ED-71 may increase P-PI3K and P-AKT expression via EphrinB2-EphB4 pathway. In addition, in a high-glucose inflammatory environment, C-reactive protein promoted the expression of RANKL in periodontal stem cells by inhibiting PI3K/ART pathway.Citation40 Our results are similar to this study. After application of P-PI3K and P-AKT inhibitor LY294002 or ARQ092, the inhibitory effect of ED-71 on the secretion of RANKL in osteoblasts was reduced. There is no evidence regarding the mechanism of EphB4 on regulating RANKL. In the present study, EphB4 regulated the RANKL/OPG ratio via PI3K/AKT signaling pathway in ED-71-treated osteoporosis, which filled the gap in research on this aspect.
In addition, as previously mentioned, the interaction of EphrinB2 and EphB4 in osteoblasts plays an important role in regulating the maturation of osteoblasts and inhibiting the differentiation of osteoclasts.Citation13 In the present study, ED-71 could increase EphrinB2 and EphB4 in osteoblasts under the action of H2O2, and inhibited osteoclastogenesis by EphrinB2-EphB4-RANKL/OPG axis. However, it is not clear whether ED-71 regulates the function and activity of osteoblasts through EphrinB2-EphB4 pathway, it also will be explored in the future.
In summary, our current study provide evidence that ED-71 suppresses osteoclastogenesis through the juxtacrine (EphB4/EphrinB2) and paracrine (RANKL/RANK/OPG) signaling pathways, as well as the synergy of the two pathways between osteoclasts and osteoblasts. In osteoporosis, ED-71 regulates the bone homeostasis by EphrinB2-EphB4-RANKL/OPG axis. This process was achieved by the regulation of PI3K/AKT signaling pathway. This finding clarified the specific mechanism of ED-71 increasing bone mass in OVX rats. However, it still needs further investigation for the clinical use of ED-71 for clinical treatment of osteoporosis.
Ethics Approval
All animal experiments in this study were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of the National Institutes of Health. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC), School and Hospital of Stomatology, Shandong University (Approval No. 20230502).
Author Contributions
All authors made contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation. All authors took part in drafting, revising or critically reviewing the article, and gave final approval of the version to be published. All authors have agreed on the journal to which the article has been submitted, and agreed to be accountable for the contents of the article.
Disclosure
The authors declare that they have no conflicts of interest in this work.
Additional information
Funding
References
- Lane JM, Russell L, Khan SN. Osteoporosis. Clin Orthopaedics Related Res. 2000;372(372):139–150. doi:10.1097/00003086-200003000-00016
- Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301(5):513–521. doi:10.1001/jama.2009.50
- Gosset A, Pouillès JM, Trémollieres F. Menopausal hormone therapy for the management of osteoporosis. Best Pract Res Clin Endocrinol Metab. 2021;35(6):101551. doi:10.1016/j.beem.2021.101551
- Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol Metab. 2012;23(11):576–581. doi:10.1016/j.tem.2012.03.008
- Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142:155–170. doi:10.1016/j.jsbmb.2013.09.008
- Shieh A, Ishii S, Greendale GA, et al. Urinary N-telopeptide and rate of bone loss over the menopause transition and early postmenopause. J Bone Miner Res. 2016;31(11):2057–2064. doi:10.1002/jbmr.2889
- Cauley JA, Danielson ME, Greendale GA, et al. Bone resorption and fracture across the menopausal transition: the study of women’s health across the nation. Menopause. 2012;19(11):1200–1207. doi:10.1097/gme.0b013e31825ae17e
- Wu H, Hu B, Zhou X, et al. Artemether attenuates LPS-induced inflammatory bone loss by inhibiting osteoclastogenesis and bone resorption via suppression of MAPK signaling pathway. Cell Death Dis. 2018;9(5):498. doi:10.1038/s41419-018-0540-y
- Taylor S, Hu R, Pacheco E, et al. Differential time-dependent transcriptional changes in the osteoblast lineage in cortical bone associated with sclerostin antibody treatment in ovariectomized rats. Bone Rep. 2018;8:95–103. doi:10.1016/j.bonr.2018.03.002
- Kim JM, Lin C, Stavre Z, et al. Osteoblast-osteoclast communication and bone homeostasis. Cells. 2020;9(9):2073. doi:10.3390/cells9092073
- Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473(2):139–146. doi:10.1016/j.abb.2008.03.018
- Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9(Suppl 1(Suppl 1)):S1. doi:10.1186/ar2165
- Martin TJ, Allan EH, Ho PW, et al. Communication between ephrinB2 and EphB4 within the osteoblast lineage. Adv Exp Med Biol. 2010;658:51–60.
- Takyar FM, Tonna S, Ho PW, et al. EphrinB2/EphB4 inhibition in the osteoblast lineage modifies the anabolic response to parathyroid hormone. J Bone Miner Res. 2013;28(4):912–925. doi:10.1002/jbmr.1820
- He Y, Sun MM, Zhang GG, et al. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6(1):425. doi:10.1038/s41392-021-00828-5
- Huang X, Liu G, Guo J, et al. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. 2018;14(11):1483–1496. doi:10.7150/ijbs.27173
- Ma Y, Ran D, Zhao H, et al. Cadmium exposure triggers osteoporosis in duck via P2X7/PI3K/AKT-mediated osteoblast and osteoclast differentiation. Sci Total Environ. 2021;750:141638. doi:10.1016/j.scitotenv.2020.141638
- Zhu M, Shi X, Gong Z, et al. Cantharidin treatment inhibits hepatocellular carcinoma development by regulating the JAK2/STAT3 and PI3K/Akt pathways in an EphB4-dependent manner. Pharmacol Res. 2020;158:104868. doi:10.1016/j.phrs.2020.104868
- Reid IR, Billington EO. Drug therapy for osteoporosis in older adults. Lancet. 2022;399(10329):1080–1092. doi:10.1016/S0140-6736(21)02646-5
- LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022;33(10):2049–2102.
- Miyamoto K, Murayama E, Ochi K, et al. Synthetic studies of vitamin D analogues. XIV. Synthesis and calcium regulating activity of vitamin D3 analogues bearing a hydroxyalkoxy group at the 2 beta-position. Chem Pharm Bull. 1993;41(6):1111–1113. doi:10.1248/cpb.41.1111
- Okano T, Tsugawa N, Masuda S, et al. Regulatory activities of 2 beta-(3-hydroxypropoxy)-1 alpha, 25-dihydroxyvitamin D3, a novel synthetic vitamin D3 derivative, on calcium metabolism. Biochem Biophys Res Commun. 1989;163(3):1444–1449. doi:10.1016/0006-291X(89)91140-6
- Uchiyama Y, Higuchi Y, Takeda S, et al. ED-71, a vitamin D analog, is a more potent inhibitor of bone resorption than alfacalcidol in an estrogen-deficient rat model of osteoporosis. Bone. 2002;30(4):582–588. doi:10.1016/S8756-3282(02)00682-8
- Kubodera N, Tsuji N, Uchiyama Y, et al. A new active vitamin D analog, ED-71, causes increase in bone mass with preferential effects on bone in osteoporotic patients. J Cell Biochem. 2003;88(2):286–289. doi:10.1002/jcb.10346
- Arifin WN, Zahiruddin WM. Sample size calculation in animal studies using resource equation approach. Malays J Med Sci. 2017;24(5):101–105. doi:10.21315/mjms2017.24.5.11
- Kou Y, Rong X, Tang R, et al. Eldecalcitol prevented OVX-induced osteoporosis through inhibiting BMSCs senescence by regulating the SIRT1-Nrf2 signal. Front Pharmacol. 2023;14:1067085. doi:10.3389/fphar.2023.1067085
- Zhang Y, Kou Y, Yang P, et al. ED-71 inhibited osteoclastogenesis by enhancing EphrinB2-EphB4 signaling between osteoclasts and osteoblasts in osteoporosis. Cell Signal. 2022;96:110376. doi:10.1016/j.cellsig.2022.110376
- Okamura H, Yang D, Yoshida K, et al. Protein phosphatase 2A Cα is involved in osteoclastogenesis by regulating RANKL and OPG expression in osteoblasts. FEBS Lett. 2013;587(1):48–53. doi:10.1016/j.febslet.2012.10.041
- Wang R, Luo H, Yang D, et al. Osteoblast Jmjd3 regulates osteoclastogenesis via EphB4 and RANKL signalling. Oral Dis. 2023;29(4):1613–1621. doi:10.1111/odi.14160
- Väänänen HK, Härkönen PL. Estrogen and bone metabolism. Maturitas. 1996;23(Suppl):S65–69. doi:10.1016/0378-5122(96)01015-8
- Matsumoto T, Miki T, Hagino H, et al. A new active vitamin D, ED-71, increases bone mass in osteoporotic patients under vitamin D supplementation: a randomized, double-blind, placebo-controlled clinical trial. J Clin Endocrinol Metab. 2005;90(9):5031–5036. doi:10.1210/jc.2004-2552
- Zhao F, Guo L, Wang X, et al. Correlation of oxidative stress-related biomarkers with postmenopausal osteoporosis: a systematic review and meta-analysis. Arch Osteoporos. 2021;16(1):4. doi:10.1007/s11657-020-00854-w
- Arthur A, Gronthos S. Eph-Ephrin signaling mediates cross-talk within the bone microenvironment. Front Cell Dev Biol. 2021;9:598612. doi:10.3389/fcell.2021.598612
- Udagawa N, Koide M, Nakamura M, et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab. 2021;39(1):19–26. doi:10.1007/s00774-020-01162-6
- Yasuda H. Discovery of the RANKL/RANK/OPG system. J Bone Miner Metab. 2021;39(1):2–11. doi:10.1007/s00774-020-01175-1
- Harada S, Mizoguchi T, Kobayashi Y, et al. Daily administration of eldecalcitol (ED-71), an active vitamin D analog, increases bone mineral density by suppressing RANKL expression in mouse trabecular bone. J Bone Miner Res. 2012;27(2):461–473. doi:10.1002/jbmr.555
- Ge YW, Feng K, Liu XL, et al. The recombinant protein EphB4-Fc changes the ti particle-mediated imbalance of OPG/RANKL via EphrinB2/EphB4 signaling pathway and inhibits the release of proinflammatory factors in vivo. Oxid Med Cell Longev. 2020;2020:1404915. doi:10.1155/2020/1404915
- Abdurahman A, Li X, Li J, et al. Loading-driven PI3K/Akt signaling and erythropoiesis enhanced angiogenesis and osteogenesis in a postmenopausal osteoporosis mouse model. Bone. 2022;157:116346. doi:10.1016/j.bone.2022.116346
- Shi X, Zhu M, Gong Z, et al. Homoharringtonine suppresses LoVo cell growth by inhibiting EphB4 and the PI3K/AKT and MAPK/EKR1/2 signaling pathways. Food Chem Toxicol. 2020;136:110960. doi:10.1016/j.fct.2019.110960
- Zhou M, Xu X, Li J, et al. C-reactive protein perturbs alveolar bone homeostasis: an experimental study of periodontitis and diabetes in the rat. J Clin Periodontol. 2022;49(10):1052–1066. doi:10.1111/jcpe.13667

