Abstract
Spinal cord stimulation (SCS) applications and technologies are fast advancing. New SCS technologies are being used increasingly in the clinical environment, but often there is a lag period between the clinical application and the publishing of high-quality evidence on safety and efficacy. Recent developments will undoubtedly expand the applicability of SCS, allowing more effective and individualized treatment for patients, and may have the potential to salvage patients who have previously failed neuromodulation. Already, high-level evidence exists for the safety, efficacy, and cost-effectiveness (Level I–II) of traditional SCS therapies in the treatment of chronic refractory low back with predominant limb pain (regardless of surgical history). More than half of all patients with chronic painful conditions experience sustained and significant levels of pain reduction following SCS treatment. Although only limited evidence exists for burst stimulation, there is now Level I evidence for both dorsal root ganglion SCS and high-frequency SCS that demonstrates compelling results compared with traditional therapies. The body of evidence built on traditional SCS research may be redundant, with newer iterations of SCS therapies such as dorsal root ganglion SCS, high-frequency SCS, and burst SCS. A number of variables have been identified that can affect SCS efficacy: implanter experience, appropriate patient selection, etiologies of patient pain, existence of comorbidities, including psychiatric illness, smoking status, and delay to SCS implant following pain onset. Overall, scientific literature demonstrates SCS to be a safe, effective, and drug-free treatment option for many chronic pain etiologies.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction to chronic pain
Chronic pain affects up to 20% of the population in developed nations.Citation1–Citation4 This represents a profound impact on individuals and their families alongside the sizeable burden on employers, health care systems, and society in general.Citation3 When chronic pain occurs, it has the potential to become disease itself, and subsequently, chronic pain has emerged as a distinct phenomenon.Citation5
Management of chronic pain varies greatly between nations and even within nations. Literature supports a multidisciplinary approach as the standard of care, although various health care systems may not always support this concept consistently.Citation2
The current standard of care for chronic, noncancer pain typically includes many disciplines with the clinician developing an individualized treatment plan with the options of utilizing surgical interventions, pharmacology, and psychological and physical therapies. Opioid analgesics are often prescribed, despite the lack of clinical evidence supporting their long-term use in the management of chronic pain.Citation6 However, for many patients, this multidisciplinary approach is inadequate or ineffectual or is accompanied by the burden of side effects that are unacceptable and debilitating. Only at this late stage, referral for advanced neuromodulation techniques is considered and potentially trialed.
Neuromodulation
The field of neuromodulation for the treatment of pain has developed rapidly since the seminal paper on the electrical inhibition of pain by the stimulation of the dorsal column almost 50 years ago.Citation7 The original term of dorsal column stimulation has evolved to become known as spinal cord stimulation (SCS).Citation8 SCS has been particularly effective as an adjunct in treating mixed neuropathic/nociceptive and neuropathic/radicular pain conditions such as failed back surgery syndrome (FBSS) and complex regional pain syndrome (CRPS). Neuromodulation therapies offer a treatment option that has minimal side effects and that is relatively safe and potentially reversible.Citation9 SCS has been used to treat various pain conditions for many decades.Citation8,Citation10–Citation13
In traditional SCS therapies, the objective has been to replace the pain sensation with paresthesia that requires mapping of stimulation to the region of pain.Citation14 The anticipation is that the electrical current alters pain processing by masking the sensation of pain with a comfortable tingling or paresthesia. Although patients mostly cope with paresthesia, a significant proportion report that the sensation is unpleasant, particularly with positional changes.
The stimulation is provided either through electrodes that are placed percutaneously into the epidural space or through a surgical paddle lead that is delivered via a laminotomy.Citation8 These devices are capable of delivering pulse frequencies in the range of 2–1,200 Hz but are regularly utilized at 40–60 Hz.
Patients typically undergo a trial of neuromodulation with an externalized power source and if this trial proves to be positive and compelling, they subsequently have a subcutaneously implantable pulse generator for the long-term therapy.
In recent years, the next phase in the evolution of neuromodulation has become available with the development of dorsal root ganglion (DRG) SCS and the emerging use of two novel advances in stimulation frequencies, being high-frequency SCS (at 10,000 Hz) and burst SCS.Citation14–Citation19 These recent advances have improved the efficacy and expanded the applicability of SCS.
DRG SCS is a highly targeted form of neuromodulation therapy.Citation20 Studies indicate that the DRG plays a key role in both nociceptive and neuropathic pain.Citation14,Citation21 DRG SCS is particularly useful in treating focal areas of pain, in particular those that have been difficult to target with traditional SCS systems such as groin and foot pain, by applying an innovative lead configuration and delivery system around the DRG.Citation8,Citation9,Citation15
High-frequency 10 SCS (HF10) presents a significant development in the evolution of SCS technologies.Citation19 This involves application of a unique waveform at 10,000 Hz at a subthreshold level and therefore provides pain relief without any paresthesia.Citation18,Citation22 The majority of patients have a clear preference for paresthesia-free stimulation, and HF10 has been approved for clinical use in Australia and Europe since 2011 and has received Food and Drug Administration approval for the United States in 2015 for patients with chronic refractory pain of the trunk and/or limbs.Citation22,Citation23
Burst SCS offers another novel mode of stimulation whereby conventional frequency parameters are provided in bursts of five pulses. The burst frequency is 40 Hz, and the pulse frequency is 500 Hz. Amplitude is reduced to try and achieve subthreshold stimulation, thereby providing pain relief with either reduced or no paresthesia.Citation16,Citation17,Citation24
Efficacy
The most recent systematic and comprehensive review of the effectiveness of SCS in treating chronic spinal pain demonstrated that there is a significant (Level I–II) evidence for SCS as a treatment for lumbar FBSS, where conventional medical management has failed.Citation23 Furthermore, there is now Level I evidence for high-frequency stimulation but only limited evidence for burst stimulation.Citation23
In another recent and extensive review and meta-analysis of conventional SCS, more than half of all patients experienced significant pain relief.Citation25 The authors observed that this was maintained for a mean follow-up period of 24 months.Citation25 These reviews demonstrate that traditional SCS is an effective treatment option for a cohort that is notoriously difficult to treat.
The existing SCS literature has a large number of case series reports and only a limited number of high-quality, large prospective, consecutively recruited, randomized, or controlled comparative trials ().Citation8,Citation23,Citation25 Furthermore, the literature, when viewed historically, must be tempered by the developments in skills, application, and technological advances.Citation26 Hence, the traditional SCS papers have often reported successful pain relief as an undifferentiated generic pain that is not specific to the site of the primary or greatest pain (eg, back or leg).Citation25 This observation is important because conventional SCS therapy has historically been prescribed for limb pain and has had only limited success in managing back pain.Citation10,Citation27–Citation29 Indeed, predominant back pain has been an exclusionary factor in many studies.Citation25 Recent studies that have included back pain as the primary source have involved HF10 therapy at 10,000 Hz; this therapy has evolved to better capture significant back, leg, and radicular pain.Citation10,Citation22
Table 1 Selection of SCS literature with focus on back ± lower limb pain studies
Tolerance to SCS has been observed in patients where pulse amplitude needs to be increased to achieve the same analgesic benefit over time and/or efficacy has been lost.Citation30,Citation31 Tolerance cannot be predicted, and although the rate has not been widely reported in the literature, one study that researched over 10 years found it to be in the order of 29%.Citation32 Possible causes for stimulation tolerance include neuroplasticity of pain transmission pathways, cellular or fibrotic changes in the tissues around the electrodes, patients reframing their pain over time, and psychological or psychiatric affective disorders.Citation30 However, data pertaining to HF10 SCS have demonstrated no tolerance at this point.Citation22
Despite strict criteria for patient selection, a substantial number of patients fail to achieve optimal pain relief with SCS.Citation30,Citation33 A number of factors have been identified as possible indicators for treatment failure including tobacco and drug use, age, and lengthy delay between times of original pain onset to SCS implant.Citation30,Citation33
Food and Drug Administration requirements for labeling the recent advanced iterations of SCS systems have led, for the first time, to Level I noninferiority comparative studies being undertaken to achieve labeling.
DRG SCS has been demonstrated as effective in multiple etiologies, including FBSS, CRPS, and chronic postsurgical pain.Citation15 A recent study reported 1 year outcomes for DRG with overall pain scores reducing from 77.6 to 33.6 (P<0.005). Back pain reduced from 74.5 to 39.7 (P<0.05), and leg pain reduced from 74.6 to 28.7 (P<0.0005). The most compelling pain reduction happened for foot pain with scores reducing from 81.4 to 22.0 (P<0.05).Citation15 Approximately 60% of the DRG SCS patients reported >50% improvement in their pain, and the pain localized to the back, legs, and feet was reduced by 42%, 62%, and 80%, respectively.Citation15 Other outcome parameters including quality of life, mood, and satisfaction were improved and maintained throughout the 12 months.Citation15
The Accurate study is a US pivotal, noninferiority, randomized controlled trial (RCT) between DRG SCS and traditional SCS Medtronic system (Medtronic, Inc., Fridley, MN, USA). It is the largest RCT in the history of CRPS and causalgia, running from 2013 with primary completion estimated for 2018. The sample size for the study is 152; with 76 randomized to DRG SCS and 76 to the control arm using Medtronic traditional SCS. The inclusion criterion was leg pain for more than 6 months duration with a visual analog scale (VAS) score >6/10.Citation34
In the intention-to-treat analysis, superiority was demonstrated in the DRG SCS group with 81% of patients achieving >50% pain reduction and meeting the primary endpoint at the 3-month mark, and 74% maintaining that primary endpoint at 12-month follow-up. The traditional SCS arm demonstrated 56% of patients having >50% pain reduction at 3 months and 53% maintaining this through 12 months.Citation34
In the subset analysis of the implant-only group, the data were even more impressive with 93% of the DRG SCS group meeting the primary endpoint at 3 months and 86% at 12 months. The traditional SCS arm had 72% meeting the primary endpoint and 70% at 12 months.Citation34 The statistical analysis demonstrated noninferiority and, beyond that, superiority in the intention-to-treat, modified intention-to-treat, and implant-only groups. Furthermore, it was noted that 70% of patients achieved >80% pain reduction in the DRG group versus 52% in the Medtronic group. Target specificity for stimulation and pain relief was achieved in 94.5% of the DRG group and 61% of the Medtronic group.Citation34
The Sunburst study is set to run from 2013, with primary completion in 2016. It is a prospective randomized, non-inferiority controlled trial with the St Jude Medical Company (St Jude Medical, Inc. St Paul, MN, USA). Patients with intractable pain were randomized for the order they would receive either traditional or burst SCS. The study was performed with a one-to-one crossover at 12 weeks to the alternate therapy. The outcome measures included safety, effectiveness, and non-inferiority. The study was applied to an enriched cohort with patients who required to have pre-existing pain scores >6/10 and a >50% pain reduction in a traditional SCS trial using tonic stimulation. The sample size for the study was 121 with 100 people randomized. The sample size reported to date is 85 with a 24-week follow-up. The mean age of patients was 59 years, with a median duration of pain being 13 years.Citation35
The trial demonstrated noninferiority, and further statistical analysis demonstrated superiority for burst stimulation over tonic stimulation (P=0.035).Citation35 The mean difference in burst pain reduction compared with tonic stimulation was 6 mm VAS points. This difference, while being statistically significant, does not meet the well-defined criteria for minimal clinical important difference.Citation36
Approximately 65% of the burst cohort experienced paresthesia-free stimulation and 69% of the cohort chose a preference for burst, with the majority of these having their preference related to no paresthesia, more so than better pain reduction.Citation34
The Senza RCT is a Level I study design run from 2012 with an estimated primary completion in 2015.Citation22,Citation37 This is the first-ever RCT of two SCS therapies with patients randomized to HF10 SCS (Senza System; Nevro Corp., Redwood City, CA, USA) or traditional SCS commercially available, Precision Plus, SCS system (Boston Scientific Corporation, Marlborough, MA, USA). It is a noninferiority study with the statistical capability of demonstrating superiority supervised by the Food and Drug Administration, and patients were monitored and programmed by the technicians associated with the respective devices. One hundred and ninety-eight patients were randomized with 101 to the HF10 SCS group and 97 to traditional. Of these, 90 HF10 SCS patients and 81 traditional SCS patients were subsequently implanted.
The primary endpoint of >50% back pain reduction at 3 months was achieved in 80.9% of the HF10 SCS group versus 42.5% of the traditional SCS group.Citation37 This met the criteria for noninferiority and statistical superiority (P<0.001). Furthermore, at 12 months, this primary endpoint was met in 78.7% versus 51.3% of the patients.
Similarly, the primary endpoint for leg pain reduction was met in 80.0% of the HF10 SCS group versus 49.4% of the traditional SCS group.Citation37 The responder rates for >50% leg pain reduction at 3 months was 83.1% in the HF10 SCS group and 55.0% in the traditional SCS group. The 12-month outcome data for the same groups were 78.7% versus 51.3% (superiority P value, P<0.001).Citation37 This study demonstrated superiority of HF10 SCS to traditional SCS in all primary and secondary endpoints that has led to the labeling of HF10 therapy as superior to traditional low-frequency SCS by the Food and Drug Administration.
In further analysis of pain etiology, it was demonstrated that for the conditions of FBSS, radiculopathy, degenerative disc disease, and spondylosis, the relative ratio of patients meeting the primary endpoint with HF10 SCS was approximately 2.0 times the traditional SCS (eg, FBSS 85.7% versus 41.4%).Citation37
Further subset analysis of patients who achieved VAS pain scores of ≤2.5 showed that for those with back pain, relief was maintained for 12 months 68.5% of the time for HF10 SCS, but only 35.8% of the time using traditional SCS. Whereas, for those with leg pain it was achieved 67.4% of the time for HF10 SCS versus 42.5% of the time with traditional SCS. Superiority P-values for HF10 were significant (P<0.001).Citation37
These data demonstrate compelling evidence for treating complex back pain that was previously unheralded in the literature. Furthermore, paresthesia-free options allow the patient to keep stimulation on potentially 24 hours a day and hence sleep with the stimulator on and also perform activities such as driving.
Economical or cost efficiency
Health care policy and funding decisions require evidence of clinical efficacy and information around cost-effectiveness of treatments.Citation38,Citation39 Consequently, there have been a number of studies considering the economic factors associated with SCS. Most recently, a 2015 study investigating the cost-effectiveness of conventional medical management with or without SCS in patients with FBSS compared a summary of the total direct and indirect costs incurred in the 12 months prior and 24 months following SCS.Citation40 The costs were scaled to values of €2,009. The total pre-SCS treatment costs were equivalent to €6,567/patient-year. The year of implant incurred a significant increase in costs of €20,902/patient-year, mainly attributed to the high cost of the SCS devices. In the following 12–14 months, SCS implant had dropped to €5,430/patient-year.Citation40 Applying the current United Kingdom National Health Service threshold (intervention considered not cost-effective if the incremental cost-effectiveness ratio is higher than €45,000/quality-adjusted life year [QALY]), SCS with conventional medical management would be considered cost-effective around 40% of the time.Citation40 However, if the willingness-to-pay threshold was shifted to €60,000/QALY, SCS would be considered cost-effective by the National Health Service with an average of 80% of the time.
In 2013, a study developed models to evaluate the cost-effectiveness of SCS and conventional medical management together compared with conventional medical management alone for patients with FBSS and CRPS.Citation41 Health effects were expressed as QALYs and costs were expressed as Canadian dollars (CAN$) scaled to 2012. The models were extrapolated over a 20 year-time period with 3.5% discounts annually (as per National Institute of Clinical Excellence suggestion). The modeling data showed that SCS with conventional medical management is cost-effective compared with conventional medical management alone for all presentations, with a cost-effectiveness ratio for SCS of CAN$9293 for FBSS and CAN$11,216 for CRPS per QALY gained.Citation41
In a study in 2010, the cost-effectiveness of SCS with conventional medical management was compared with conventional medical management alone in CRPS patients.Citation42 This study models economic costs using a simulated model population, employing parameters and assumptions set from previously published randomized trials. Here, SCS was shown to be cost-effective in select CRPS patients, with a probability exceeding 80% that SCS is cost-effective where the willingness to pay is set for a maximum of £30,000 per QALY.Citation42
Another cost-effectiveness study of SCS was performed in 2010 using a FBSS cohort.Citation42 Here, the authors compared SCS versus conventional medical management versus reoperation. Rechargeable and nonrechargeable implantable pulse generators were also assessed for cost efficacy. This study showed that in selected patients, SCS is cost-effective both as an adjunct to conventional medical management and as an alternative to reoperation; that the likelihood SCS would be cost-effective versus conventional medical management and versus reoperation exceeds 80%, where the willingness to pay is set for a maximum of £20,000 per QALY.Citation42
In 2008, a systematic review for cost-effectiveness of three studies where FBSS patients had been treated with SCS demonstrated that SCS is both more effective and less costly than conventional medical management alone in the long-term but there are high upfront implant costs associated with SCS implantation and maintenance.Citation43
In 2008, another study reported the generic health-related quality of life and costs of SCS at 6 months follow-up (using data from the PROCESS trial) compared to the quality of life, resource consumption, and costs of conventional medical management alone in patients with FBSS.Citation39 The study found that the mean total health care costs for the SCS group were significantly higher (€12,653) than the conventional medical management group (€2,594), when scaled for UK 2005–2006 national data.Citation39
This result reflects the high upfront costs of SCS over the limited 6-month follow-up period. The authors showed that 15% of the additional mean cost of SCS was offset within 6 months by a reduced use of drug and nondrug therapies. They also demonstrated a gain in quality of life over the same period which was significantly greater for the SCS group. The study concludes that, over the short term, SCS treatment results in greater healthcare costs but also generates important health improvements for the patients over the same period.Citation39
Cost-efficacy studies show that despite significant initial costs, SCS compared with other conventional treatments available to chronic pain patients results in long-term reductions in health care costs, which offset the high initial treatment costs over time.Citation44
Safety and tolerability
In the literature, SCS is reported as a safe procedure due to its reversible and minimally invasive characteristics.Citation30 Although catastrophic complications are possible, they are very rare. However, the incidence of minor complications of SCS has been reported at around 30%–40%.Citation13,Citation30,Citation31,Citation45–Citation47 These minor complications tend to occur within 12 months of implantation and are readily reversible and generally resolved.Citation13
The complications are divided into three main categories: mechanical, biological, and technique-related.Citation32 Complications of a mechanical origin are more common than those of biological origin.Citation31,Citation45 Historically, hardware-related complications occurred at a rate of between 24%–50%, whereas adverse biological events occurred in 7.5% of cases.Citation30
Mechanical complications include lead fracture or disconnection, which has a reported incidence of between 5% and 9%; lead migration has a reported incidence between 0% and 27%; implantable pulse generator failure occurred at a reported frequency of 1.7%.Citation11,Citation30 These complications can be minimized by using appropriate leads, anchoring, and suturing techniques. Furthermore, minimizing patient movements in the first 3 months after surgery allows for postoperative scarring of leads into place.Citation30
Kapural et alCitation22 demonstrated that lead migration of significance and requiring intervention in both the HF10 and traditional SCS arms occurred <5%. This most likely reflects improvements in both lead design and the anchoring systems used (–).Citation14,Citation22,Citation26,Citation48
Figure 1 (A) Posterior anterior fluoroscopy image and (B) lateral fluoroscopy image of T8-T10 placement of linear leads (Nevro Corp.) for the treatment of chronic back and leg pain.
Abbreviations: L, left; R, right.
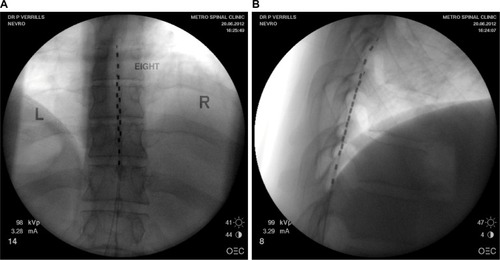
Figure 2 (A) Posterior anterior fluoroscopy image and (B) lateral fluoroscopy image of leads placed bilaterally at T12 and L1 dorsal root ganglions for the treatment of chronic idiopathic orchialgia pain.
Abbreviations: L, left; R, right.
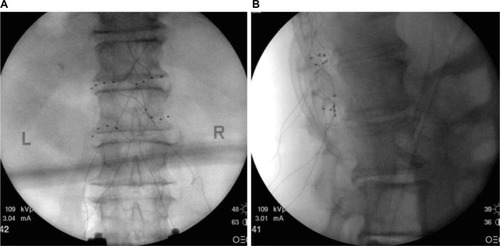
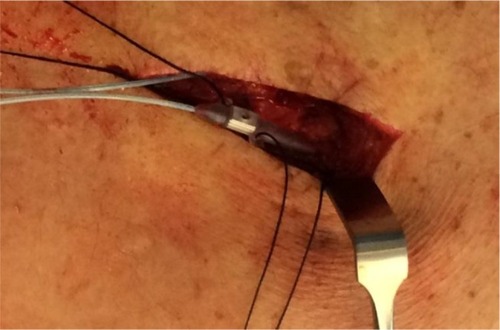
Figure 3 Intraoperative image of NX3000 anchor system by Nevro Corp.
Biological complications include infection, allergic reaction, pain at implant site, implantable pulse generator seroma, epidural fibrosis, epidural hematoma, dural puncture, and, rarely, neurological injury.Citation30,Citation31,Citation49–Citation52,Citation57–Citation60 The most common biological complication is infection with a rate between 3% and 8%, and the majority of these are superficial.Citation30 As is seen in the more recent literature,Citation30 incidence of infection can be minimized via pre and postoperative antibiotic use and appropriate skin preparation. The occurrence of dural puncture is reported as between 0.3% and 2%.Citation53 Other adverse biological events such as epidural fibrosis, compressive phenomenon, or spinal cord injury, while serious, are rare.
The Neuromodulation Appropriateness Consensus Committee has recommended a number of criteria and adaptations to practice to help reduce complications: physician training and mentoring, the appropriate and careful selection of patients, a continued focus on equipment development and innovation, as well as the dissemination and application of practice and research-based advances.Citation30
Patient-focused perspectives
As this field strives to provide high-quality, transparent, and independent empirical evidence for SCS therapies, it is possible to overlook the primary rationale for SCS treatment in the first place: patient benefit.
Patients suffering from chronic pain are often misdiagnosed or treated inappropriately. Improvements to training in pain management are needed, beginning at the undergraduate medical level. At present, the time allocated to pain management is generally inadequate; for instance, in the United Kingdom, the median time spent on pain management by a medical student is 13 hours, and sometimes it is as little as 6 hours.Citation2 Indeed, when
“the undergraduate training of all healthcare professionals is analyzed, education about the identification, assessment, and treatment of pain represents less than 1% of university-based teaching – yet pain is the most common reason for patients to consult their general practitioner.”Citation2
The ensuing repercussions may include inadequate diagnosis, inappropriate treatments, and extended periods of mismanagement. Further complicating the treatment of this cohort has been the inclusion of neuromodulation therapies as a treatment of last resort.Citation54 Yet research has shown that shifting SCS forward in the treatment algorithm of refractory chronic pain is associated with better patient outcomes.Citation44,Citation55
In recent years, many researchers and practitioners have included patient satisfaction (alongside empirical measures) as a clinically useful metric for assessing SCS treatment success.Citation23 When reported, overall patient satisfaction is high for the vast majority of SCS patients, perhaps reflecting the efficacy of SCS treatment, but may also reflect factors such as limitations of alternative treatments, safety, and tolerability of SCS and the rapidity of onset and durability of SCS treatment.Citation56
Moreover, under current arrangements, most patients access SCS treatment through private health insurance schemes or through compensatory bodies such as workers compensation schemes.Citation57 For patients who do not qualify for these funding systems, the immediate cost outlay would likely post a significant financial barrier to treatment.Citation57 The SCS specialty should aim to support the provision of equitable and accessible treatment for all.
Conclusion
Significant evidence exists for traditional SCS as a safe, clinical, and cost-effective treatment for many chronic pain conditions. Indeed, the field is rapidly evolving, and there is now Level I evidence for newer techniques including HF10 SCS and DRG SCS, which demonstrate dramatic improvements in overall efficacy in reducing pain in specific conditions, including failed back surgery, back pain, neuropathic leg pain, CRPS, and causalgia.
Furthermore, the field has increasingly met the challenge of not only having newer devices to achieve these outcomes but concurrently reducing the risks of complications and adverse events.
The data supporting SCS in its multiple forms are compelling and have reached a level that now demands that this therapy be considered earlier in the treatment continuum and to be no longer regarded as simply an end-stage salvage therapy.
Acknowledgments
The authors wish to thank anonymous reviewers for constructive comments that have led to an improved paper.
Disclosure
Paul Verrills is a consultant to NEVRO Corp and St Jude Medical Advisory and peer to peer teaching. The other authors (AB and CS) declare no conflicts of interest.
References
- ElzahafRATashaniOAUnsworthBAJohnsonMIThe prevalence of chronic pain with an analysis of countries with a human development index less than 0.9: a systematic review without meta-analysisCurr Med Res Opin20122871221122922697274
- PergolizziJAhlbeckKAldingtonDThe development of chronic pain: physiological CHANGE necessitates a multidisciplinary approach to treatmentCurr Med Res Opin20132991127113523786498
- LangleyPCThe prevalence, correlates and treatment of pain in the European UnionCurr Med Res Opin201127246348021194390
- McBethJJonesKEpidemiology of chronic musculoskeletal painBest Pract Res Clin Rheumatol200721340342517602991
- MerskeyHBogdukNClassification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain TermsSeattleIASP Press1994
- HauserWBockFEngeserPTolleTWillweber-StrumpfeAPetzkeFLong-term opioid use in non-cancer painDtsch Arztebl Int20141114373274025404530
- ShealyCNMortimerJTReswickJBElectrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical reportAnesth Analg19674644894914952225
- DeerTRMekhailNProvenzanoDThe appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus CommitteeNeuromodulation201417651555025112889
- SongJJPopescuABellRLPresent and potential use of spinal cord stimulation to control chronic painPain Physician201417323524624850105
- RoulaudMDurand-ZaleskiIIngrandPMulticolumn spinal cord stimulation for significant low back pain in failed back surgery syndrome: design of a national, multicentre, randomized, controlled health economics trial (ESTIMET Study)Neurochirurgie201561120
- CameronTSafety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature reviewJ Neurosurg20041003 Suppl25426715029914
- DeerTKimCBowmanRRansonMDouglasCSTolentinoWSpinal cord stimulation as a method of reducing opioids in severe chronic pain: a case report and review of the literatureW V Med J20101064 Spec565921932754
- TurnerJALoeserJDDeyoRASandersSBSpinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complicationsPain20041081–213714715109517
- DeerTRKramesEMekhailNThe appropriate use of neurostimulation: new and evolving neurostimulation therapies and applicable treatment for chronic pain and selected disease statesNeuromodulation201417659961525112892
- LiemLRussoMHuygenFJOne-year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic painNeuromodulation2015181414825145467
- De RidderDPlazierMKamerlingNMenovskyTVannesteSBurst spinal cord stimulation for limb and back painWorld Neurosurg201380564264923321375
- De RidderDVannesteSPlazierMvan der LooEMenovskyTBurst spinal cord stimulation: toward paresthesia-free pain suppressionNeurosurgery201066598699020404705
- Van BuytenJPAl-KaisyASmetIPalmisaniSSmithTHigh-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical studyNeuromodulation2013161596523199157
- TiedeJBrownLGekhtGVallejoRYearwoodTMorganDNovel spinal cord stimulation parameters in patients with predominant back painNeuromodulation201316437037523433237
- ShanthannaHChanPMcChesneyJPaulJThabaneLAssessing the effectiveness of “pulse radiofrequency treatment of dorsal root ganglion” in patients with chronic lumbar radicular pain: study protocol for a randomized control trialTrials2012135222540851
- WallPDDevorMSensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured ratsPain19831743213396664680
- KapuralLYuCDoustMWNovel 10-kHz High-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trialAnesthesiology2015123485186026218762
- GriderJSManchikantiLCarayannopoulosAEffectiveness of spinal cord stimulation in chronic spinal pain: a systematic reviewPain Physician2016191E33E5426752493
- de VosCCBomMJVannesteSLendersMWde RidderDBurst spinal cord stimulation evaluated in patients with failed back surgery syndrome and painful diabetic neuropathyNeuromodulation201417215215924655043
- TaylorRSDesaiMJRigoardPTaylorRJPredictors of pain relief following spinal cord stimulation in chronic back and leg pain and failed back surgery syndrome: a systematic review and meta-regression analysisPain Pract201414648950523834386
- GeurtsJWSmitsHKemlerMABrunnerFKesselsAGHvan KleefMSpinal cord stimulation for complex regional pain syndrome type I: a prospective cohort study with long-term follow-upNeuromodulation201316652352923363081
- PerruchoudCEldabeSBatterhamAMAnalgesic efficacy of high-frequency spinal cord stimulation: a randomized double-blind placebo-controlled studyNeuromodulation201316436336923425338
- BarolatGOakleyJCLawJDNorthRBKetcikBSharanAEpidural spinal cord stimulation with a multiple electrode paddle lead is effective in treating intractable low back painNeuromodulation200142596622151612
- RussoMVerrillsPMitchellBSalmonJBarnardASantarelliDHigh frequency spinal cord stimulation at 10 kHz for the treatment of chronic pain: 6-month Australian clinical experienceNeuromodulation. Pain Physician201619426728027228514
- DeerTRMekhailNProvenzanoDThe appropriate use of neurostimulation: avoidance and treatment of complications of neurostimulation therapies for the treatment of chronic painNeuromodulation201417657159825112891
- HayekSMVeiziEHanesMTreatment-limiting complications of percutaneous spinal cord stimulator implants: a review of eight years of experience from an academic center databaseNeuromodulation201518760360826053499
- KumarKWilsonJRTaylorRSGuptaSComplications of spinal cord stimulation, suggestions to improve outcome, and financial impactJ Neurosurg Spine20065319120316961079
- De La CruzPFamaCRothSPredictors of spinal cord stimulation successNeuromodulation201518759960226119040
- LevyRPlenary presentationPaper presented at: 19th Annual North American Neuromodulation Society (NANS)2015Las Vegas, NV
- DeerTPlenary presentationPaper presented at: 19th Annual North American Neuromodulation Society (NANS)2015Las Vegas, NV
- FarrarJTPortenoyRKBerlinJAKinmanJLStromBLDefining the clinically important difference in pain outcome measuresPain200088328729411068116
- KapuralLComparison of 10 kHz high-frequency SCS to traditional low-frequency SCS: the SENZA-RCT U.S. Pivotal StudyNorth American Neuromodulation Society (NANS)2014Las Vegas, NV
- NorthRBShipleyJWangHMekhailNA review of economic factors related to the delivery of health care for chronic low back painNeuromodulation201417697625395118
- MancaAKumarKTaylorRSQuality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial)Eur J Pain20081281047105818359255
- ZuccoFCiampichiniRLavanoACost-effectiveness and cost-utility analysis of spinal cord stimulation in patients with failed back surgery syndrome: results from the PRECISE studyNeuromodulation201518426627625879722
- KumarKRizviSCost-effectiveness of spinal cord stimulation therapy in management of chronic painPain Med201314111631164923710759
- KemlerMARaphaelJHBentleyATaylorRSThe cost-effectiveness of spinal cord stimulation for complex regional pain syndromeValue Health201013673574220561326
- BalaMMRiemsmaRPNixonJKleijnenJSystematic review of the (cost-)effectiveness of spinal cord stimulation for people with failed back surgery syndromeClin J Pain200824974175618936591
- LadSPPetragliaFWIiiKentARLonger delay from chronic pain to spinal cord stimulation results in higher healthcare resource utilizationNeuromodulation2016291012389
- EldabeSBuchserEDuarteRVComplications of spinal cord stimulation and peripheral nerve stimulation techniques: a review of the literaturePain Med Epub20151214
- PetragliaFW3rdFarberSHGramerRThe incidence of spinal cord injury in implantation of percutaneous and paddle electrodes for spinal cord stimulationNeuromodulation2016191859026644210
- KumarKTaylorRSJacquesLSpinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndromePain20071321–217918817845835
- MironerYESatterthwaiteJRLewisEMEfficacy of a single, percutaneous, across midline, Octrode® lead using a “midline anchoring” technique in the treatment of chronic low back and/or lower extremity pain: a retrospective studyNeuromodulation200811428629522151143
- LennarsonPJGuillenFTSpinal cord compression from a foreign body reaction to spinal cord stimulation: a previously unreported complicationSpine20103525E1516E151921102282
- LevyRHendersonJSlavinKIncidence and avoidance of neurologic complications with paddle type spinal cord stimulation leadsNeuromodulation201114541242221967534
- DeerTRLevyRMVerrillsPMackeySAbejonDPerspective: peripheral nerve stimulation and peripheral nerve field stimulation birds of a different featherPain Medicine201516341141225599816
- ScrantonRASkaribasIMSimpsonRKJrSpinal stimulator peri-electrode masses: case reportJ Neurosurg Spine2015221707425380541
- DeerTRStewartCDComplications of spinal cord stimulation: identification, treatment, and preventionPain Medicine20089S1S93S101
- KramesESInterventional pain management. Appropriate when less invasive therapies fail to provide adequate analgesiaMed Clin North Am199983378780810386125
- KumarKRizviSBnursSBSpinal cord stimulation is effective in management of complex regional pain syndrome I: fact or fictionNeurosurgery201169356658021441839
- KumarKTaylorRSJacquesLThe effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month followup of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulationNeurosurgery200863476277018981888
- ElleryBParsonsJMerlinTHigh Frequency Spinal Cord Stimulation and Dorsal Root Ganglion Stimulation for Chronic PainHerston QLDDepartment of Health, Queensland2015
- KemlerMADe VetHCWBarendseGAMVan Den WildenbergFAJMVan KleefMThe effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: two years’ follow-up of the randomized controlled trialAnn Neurol2004551131814705107
- NorthRBKiddDHFarrokhiFPiantadosiSASpinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trialNeurosurgery20055619810615617591
- TurnerJAHollingworthWComstockBDeyoRAComparative effectiveness research and policy: experiences conducting a coverage with evidence development study of a therapeutic deviceMed Care2010486 SupplS129S13620473203
- TurnerJAHollingworthWComstockBADeyoRASpinal cord stimulation for failed back surgery syndrome: outcomes in a workers’ compensation settingPain20101481142519875232
- de VosCCDijkstraCLendersMWPMHolsheimerJSpinal cord stimulation with hybrid lead relieves pain in low back and legsNeuromodulation201215211812322074412
- MoriyamaKMurakawaKUnoTA prospective, open-label, multicenter study to assess the efficacy of spinal cord stimulation and identify patients who would benefitNeuromodulation201215171222151729
- Al-KaisyAVan BuytenJPSmetIPalmisaniSPangDSmithTSustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter studyPain Med201415334735424308759
- De RidderDLendersMWDe VosCCA 2-center comparative study on tonic versus burst spinal cord stimulation: amount of responders and amount of pain suppressionClin J Pain201531543343724977394

