Abstract
3D bioprinting is a powerful enabling technology for the automated fabrication of biomimetic constructs for skin modelling and repair with high resolution and reproducibility. Bioinks, which often comprise the combination of cells and printable biomaterials, are essential for bioprinting 3D cell-laden constructs emulating the architecture, composition and function of native skin. Tissue- and organ-specific decellularized extracellular matrix (dECM) materials are assuming a significant role in bioink design owing their capacity to provide native biophysical and biochemical signals that can elicit distinct cellular responses toward tissue development. Herein, we discuss the rational design of dECM-based bioinks, focusing on processing methods to obtain dECM as well as on bioprinting strategies to engineer 3D constructs for applications in wound healing and in vitro skin modelling.
Skin is the outermost organ of the human body acting as the primary line of defense for internal tissues and organs, while providing protection against external agents and regulating body temperature. To exert these vital functions, skin has a multilayered architecture and complex composition at cellular and compositional levels. As the skin interfaces with the external environment, it is prone to trauma and injury which, depending on the type and severity of such incidents, can affect skin structure and function, leading to acute or chronic wounds. As a spontaneous response to injury, skin triggers a well-orchestrated healing cascade towards the restoration of the native tissue architecture, composition and function [Citation1,Citation2]. However, the innate self-healing ability of human skin is restricted to small and superficial injuries. Full-thickness wounds affecting deep skin layers display a limited regenerative capacity and require the use of therapies to promote de novo tissue formation [Citation3]. The predominant clinical approach for managing full-thickness and chronic wounds presently revolves around the application of dressings [Citation4], which create a suitable environment for effective wound healing by safeguarding the wound area, maintaining appropriate moisture levels, and absorbing surplus exudate. Nevertheless, due to the limited clinical evidence regarding their ability to accelerate wound closure, these current clinical approaches may be regarded primarily as adjunctive means to establish an ideal local healing environment [Citation5].
Skin tissue engineering aims to develop clinically effective strategies capable of promoting skin formation, while attempting to avoid pathological extracellular matrix (ECM) remodeling. The traditional approach uses natural (e.g., collagen, alginate, gelatin) and/or synthetic (e.g., PLGA (poly(lactic-co-glycolic acid)), PCL (polycaprolactone)) polymers with controllable biophysical and biochemical cues that provide mechanical support and regulate cell fate to induce tissue morphogenesis and rebuild a functional tissue [Citation6]. Nevertheless, existing strategies for wound healing still present critical limitations and poor clinical outcomes [Citation5]. These include host integration and tissue maturation issues, lack of vascularization and innervation, insufficient mechanical properties, poor reconstruction of skin appendages (e.g., sweat glands and hair follicles) and limitations regarding the incorporation of immune cells, which limit our ability in recapitulating the properties and function of native skin [Citation7]. To overcome some of these issues, the use of tissue- and organ-specific decellularized extracellular matrix (dECM) is currently under the spotlight as a promising alternative for the conventional biomaterials in the field. Owing its ability to act as a physiological reservoir of bioactive factors and preserve native ECM components and key bioactive signaling molecules, dECM is able to trigger specific cellular responses and induce tissue development [Citation8]. Remarkably, dECM-based biomaterials retain the intrinsic ability to provide cells with tissue-specific microenvironments and regulate cell phenotype and function, being an attractive source of biomaterials for wound healing applications [Citation9–11]. However, it is crucial to understand not only the impact of decellularization process on dECM, but also its potential as a natural biomaterial for skin tissue engineering.
3D bioprinting is a revolutionizing technology that enables the fabrication of biomimetic and patient-specific constructs [Citation12]. It allows the automated deposition of living or non-living materials into 3D constructs with unprecedented level of biomimicry, opening new avenues in the fabrication of grafts and physiologically relevant models that can be used as in vitro microphysiological platforms to evaluate biological responses [Citation13,Citation14]. In the most common approach, bioinks which encompass a printable formulation of cells eventually combined with biomaterials and bioactive molecules, are bioprinted into pre-defined spatial locations to create 3D constructs with structural organization and biological function. In another approach, cell-free biomaterials (i.e., biomaterial inks) are deposited via bioprinting technologies to generate 3D constructs with the ability to guide and influence cell response [Citation15]. Although there is substantial interest regarding the utilization of dECM in the context of 3D bioprinting, the development of dECM-based (bio)inks that can effectively satisfy the processing requirements of diverse bioprinting technologies, while concurrently promoting cellular functionality continues to present a formidable and ongoing challenge.
This review aims to discuss the emerging role of dECM-based (bio)inks in the bioprinting of therapeutic products for skin repair as well as 3D in vitro models of healthy and diseased skin as versatile research platforms for pathophysiology studies and drug testing (). We first discuss design strategies for the development of dECM-based (bio)inks, ranging from the ECM source to methods of decellularization, dECM modification, characterization and sterilization. Then, we highlight recent advances in the bioprinting of dECM-based (bio)inks for skin tissue engineering, focusing on the role of dECM and how it contributes to improved cellular responses. We also discuss existing evidence on whether the design of multimaterial bioinks by combining dECM with natural polymers has significant advantages compared with dECM-only bioinks. Finally, we provide insights into the challenges and opportunities in the field.
Several sources can be used to obtain dECM, which is extracted and processed via a combination of chemical, physical and/or biological methods, followed by sterilization and quality control steps. For (bio)ink design, the dECM can be combined with cells and used as either a single material or combined with other biomaterials that impart specific properties at rheological, biomechanical and/or biological levels. In the context of skin bioprinting, extrusion- and inkjet-based bioprinting have been used to create acellular and cell-laden constructs as in vitro skin models and grafts for wound healing.
dECM: Decellularized extracellular matrix; ECM: Extracellular matrix.
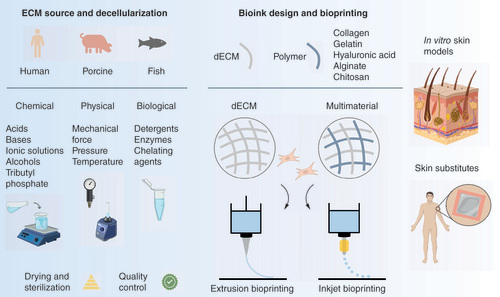
Decellularized extracellular matrix: from source to bioink design
Influence of tissue/organ source on dECM application & scalability
Considerations on dECM bioink design based on source selection
Several tissues obtained from human and animal sources have been employed in the development of dECM-based hydrogels for skin applications [Citation16]. The selection of the tissue source is an important consideration in bioink design, as intrinsic differences in tissue characteristics across various species can influence the biological outcome [Citation17]. The selection of ECM source requires a comprehensive assessment of multiple factors such as tissue or organ dimensions, accessibility of the source material (e.g., gestation period, litter size, and reproductive maturity), safety (e.g., infection risk, immune-rejection potential) and ethical considerations.
Human-derived tissues have been used for the development of dECM-bioinks due to their relevant biological, physiological and biochemical properties [Citation17,Citation18]. The use of human sources exhibits beneficial noteworthy attributes that bear direct relevance to safety in processing, thereby minimizing the risk of disease transmission. This important safety factor is primarily related to the comprehensive infrastructural frameworks that oversee the acquisition and collection of these biological materials [Citation19]. Additionally, certain human-derived tissues, such as the placenta, occupy a distinctive status in the biomedical landscape as they are categorized and disposed of as medical waste following childbirth procedures [Citation20,Citation21]. Consequently, tissue extraction can be accomplished without inflicting any direct harm upon the donor, thus minimizing ethical difficulties associated to their utilization [Citation21]. Additional tissues of interest, such as adipose tissue, derive from pre-existing interventions and do not pose additional welfare concerns for the donor beyond those previously established by the approved procedure. Cadaveric tissue is also explored for skin applications, in particular those obtained from skin [Citation22–24], small intestinal submucosa (SIS) [Citation25] and pericardium [Citation26,Citation27]. However, regarding human-source tissue it is imperative to deliberate the implications related with major histocompatibility complex (MHC) antigens [Citation26].
ECM-based biomaterials have also been developed by using various animal sources, including porcine, bovine, murine, leporidae, and piscean origins. In the context of bioink design, porcine and bovine tissue have traditionally emerged as the prevalent dECM sources [Citation28]. These tissue sources represent larger animal models, resulting in a greater ECM supply [Citation29]. However, it is important to acknowledge that housing and maintaining these animals entail increased infrastructure costs compared with smaller animal models. In comparison, murine and leporidea are less expensive, but their smaller sizes also equate to less raw material per unit [Citation30]. Nonetheless, when these animal sources are used, it is imperative to address the issue of human immunological rejection of Gal antigens (α1,3 Gal epitopes) present in the animal sources [Citation31]. These xenogeneic antigens have been extensively documented and are linked to complications such as hyperacute rejection (HAR), thereby impeding healing, regeneration and graft viability [Citation32,Citation33]. Moreover, animal-derived bioinks also raise concerns of xenozoonoses disease transmission [Citation34]. Alternatively, acellular fish skin has emerged as a promising resource for skin replacement due to several advantageous characteristics. It exhibits a low risk of transmitting viral diseases, contains omega-3-fatty acids within the matrix that impart antiviral and antibacterial properties, and is associated with expedited healing times [Citation35,Citation36]. Furthermore, the use of fish skin encounters relatively few ethical and cultural barriers, while its abundance as a byproduct of the fishing industry renders it a sustainable and readily available resource, featuring a cost-effective manufacturing process [Citation37].
Considerations on dECM bioink design based on tissue/organ selection
Skin-derived ECM presents itself as an interesting source for skin applications since its unique biochemical fingerprint matches the intended microenvironment [Citation38]. Consequently, skin-derived dECM has been shown to modulate skin cell fate, improving morphology and function of bioprinted cells [Citation10,Citation39]. However, skin-derived dECM lacks some properties of interest such as anti-inflammatory, anti-bacterial and antioxidant. This limitation can be addressed by integrating bioactive molecules into the dECM that could prove crucial in the production of an ideal tissue matrix that aims for chronic or burn wound management [Citation13]. In contrast, placental tissue already presents anti-inflammatory [Citation40], antibacterial [Citation40], anti-oxidative [Citation40,Citation41], anti-scarring [Citation42], and fibrosis suppression capabilities [Citation43]. Similar properties have been attributed to the amniotic membrane namely anti-angiogenic and anti-inflammatory [Citation44], as well as anti-microbial properties [Citation45]. Like placenta, other raw materials such as adipose tissue are usually considered for the development of skin constructs. Adipose tissue’s unique composition has been shown to promote a strong angiogenetic response when implanted subcutaneously in mice [Citation46]. Moreover, acellular adipose tissue has been demonstrated to promote adipogenesis and accelerate wound healing [Citation47]. In addition to skin-derived ECM, placenta and adipose tissue, decellularized membranes derived from the SIS, and mesothelium have been studied for skin wound healing. The SIS’s composition has been associated with capacity to stimulate immunological mediators, induce angiogenesis, facilitate reepithelialization and inhibit matrix metalloproteinases [Citation48–51]. Beyond SIS, mesothelium derived from various body cavity linings such as the pleura, peritoneum, and pericardium, possesses a substantial content of collagen type IV, laminin, and fibronectin, contributing to enhanced cellular proliferation, micro-vessel formation, and resistance to enzymatic degradation [Citation52–54].
Production of dECM
Decellularization process
Decellularization procedures commonly involve the combination of various decellularization agents, categorized as chemical (acids, bases, ionic solutions, alcohols, and tributyl phosphate), biological (detergents, enzymes, and chelating agents), and physical agents (temperature, pressure, and mechanical force) [Citation55,Citation56]. These agents can be applied through a variety of methodologies, including immersion and agitation, the utilization of pressure gradients, whole organ perfusion, and the use of supercritical fluids [Citation55,Citation56]. The effectiveness of the procedure relies not solely on the selected protocol, but also on the tissue subjected to decellularization. This is due to the fact that tissue-specific characteristics, including cellularity, density and thickness, have the potential to impact the process outcome [Citation55]. Furthermore, variables such as economic implications, scalability and regulatory factors may additionally contribute to determine the most appropriate decellularization methodology.
Several decellularization methodologies and tissue sources have been used in the design of dECM-based bioinks for skin tissue engineering (). Current protocols exhibit an excessive dependence on aggressive chemical agents and detergents, specifically trypsin, sodium dodecyl sulfate (SDS), sodium deoxycholate (SDC) and Triton X (TX)-100. Previous works have shown that trypsin and SDS can detrimentally affect the structural integrity of ECM, including depletion of collagen, laminin, elastin, fibronectin, glycosaminoglycans (GAGs) and growth factors [Citation57–66]. Moreover, although SDC is generally regarded as causing less harm compared with SDS, it has also been associated with the disruption of ECM ultrastructure and the extraction of GAGs [Citation67–70]. The use of TX-100 has been documented to result in substantial depletion of elastin, GAGs, and alterations in collagen structure [Citation71–74]. Moreover, preserving GAGs and elastin throughout the decellularization process is also critical to improve the rheological properties of dECM-based bioinks such as shape fidelity and stacking ability, as well as to contribute for improved the cell viability [Citation63].
Table 1. Examples of processing methodologies applied for the design of dECM-based bioinks for skin bioprinting.
In the context of bioink design, there are steps that could be taken to enhance the protocol efficiency and reduce the processing time, resource consumption and potential negative impacts on dECM composition. Methods such as tissue fragmentation, including mincing, homogenization or slicing, can facilitate the extraction of target molecules, while the use of penetration enhancers, which are substances that enhance molecule delivery into tissues, can improve absorption and effectiveness of decellularization agents. Tissue mincing serves to augment the tissue’s surface area, enhancing the penetration of decellularization agents and ensuring the complete removal of cellular constituents [Citation86]. This approach further offers the potential for a reduction in the required time and/or concentration of used agents. Additionally, another option to increase tissue’s surface area and reduce processing time is tissue homogenization using a blender/ultra-turrax and slicing [Citation85,Citation87,Citation88]. However, it is important to achieve a suitable balance regarding mechanical tissue disruption, since this can result in increased release of macromolecule-bound proteins (e.g., growth factors), thereby potentially leading to the unintended depletion of these essential components [Citation89]. Alternatively, enhanced diffusion of decellularization additives may be realized through the use of agents such as dimethyl sulfoxide (DMSO), which increases penetration of detergents in the cells by promoting membrane thinning and fluidity at low concentrations [Citation90]. A prevailing challenge within the decellularization process is the inherent complexity associated to the effective removal of detergents following extended exposure periods [Citation91]. The presence of residual amounts of detergents such as SDS, have been shown to induce cell lysis [Citation92] and immune response after implantation [Citation67,Citation93]. Therefore, the use of DMSO leads to a decrease in overall exposure time, thereby favorably impacting the process of detergent removal by decreasing the timeframe available for chemical binding of the detergent to the matrix [Citation94,Citation95]. Other penetration agents can be employed such as ethylenediaminetetraacetic acid (EDTA) that transiently loosen the tight junctions between cells opening the matrix [Citation96]. A balance between mechanical tissue disruption and enhanced delivery of active ingredients can generate protocols that are more effective in achieving desired outcomes with minimal ECM damage.
Overall, decellularization procedures for bioink production demand careful consideration of agents and methods to preserve ECM integrity. The overreliance on established protocols that employ harsh reagents leads to a significant removal of ECM components from the matrix [Citation97]. Alternative methods such as dimethyl ether show promise in maintaining biochemical composition, while penetration enhancers like DMSO offer efficient diffusion. Striking a balance between tissue disruption and active ingredient delivery is key for optimized protocols with minimal tissue damage.
Safety & post-processing
The control regarding the safety and effectiveness of the decellularization procedures is crucial for obtaining viable dECM and reduce the risk of potential immune reactions. Moreover, since the applied decellularization protocol impacts bioink production, quality control at this stage can reduce further optimization steps and improve bioprinting reproducibility. Therefore, considering the findings concerning immune rejection, a quantitative decellularization metric should be determined. In 2011, Crapo et al. [Citation55] established a set of quantitative metrics for decellularization, which included evaluating the presence of visible nuclear material and DNA quantity and length through Hematoxylin and Eosin (H&E) and 4′,6-diamidino-2-phenylindole (DAPI) stainings. Additional metrics based on tissue origin can be added. As mentioned in the previous section, allograft rejection is commonly associated with donor-specific MHC molecules [Citation98], and hyperacute xenograft rejection of porcine grafts has been predominantly linked to the xenoantigenic Galα1–3-galβ1-(3)4GlcNAc-R epitope (α-Gal) [Citation99]. Consequently, it is possible to extend the aforementioned decellularization criteria: (1) <50 ng dsDNA per mg ECM dry weight [Citation55], (2) <200 bp DNA fragment length [Citation55], (3) lack of visible nuclear material in tissue sections [Citation55], (4) Galα1–3-galβ1-(3)4GlcNAc-R epitope absence, and (5) MHC I and II antigens absence.
Following the successful decellularization process, it is expected to subject the dECM material to drying and sterilization procedures. ECM drying is another essential step for stability, efficacy and safety in bioprinting. It can prevent protein denaturation, degradation and microbial growth, while retaining bioactivity and reducing cold chain requirements [Citation100]. Techniques like freeze-drying, vacuum-drying, spray-drying and supercritical fluids can be used to obtain a dry product [Citation100]. Controlling drying parameters like temperature, pressure and excipients ensures consistency and protein structure stability. Temperature before and during the freeze-drying process can have a significant impact on matrix proteins [Citation101]. An additional emerging technological advancement concerns to the utilization of supercritical carbon dioxide (scCO2) to dry acellular porcine esophageal matrix. This innovative approach effectively preserves the integral macro- and microstructural elements, collagen, and GAGs within the matrix. Additionally, the application of this protocol facilitates the elimination of residual debris, DNA remnants and decellularization agents, enhancing the inherent biocompatibility and making of this protocol a unique approach [Citation102,Citation103]. However, it is worth noting that the majority of dECM-based bioinks undergo a freeze-drying process, which is widely regarded as the gold standard technology in the field. Nevertheless, the absence of comprehensive data regarding the methodologies and protocols employed in drying process presents a significant challenge in terms of making meaningful comparisons between the various drying methods. It is crucial to recognize that the choice of drying methodology can exert a substantial influence on the ultimate characteristics of the resulting product. Therefore, future research endeavors should prioritize the exploration and evaluation of drying protocols aimed at enhancing the material properties of dECM-based bioinks.
Regarding dECM materials, it is typical to apply sterilization methods consistent with those commonly employed in medical devices and biomaterials for clinical applications, adhering to Good Manufacturing Practice (GMP) guidelines and complying with the European directive CEN-EN 556-1 [Citation104]. The methods employed must be efficacious in inactivating microorganisms on the device to an extent where the likelihood of infection transmission becomes negligible [Citation105,Citation106]. Sterilization effectiveness is typically assessed by sterility assurance level (SAL) assays [Citation95,Citation96]. In addition to effectiveness, the sterilization method should avoid inducing substantial alterations in the material that may trigger an immune response. This is essential for maintaining the material’s biochemical and mechanical characteristics, ensuring that its intended functionality remains unimpaired [Citation107]. Currently approved terminal sterilization methods encompass thermal-based techniques, specifically dry heat and autoclaving, as well as chemical-based ethylene oxide (EtO) sterilization, and radiation-based gamma-irradiation [Citation108]. Notably, gamma irradiation has been associated to the generation of free hydroxyl radicals and other radiotoxins, which can induce toxigenic and mutagenic effects, potentially contributing to carcinogenesis [Citation109]. Even when administered at low dosages, gamma irradiation has been demonstrated to cause significant damage to the amnion basement membrane, as well as to the elastic and collagen fibers of papillary dermis [Citation110,Citation111]. Similarly, chemical sterilization by EtO was found to have an effect on the mechanical properties and ultrastructure of decellularized bladder matrix [Citation112], impair bone-forming activity [Citation113], and affect tissue remodeling upon implantation [Citation114]. Given that EtO is recognized as a toxic and carcinogenic substance, stringent measures are required to assess and eliminate residual amounts prior to its use in cell culture or human applications [Citation115]. Moreover, EtO constitutes a health hazard and an occupational exposure risk [Citation116]. An alternative approach employs peracetic acid for the inactivation of bacterial spores. This method uses peracetic acid concentrations ranging from 0.05 to 1% and requires a duration between 15 s to 30 min [Citation106]. Moreover, immersion in peracetic acid and ethanol is generally considered less harmful to biological tissue, though studies have reported impaired tissue remodeling post-implantation, altered biochemical composition and mechanical properties [Citation111], as well as significant changes in tissue microarchitecture [Citation117]. Presently, bioinks derived from dECM are commonly sterilized using 0.1% peracetic acid and 4% ethanol, followed by rinsing with PBS and/or water [Citation10,Citation47,Citation62,Citation78,Citation80]. This sterilization approach may be attributed to the limited range of sterilization techniques applicable to biological materials, coupled with the practical advantage of on-site availability, as peracetic acid immersion requires no additional equipment. In this sense, the need for effective terminal sterilization techniques that do not significantly impair biological based materials is clear. From the aforementioned techniques, scCO2 presents as an attractive alternative as it has been applied to a myriad of biological tissues, namely bone [Citation118,Citation119], tendon [Citation119], skin [Citation120,Citation121], lung [Citation122] and aorta [Citation123] without significantly compromising the mechanical and biochemical properties. Moreover, Wehmeyer et al. [Citation124] reported amniotic membrane sterilization with a 10-6 SAL reduction using scCO2 and a well preserved structure and composition.
Bioprinting of dECM-based bioinks for skin applications
Within the field of biofabrication, bioprinting is assuming a leading position in the production of 3D constructs that recapitulate, in some extent, the heterogeneity, architecture and function of human skin [Citation10,Citation79,Citation125–127]. Bioprinting technologies can be classified into light-assisted, inkjet- and extrusion-based bioprinting [Citation12]. These technologies present distinct operating principle and resolution, often requiring the design of bioinks with specific rheological properties. Light-assisted bioprinting technologies, including stereolithography, digital light processing and volumetric bioprinting, inherently use light to induce sol-gel conversion of a low viscosity and photocrosslinkable bioresin into a 3D construct with high resolution [Citation128–130]. On the other hand, inkjet-based bioprinting uses either thermal or piezoelectric effects to promote the formation and ejection of small droplets of a bioink onto a receiving substrate. Despite enabling rapid printing and high resolution, inkjet bioprinting requires bioinks with low viscosity and cell density to preclude nozzle clogging and cell sedimentation [Citation131,Citation132]. Extrusion-based bioprinting is the most versatile technology for cell printing as it affords the continuous deposition of bioinks with a wide range of rheological properties [Citation79,Citation133]. The direct writing approach involves the deposition of self-supporting bioinks, becoming a challenge for the development of bioinks that afford the accurate and reproducible fabrication of centimeter-sized 3D constructs with high resolution. This is particularly relevant for dECM bioinks as most of these bioinks suffer from poor printability due to their low viscosity, which is a common consequence of the loss of ECM proteins during decellularization [Citation134]. To address this issue, the versatility of extrusion-based bioprinting has allowed the development of alternative strategies toward the bioprinting of complex 3D constructs from low viscosity bioinks [Citation12]. One example is the embedded bioprinting, which uses a support material that behaves as a solid at low shear stress but flows under conditions of high shear stress, overcoming the need for self-supporting bioinks and enabling the fabrication of complex constructs [Citation7]. In this regard, a 3D bioprinting technique termed as freeform reversible embedding of suspended hydrogels (FRESH) is attracting a considerable interest to fabricate complex 3D constructs through the deposition of low viscosity bioinks within a thermoreversible support bath composed of gelatin microparticles [Citation135]. Alternative strategies rely on (i) the use of light-activated bioinks, which can be rapidly photocrosslinked during or post-deposition to improve print fidelity [Citation39,Citation79,Citation136,Citation137], (ii) the design of granular and self-healing bioinks [Citation138,Citation139], as well as (iii) the addition of viscosity enhancing components (e.g., alginate, carbon nanotubes, nanosilicates) to tune bioink rheology [Citation140,Citation141].
Design & bioprinting of dECM bioinks
In recent years, the use of dECM opened new opportunities in the development of therapeutic products for skin tissue engineering and biomimetic in vitro systems for skin modelling. The synergy between 3D bioprinting technology and dECM holds tremendous potential and has shown promising outcomes for skin wound healing and in vitro skin modelling [Citation142–144]. Bioprinted skin grafts should recapitulate the composition and properties of native skin layers in order to promote de novo tissue formation with functional properties. In this regard, dECM obtained from porcine dermis via a combination of chemical and enzymatic treatments was used to develop a printable bioink loaded with human dermal fibroblasts (HDFs) [Citation125]. The bioink was prepared at different concentrations (2% and 3%) and employed for the extrusion bioprinting of a dermal construct that underwent thermal gelation. The dECM preserved not only collagen and GAGs, but also bioactive molecules and growth factors (A), providing the cells with a dermal-like microenvironment that enhanced cell viability and proliferation [Citation125]. To better mimic the multilayer architecture of human skin and promote tissue repair, a porcine-derived dECM containing HDFs was employed to reconstruct the dermis via extrusion bioprinting, followed by the manual seeding of human keratinocytes [Citation78]. Upon implantation in a mouse chimney wound model, cellularized constructs showed rapid re-epithelialization and enhanced tissue regeneration compared with cell-free dECM constructs. As collagen is the major component of skin ECM, it has been the gold standard for skin reconstruction both in vitro and in vivo. For this reason, Hubert et al. [Citation97] characterized the impact of using either collagen or dECM from porcine-decellularized dermis on the mechanical and biological properties of bioprinted 3D skin models. It was found that fibroblasts showed higher focal adhesion and ECM deposition in skin models prepared from the dECM bioink than collagen type-I. Moreover, the stiffness and viscoelasticity of skin models fabricated with the dECM bioink also showed improved similarity to ex vivo human skin. Notably, despite the collagen being the major structural component of the ECM, these data suggest the role of other ECM components, such as elastin and GAGs, in the biological function of reconstructed skin at mechanical and cellular levels.
(A) Quantification of the major ECM components (collagen, GAG, and elastin) after the decellularization process. (B) Representative images of 3D cell-printed in vitro skin equivalents using type I collagen (C-HSE) and skin-derived bioink (S-HSE). Scale bar: 2 mm. (C) Morphological changes occurring in the diverse types of skin grafts during 28 days after the surgery. (D) Bioprinted skin model recreating diabetic hallmarks by the inclusion of perfusable and vascularized hypodermal compartment and augmented diabetic features.
C-HSE: Human skin equivalent using collagen bioink; dECM: Decellularized extracellular matrix; ECM: Extracellular matrix; GAG: Glycosaminoglycans; IS: Inguinal split-thickness skin grafts; PCL: Polycaprolactone; PDA: 3D-printed dermal analogues; PL: Pelnac Dermal Substitute; ROS: Reactive oxygen species; S-HSE: Human skin equivalent using skin-derived extracellular matrix bioink.
Modified with permission from [Citation10,Citation125,Citation126,Citation145].
![Figure 2. Design and bioprinting of dECM bioinks for skin tissue engineering.(A) Quantification of the major ECM components (collagen, GAG, and elastin) after the decellularization process. (B) Representative images of 3D cell-printed in vitro skin equivalents using type I collagen (C-HSE) and skin-derived bioink (S-HSE). Scale bar: 2 mm. (C) Morphological changes occurring in the diverse types of skin grafts during 28 days after the surgery. (D) Bioprinted skin model recreating diabetic hallmarks by the inclusion of perfusable and vascularized hypodermal compartment and augmented diabetic features.C-HSE: Human skin equivalent using collagen bioink; dECM: Decellularized extracellular matrix; ECM: Extracellular matrix; GAG: Glycosaminoglycans; IS: Inguinal split-thickness skin grafts; PCL: Polycaprolactone; PDA: 3D-printed dermal analogues; PL: Pelnac Dermal Substitute; ROS: Reactive oxygen species; S-HSE: Human skin equivalent using skin-derived extracellular matrix bioink.Modified with permission from [Citation10,Citation125,Citation126,Citation145].](/cms/asset/a48e71d1-85fb-452e-9649-c08f1a820ec2/idpm_a_12366990_f0002.jpg)
The combination of bioprinting technologies is emerging as an effective approach to generate skin tissue constructs by exploring the advantages of each technology [Citation62,Citation146]. Following this approach, Fang et al. [Citation147] created a bilayer construct to promote wound healing and reduce scarring by combining electrospun PLGA nanofibers with extrusion printed decellularized pig dermis. PLGA nanofibers were first fabricated to recreate the epidermis, followed by the deposition of an acellular dECM ink to recreate the dermis. The bilayer construct reduced the number of fibroblasts, collagen deposition and scar thickness in a rabbit ear hypertrophic scar model when compared with PLGA and dECM alone. In another work, Kim et al. [Citation10] used porcine skin tissue to design a dECM bioink with intrinsic growth factors and cytokines for the bioprinting of an in vitro 3D skin model and a pre-vascularized patch for skin reconstruction. To generate the skin model, HDFs were embedded within the dECM bioink and extrusion bioprinted to recreate the dermal layer. After 14 days of maturation, epidermal keratinocytes were inkjet bioprinted on top of the dermis to reconstruct the epidermis. The 3D model presented superior barrier function, improved epidermal organization and a more stabilized dermal compartment with increased dermal ECM secretion when compared with a 3D skin model generated using collagen type-I (B). To create the skin patch, human adipose-derived mesenchymal stem cells (ASCs) and endothelial progenitor cells (EPCs) were loaded in the dECM bioink and deposited via extrusion bioprinting [Citation10]. The wound healing ability of bioprinted ASCs/EPCs-laden dECM patch was evaluated in vivo by implantation of the pre-cultured patch in excisional wounds created on BALB/c mice. The cell-laden dECM patch promoted superior wound healing by accelerating hemostasis, epithelialization and angiogenesis when compared with other experimental groups, including (i) no treatment (Hank’s Balanced Salt Solution), (ii) cell-free dECM, (iii) ASCs/EPCs injection, (iv) ASCs-laden patch, and (v) ASCs/EPCs-laden pre-vascularized patch.
In addition to recreating the multilayer skin structure, a crucial process to enhance the biological function of new skin relies on the immunomodulatory properties of bioprinted skin substitutes and their integration into the host tissue. In this regard, Chen et al. [Citation126] engineered a cell-free 3D bioprinted dermal skin graft using a biomaterial ink composed of porcine dermal dECM that was crosslinked using glutaraldehyde. In vivo experiments in rats showed that graft implantation reduced wound contraction and scarring, while promoting the polarization of macrophages toward the M2 phenotype, leading to a reduced inflammation compared with a standard implantable artificial dermis (Pelnac®) (C). Pioneer work using dECM bioinks has also been done to create in vitro 3D models for skin disease modelling. Kim et al. [Citation145] combined extrusion and inkjet-based bioprinting, along with dECM bioinks obtained from healthy porcine dermis, hypodermis and vascular tissues to generate a diabetic skin model. The multilayered model comprising vessel structures reproduced several hallmarks of native diabetic skin, including delayed re-epithelialization, vascular dysfunction, increased insulin resistance, adipose hypertrophy and inflammatory response (D).
Besides the advances regarding the design of dECM-based bioinks for tissue-engineered skin, progress has also been done in the development of bioprinting strategies to create 3D constructs with specific properties. One example includes the extrusion cryogenic 3D printing technology, in which the water will freeze in the extruded slurry, leading to phase separation and rapid 3D forming. This is followed by lyophilization to generate a porous structure that supports cell adhesion, growth and proliferation [Citation148]. Following this strategy, a biomaterial ink based on decellularized porcine SIS was used for bioprinting 3D constructs with controlled architecture and capable of regulating cell migration and proliferation [Citation9]. Bioprinted constructs seeded with rat normal skin fibroblasts promoted cell proliferation and the secretion of ECM proteins such as collagen I, collagen III and fibronectin. Although the dECM was obtained from porcine small intestine, these results demonstrate positive effects on skin fibroblasts suggesting a potential ECM source for skin tissue engineering. Major challenges in ECM bioink design rely on the limited knowledge regarding the impact of the decellularization methods on the cellular response to dECM as well as on the impact of tissue-specificity of dECM-based biomaterials on the biological function of bioprinted constructs. A recent study employed proteomic analyses to investigate the structural and molecular alterations during the processing of ECM obtained from different tissues (liver, heart, skin and cornea) [Citation76]. Human bone marrow mesenchymal stem cells bioprinted within dECM bioinks from different sources showed specific gene expression patterns that resembled the prominent properties of the native tissue. These data suggest that each dECM possess a unique set of tissue-specific matrisome proteins that play a crucial role in tissue-specific cellular growth and differentiation, highlighting the interplay between dECM source and cell fate.
Design & bioprinting of multimaterial dECM-containing bioinks
Decellularized extracellular matrix-based bioinks enable superior biomimicry of tissue- and cell-specific microenvironmental niches, providing a myriad of structural, biophysical and biochemical cues to the cells. Despite this is a major benefit when compared with other commonly used biomaterials (e.g., alginate, hyaluronic acid, chitosan), the methods of ECM decellularization and solubilization often result in bioinks exhibiting low viscosity and limited mechanical properties [Citation134]. Depending on the bioprinting technology, low viscosity bioinks might either translate into poor printability or require the use of alternative bioprinting strategies (e.g., embedded bioprinting), while poor mechanical properties might limit their application for hard tissue engineering or present the cells with non-tissue specific mechanical cues. A common approach to surpass these limitations relies on the design of multimaterial bioinks by combining dECM with natural polymers, which have been used as rheology modifiers to improve bioink printability being either removed post-bioprinting or integrating the bioprinted construct to enhance the mechanical properties [Citation28,Citation149].
Biomaterial selection for the design of multimaterial dECM-containing bioinks depends on several factors such as (i) the native biophysical and biochemical properties of the selected biomaterial, (ii) the biomaterial role in the (bio)ink formulation, and (iii) the target application. Some naturally-derived polymers, like collagen, gelatin and fibrinogen, inherently contain cell responsive sites in native composition promoting cell adhesion and enabling cell-mediated remodeling. On the other hand, despite alginate lacks cell responsive sites in native state, it can form physical hydrogels under cytocompatible conditions via ionic crosslinking, being mostly explored as rheology modifier or as a mechanical reinforcement. Although the use of synthetic polymers in dECM-bioink design is very limited, they exhibit a chemically-defined composition and superior mechanical properties, which can be useful in applications requiring high mechanical performance. Furthermore, depending on whether the bioprinted construct is intended to be acellular or cellularized, the selection of biomaterials and crosslinking chemistries can also differ. While acellular constructs can be bioprinted using highly viscous biomaterial inks and crosslinked under non-physiological conditions widening the range of available materials and crosslinking reactions, cell-laden constructs require that bioink formulation, bioprinting and crosslinking proceed under cell-compatible settings, which imposes additional constrains to bioink design [Citation62]. Polymers are widely used for bioink design due to their broad range of properties, along with the ability to afford biofunctionalization and crosslinking via chemical and physical reactions. By exploring biofunctionalization reactions it is possible to engineer polymers with cell responsive cues (e.g., cell adhesion sites or degradation sites) [Citation137], while its chemical modification allows the crosslinking of hydrogels via specific reactions including physical, covalent and dynamic covalent chemistries [Citation150]. Indeed, it has been demonstrated that polymer biofunctionalization and modification are efficient in tunning the hydrogel printability, viscoelasticity and cell response in 3D [Citation151]. Furthermore, the introduction of specific functional groups into polymer backbone such as aldehydes has been employed to promote reaction with amine groups in skin ECM to enhance bioadhesion and tissue integration [Citation152].
The selection of crosslinking reactions for bioink design is of utmost importance as it will dictate several hydrogel properties that affect not only the bioink printability, but also the cell response in bioprinted constructs. Physical crosslinking reactions such as ionic and thermal gelation are characterized by the reversibility of the crosslinks, contributing to the viscoelastic nature of resulting hydrogels [Citation153]. Despite the relatively low mechanical properties of physically crosslinked constructs, physical gelation has been used to modulate the bioink rheology as well as for post-bioprinting shape fixation of hydrogel constructs [Citation75,Citation154]. Chemical crosslinking commonly involves the establishment of stable and permanent covalent bonds using reactions such as photoinitiated thiol-ene click chemistry and thiol-Michael addition reaction. Despite such reactions impart long-term stability and superior mechanical properties to bioprinted constructs when compared with physical crosslinking, it has been shown that cellular responses within static hydrogels are dependent on the stiffness and network degradation [Citation155,Citation156]. Due to the non-reversible nature of stable covalent crosslinks, covalent crosslinking is often used to control the mechanical properties of bioprinted constructs [Citation137]. Recent developments have been focused on exploring dynamic chemistries for bioink design, including reversible supramolecular interactions (e.g., host–guest interactions) and reversible covalent chemical reactions (e.g., hydrazone bonds, Schiff based bonds) [Citation157]. Such chemistries result in the formation of adaptable bonds in the polymer network, which can dissociate and re-associate under physiological conditions, imparting shear thinning behavior for extrusion bioprinting and time-dependent mechanical properties that better recapitulate the mechanical behavior of native ECMs [Citation158,Citation159].
Collagen & gelatin/dECM-based bioinks
Collagen is the main ECM component remaining the gold standard in skin tissue engineering due to its cell responsive properties [Citation160]. Despite collagen type-I being the primary component in the dECM, it has been combined with dECM to enhance printability and structural integrity of bioprinted constructs, enabling the fabrication of 3D constructs with clinically relevant dimensions. Following this strategy, Behre et al. [Citation161] designed biomaterial inks by combining purified collagen type-I and dECM from urinary bladder matrix or skeletal muscle matrix for FRESH printing of personalized patches for soft tissue reconstruction. Bioprinted acellular patches (≈12 × 8 × 2 cm) demonstrated high fidelity and dimensional accuracy, being able to conform to the defect of a canine volumetric muscle loss model of a penetrating open wound consisting of skin, adipose, facia, and muscle tissue (A). In vitro assays indicated that collagen/dECM from the urinary bladder matrix performed better regarding cell proliferation and greater number of macrophages with M2 phenotype than both collagen and collagen/dECM from skeletal muscle. Despite the effect of FRESH printed patch on wound healing was not evaluated, this study demonstrates the design and bioprinting of large patient-specific patches from dECM-containing inks. Moreover, further studies are warranted to determine if an acellular and centimeter scale patch is efficient in promoting uniform tissue repair and, in particular, for large and deep skin injuries.
(A) Manipulation of the bioprinted dECM patch (i) and its ability to fit to the wound bed (ii). (B) Effect of gelatin incorporation on the printability of dECM inks and comparison to Pluronic F 127 ink (scale bar: 10 mm). (C) Schematic illustration of the design, bioprinting and implantation of ADSCs-laden ECM–GelMA–HAMA constructs. (D) Macroscopic images of the wound size after treatment with Alg/gel and 5%ECM-Alg/gel 3D printed scaffolds during 21 days upon implantation. (E) Schematic illustration of the process of fabrication of the skin model of hypertrophic scar.
ADSC: Adipose-derived stem cells; Alg: Alginate; dECM: Decellularized extracellular matrix; ECM: Extracellular matrix; GelMA: Gelatin-methacryloyl; HAMA: Methacrylated hyaluronic acid; PCA: Preformed cellular aggregates.
Modified with permission from [Citation7,Citation14,Citation79,Citation161,Citation162].
![Figure 3. Design and bioprinting of multimaterial dECM-containing bioinks for skin tissue engineering.(A) Manipulation of the bioprinted dECM patch (i) and its ability to fit to the wound bed (ii). (B) Effect of gelatin incorporation on the printability of dECM inks and comparison to Pluronic F 127 ink (scale bar: 10 mm). (C) Schematic illustration of the design, bioprinting and implantation of ADSCs-laden ECM–GelMA–HAMA constructs. (D) Macroscopic images of the wound size after treatment with Alg/gel and 5%ECM-Alg/gel 3D printed scaffolds during 21 days upon implantation. (E) Schematic illustration of the process of fabrication of the skin model of hypertrophic scar.ADSC: Adipose-derived stem cells; Alg: Alginate; dECM: Decellularized extracellular matrix; ECM: Extracellular matrix; GelMA: Gelatin-methacryloyl; HAMA: Methacrylated hyaluronic acid; PCA: Preformed cellular aggregates.Modified with permission from [Citation7,Citation14,Citation79,Citation161,Citation162].](/cms/asset/96fd6a13-76b8-4326-8947-ea2fc0ec579c/idpm_a_12366990_f0003.jpg)
Gelatin is one of the most used polymers to design multimaterial dECM-containing (bio)inks for skin bioprinting. The thermosensitive behavior of gelatin enables easy modulation of its printability, while its chemical modification with photosensitive groups, like methacrylates, yielding gelatin-methacryloyl (GelMA), makes it an attractive biomaterial in 3D bioprinting [Citation13,Citation14]. Methacrylation is the standard chemical modification of gelatin, offering a mechanically tunable system in which mechanical and biological properties can be easily controlled by varying the modification degree, polymer concentration and photopolymerization time [Citation39]. Moreover, being a collagen derivative protein, it is biodegradable and contains RGD sequences, which improves cell interaction and biological response [Citation13]. Sarmin et al. [Citation7] used gelatin to modulate the viscosity of a porcine abdominal skin dECM-based ink. Gelatin incorporation allowed the extrusion bioprinting of a free-form and well-defined 3D construct that supported the formation of a stratified epidermis in skin models (B). Although unmodified gelatin has been widely used in skin bioprinting, its thermoreversible properties make gelatin unstable at physiological temperature. In this regard, Jin et al. [Citation39] used GelMA and dermal dECM from porcine skin to produce a full thickness skin graft comprising dermal and epidermal layers by the sequential bioprinting of human umbilical vein endothelial cells (HUVECs)-loaded 10% GelMA bioink, human primary fibroblasts-loaded 1.5% dECM bioink and 20% GelMA ink to support seeded HaCaT cells for epidermis formation. Upon implantation in wounds created on dorsal mouse skin, a more efficient neovascularization and accelerated wound closure were reported on groups treated with dECM constructs when compared with the GelMA-only constructs. It was suggested that as dECM retains main skin ECM components, it promotes the in vivo growth of blood vessels, while GelMA, as a permissive biomaterial, supports vascular morphogenesis and lumen formation.
Fibrinogen/dECM-based bioinks
Fibrinogen has been widely used in bioink design for skin bioprinting due to its biocompatibility, biodegradability, nanofibrous structure and rapid crosslinking in the presence of thrombin yielding fibrin hydrogels [Citation163]. However, there is limited evidence regarding the ability of fibrin hydrogels in promoting the formation of a mature skin ECM. A prior study has reported that implantation of a bioprinted cellularized construct made of a fibrin hydrogel containing gelatin, glycerol and hyaluronic acid (HA) in skin defects created in mice resulted in the formation of immature ECM post-implantation [Citation164]. To improve the biological response, Jorgensen et al. [Citation11] developed a multimaterial ink by combining human skin-derived dECM, fibrinogen, gelatin and HA. Fibrinogen was subsequently crosslinked with thrombin improving the mechanical strength of fabricated hydrogels. Furthermore, the presence of dECM enhanced the structural integrity and biological properties of hydrogels as demonstrated by the superior storage modulus, cellularity and viability of primary human skin fibroblasts 15 days post-bioprinting. To increase the biomimicry and structural complexity of in vitro skin models, Kim et al. [Citation146] generated a perfusable tri-layer 3D skin equivalent by integrating the 3D printing of a customized PCL-based transwell, the extrusion bioprinting of bioinks and sacrificial gelatin ink, and the inkjet bioprinting of human epidermal keratinocytes. To recapitulate the site-specific cell microenvironment, fibrinogen was combined with either adipose-derived dECM bioink containing preadipocytes or skin-derived dECM bioink containing HDFs for the extrusion bioprinting of the hypodermal and dermal compartments, respectively. Afterward, inkjet bioprinting of human keratinocytes was employed for epidermis reconstruction. A vascular channel was also included in the dermis by the extrusion bioprinting of a vascular bioink comprised of HUVECs and thrombin-embedded within gelatin hydrogel. The bioprinted 3D skin model showed successful formation of mature epidermis, establishment of epidermal-dermal junction, the secretion of representative dermal ECM components, and the presence of mature adipocytes with lipid droplets, providing a biomimetic skin model that closely resembles key features of native human skin.
Hyaluronic acid/dECM-based bioinks
HA is a native component of human skin ECM and plays a significant role in wound healing, cell differentiation and angiogenesis [Citation165]. Moreover, HA has a strong moisture retaining ability, which is an important feature for wound healing, relieving the typical desiccation and contraction of bioprinted skin substitutes [Citation138]. While unmodified high molecular weight HA has been employed in the context of bioprinting to improve bioink printability [Citation166], methacrylated hyaluronic acid (HAMA) is mainly used to enhance the mechanical properties of bioprinted constructs through the formation of stable covalent bonds via photocrosslinking [Citation79]. Recently, a photocrosslinkable multimaterial bioink comprised of human adipose tissue-derived dECM (adECM), HAMA and GelMA was designed for the extrusion bioprinting of human adipose-derived stem cells (hADSCs)-laden constructs for wound healing (C) [Citation79,Citation138]. After 14 days of implantation in full-thickness excisional skin wounds created in nude mice, bioprinted cell-laden skin substitutes promoted complete wound closure and significantly reduced the wound area when compared with cell-free substitutes made of either adECM/GelMA/HAMA or GelMA/HAMA [Citation79]. Interestingly, in vivo results indicated a similar closure of wounds treated with cell-laden substitutes composed of adECM/GelMA/HAMA and GelMA–HAMA, suggesting a major contribution of hADSCs rather than adECM to wound healing. However, histopathological analysis of newly formed skin showed the positive effects of both hADSCs and adECM in promoting collagen III/I ratio, collagen deposition, and wound neovascularization [Citation79].
Alginate/dECM-based bioinks
Alginate is an attractive natural polymer that exhibits fast and cytocompatible gelation, being widely used in the field of bioprinting to improve bioink printability. Since the chemical modification of polymers for the design of multimaterial dECM-based bioinks usually involves multiple steps of synthesis, purification and characterization that increase the complexity, an alternative strategy involves the use of unmodified polymers that impart specific features to the bioink. In this regard, Bashiri et al. [Citation14] explored the shear-thinning behavior of alginate and the cell responsive properties of gelatin to modulate the printability and mechanical properties of placenta-derived dECM. While dECM-only biomaterial inks were not printable due to limited rheological properties and poor structural stability, the addition of alginate and gelatin enabled the printing of 3D dECM-containing constructs with good shape fidelity. Printed constructs were crosslinked using a combination of calcium chloride and glutaraldehyde, and evaluated for their angiogenic and wound healing properties. A significant growth in the number of blood vessels and wound size reduction were observed for dECM-containing constructs compared with alginate/gelatin scaffolds as observed through the CAM assay and construct implantation into full thickness wound model created in mouse (D). Recently, alginate, gelatin and collagen were combined with dECM obtained from human epidermis and hypodermis scar tissue for bioprinting a skin model of hypertrophic scar (E) [Citation162]. The 3D model was bioprinted with fibroblasts isolated from human scars and resembled key features of hypertrophic scars including myofibroblast activation and transforming growth factor beta 1 (TGF-β1) secretion. As alginate lacks cell binding sites in native composition, Lee et al. [Citation75] used uncrosslinked alginate to improve the viscosity of a porcine skin dECM bioink and evaluated the impact of alginate content on printability and cell response. It was found that a minimum alginate concentration of 2% is required for the extrusion bioprinting of 3D constructs without compromising the viability and metabolic activity of fibroblast cell line NIH3T3 within post-bioprinting calcium-crosslinked hydrogels. Moreover, results show enhanced cell metabolic activity and viability in bioprinted alginate constructs containing 10 mg/ml of dECM compared to 20 mg/ml dECM, suggesting that higher dECM content might inhibit cell growth. Further assessment of the shape fidelity of bioprinted constructs is warranted to evaluate the impact of uncrosslinked alginate in bioink printability and structural complexity of fabricated constructs.
Chitosan/dECM-based bioinks
Chitosan has been explored for bioink design owing to its excellent biocompatibility, hemostasis capability and inherent antibacterial activity. As the limited water-solubility of chitosan can hinder their combination with dECM and hydrophilic polymers, quaternized chitosan was recently combined with gelatin and porcine skin-derived dECM for the fabrication of 3D constructs with antibacterial properties [Citation13,Citation167]. Xu et al. [Citation13] used extrusion bioprinting to create porous scaffolds, which were crosslinked using EDC/NHS to preclude dissolution at physiological temperature, and subsequently lyophilized. Afterward, poly(ionic liquid)s (PILs) were assembled onto the scaffold surface via hydrogen bonding and lyophilized again. Scaffolds presented hemostatic properties and antibacterial activity against Gram-negative (E. coli) and Gram-positive (S. aureus) bacteria. While dECM/gelatin scaffold did not show bactericidal properties and even promoted bacterial growth, dECM/gelatin/quaternized chitosan scaffolds (74.28% of E. coli and 85.63% of S. aureus) and PILs-containing dECM/gelatin/quaternized chitosan scaffolds (almost 100%) killed more than 70% of bacteria after 24 h of culture. The excellent antibacterial activity of PILs-containing dECM/gelatin/quaternized chitosan scaffolds is attributed to their rapid release at 37°C. Despite the promising results, the antibacterial activity and wound healing ability of scaffolds should be investigated in relevant models to assess their effectiveness.
Conclusion
The ECM is emerging as an important material source to design biomimetic (bio)inks for the bioprinting of skin substitutes and 3D skin models. By leveraging dECM, it becomes possible to craft (bio)inks customized for specific cells or tissues, facilitating the replication of native biophysical and biochemical cues. The task of designing dECM-based (bio)inks is inherently challenging, as it needs to consider multiple steps ranging from the choice of the ECM source and its processing to the bioprinting technology and application.
The selection of tissue or organ source is a key decision that influences the ultimate success of dECM-based (bio)inks. Despite human- and porcine-derived skin dECM being the most common sources, other human-derived tissues, such as the placenta and adipose tissue, offer notable advantages regarding its biological relevance, safety, and ethical considerations. Moreover, fish skin has recently raised attention and can be considered a promising alternative, which combines safety, antimicrobial properties and sustainability.
The decellularization process requires a delicate balance between effective removal of cellular components and preservation of ECM integrity. Additionally, quality control measures, including quantitative metrics and antigen assessment, ensure the safety and effectiveness of dECM materials. Post-processing steps, like drying and sterilization, are crucial for stability and safety in bioprinting applications. The choice of sterilization method should consider not only effectiveness, but also potential alterations in the ECM properties.
Extrusion bioprinting is the prime technology for dermal reconstruction via bioprinting of cell-laden dECM-based bioinks, while the highthroughput nature of inkjet bioprinting is effective on enabling the deposition of keratinocytes for epidermis formation. Although extrusion bioprinting of dECM (bio)inks has proven to be challenging due to its low viscosity and limited mechanical properties, natural polymers have been combined with dECM to design multimaterial (bio)inks with superior properties. While the role of natural polymers in enhancing the rheology and printability of dECM (bio)inks is well-documented, how their incorporation contributes to improve biological response remains unclear. Despite the current knowledge suggesting that the presence of dECM is essential to improve the biological response of bioprinted multimaterial constructs [Citation11], there is a lack of strong evidence demonstrating whether multimaterial constructs are superior in promoting wound healing. Pre-clinical studies provide encouraging outcomes regarding the healing ability of bioprinted dECM-containing constructs, while reconstructed in vitro skin models offer valuable platforms for translational studies.
Future perspective
Novel developments on decellularization techniques should focus on reducing the reliance on harsh chemical agents and minimizing the damage to the ECM structure, as well as on ensuring protocol and quality control standardization, which will be essential for the widespread adoption of dECM-based bioprinting in clinical settings. From a bioprinting perspective, despite photocrosslinkable dECM-based bioinks being reported [Citation168], the use of light-based bioprinting to create dECM-based constructs remains to be explored. Such a bioprinting technology could be of interest to create 3D skin constructs with microscale resolution and functional gradients. Future studies should also focus on the assessment of bioprinted dECM-based skin substitutes using human relevant models of wound healing as well as on the characterization and validation of bioprinted in vitro skin models. The use of bioprinted 3D skin equivalents to serve themselves as standardized in vitro skin models is also an important emergent area which finds its application in drug screening and to study a diversity of skin diseases.
Biomaterials with improved biomimicry are required to create biologically functional grafts for skin repair and sophisticated in vitro skin models.
Decellularized extracellular matrix (dECM)-based bioinks are emerging as powerful building blocks for skin bioprinting.
Decellularized extracellular matrix: from source to bioink design
The selection of tissue sources for dECM bioink design is a critical factor, with human-derived tissues offering biological relevance, safety and ethical advantages, and animals offering cost-efficient alternatives.
The efficiency of the decellularization process, including the choice of agents and methods, is crucial for clinical translation.
Post-processing drying and sterilization protocols to maintain dECM integrity should be emphasized.
Bioprinting of dECM-based bioinks for skin applications
The dECM bioinks often display limited printability.
The dECM enhances the cellular response and biological function of in vitro skin models.
The combination of dECM with natural and synthetic polymers improves bioink printability and mechanical properties of bioprinted constructs.
Conclusion
The decellularization process requires a delicate balance between effective removal of cellular components and preservation of ECM integrity.
Skin grafts and in vitro models containing dECM are being bioprinted using extrusion- and inkjet-based techniques.
The dECM improves the biological function of bioprinted skin in vitro, but scientific evidence from in vivo studies is still scarce.
Future perspective
Decellularization methods should be improved regarding the standardization, safety and efficacy.
The therapeutic efficacy of bioprinted dECM-based skin grafts should be evaluated in human relevant models of wound healing.
Bioprinted 3D skin models will potentially serve as a tool for drug screening and mechanistic studies.
Acknowledgments
The authors acknowledge the BE@T – Bioeconomy for Textiles and Apparel, investment TC-C12-i01 – Sustainable Bioeconomy, funded through the Recovery and Resilience Program (PRR) and the FCT through the project UID/Multi/50016/2020. JB Costa, IV Silva and R Presa are grateful to FCT for the individual Junior Researcher contract (doi.org/10.54499/2022.02781.CEECIND/CP1745/CT0001), and Doctoral Research Grants (2021.05919.BD) and (2022.13383.BD), respectively.
Financial disclosure
This work was supported by FEDER – Fundo Europeu de Desenvolvimento Regional funds through COMPETE 2020 – Operacional Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and Portuguese funds via FCT – Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior in the framework of the projects doi.org/10.54499/2022.06048.PTDC and UID/BIM/04293/2020, as well as by the project IBEROS+ (0072_IBEROS_MAIS_1_E) via “Interreg VI A Espana – Portugal (POCTEP) 2021-2027”. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, stock ownership or options and expert testimony.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
References
- Takeo M, Lee W, Ito M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015;5(1):a023267.
- Tottoli EM, Dorati R, Genta I et al. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020;12(8):735.
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med. 2014;6(265):265sr6.
- Rezvani Ghomi E, Khalili S, Nouri Khorasani S et al. Wound dressings: current advances and future directions. J. Appl. Poly. Sci. 2019;136(27):47738.
- Dearman BL, Boyce ST, Greenwood JE. Advances in skin tissue bioengineering and the challenges of clinical translation [mini review]. Front. Surg. 2021;8.
- Chouhan D, Dey N, Bhardwaj N et al. Emerging and innovative approaches for wound healing and skin regeneration: current status and advances. Biomaterials 2019;216:119267.
- Sarmin AM, El Moussaid N, Suntornnond R et al. Multi-scale analysis of the composition, structure, and function of decellularized extracellular matrix for human skin and wound healing models. Biomolecules 2022;12(6):837.
- Booth AJ, Hadley R, Cornett AM et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am. J. Respir. Crit. Care Med. 2012;186(9):866–876.
- Shi L, Hu Y, Ullah MW et al. Cryogenic free-form extrusion bioprinting of decellularized small intestinal submucosa for potential applications in skin tissue engineering. Biofabrication 2019;11(3):035023.
- Kim BS, Kwon YW, Kong J-S et al. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: a step towards advanced skin tissue engineering. Biomaterials 2018;168:38–53.
- Jorgensen AM, Chou Z, Gillispie G et al. Decellularized skin extracellular matrix (dsECM) improves the physical and biological properties of fibrinogen hydrogel for skin bioprinting applications. Nanomaterials 2020;10(8):1484.
- Gu Z, Fu J, Lin H et al. Development of 3D bioprinting: from printing methods to biomedical applications. Asian J. Pharmaceut. Sci. 2020;15(5):529–557.
- Xu J, Fang H, Su Y et al. A 3D bioprinted decellularized extracellular matrix/gelatin/quaternized chitosan scaffold assembling with poly(ionic liquid)s for skin tissue engineering [Article]. Int. J. Biol. Macromol. 2022;220:1253–1266.
- Bashiri Z, Rajabi Fomeshi M, Ghasemi Hamidabadi H et al. 3D-printed placental-derived bioinks for skin tissue regeneration with improved angiogenesis and wound healing properties. Mat. Today Bio. 2023;20:100666.
- Groll J, Burdick JA, Cho DW et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication. 2019;11(1):013001.
- Saldin LT, Cramer MC, Velankar SS et al. Extracellular matrix hydrogels from decellularized tissues: structure and function. Acta Biomater. 2017;49:1–15.
- Saricilar EC, Huang S. Comparison of porcine and human acellular dermal matrix outcomes in wound healing: a deep dive into the evidence. Arch. Plast. Surg. 2021;48(4):433–439.
- Cicuéndez M, Casarrubios L, Feito MJ et al. Effects of human and porcine adipose extracellular matrices decellularized by enzymatic or chemical methods on macrophage polarization and immunocompetence. Int. J. Mol. Sci. 2021;22(8):3847.
- Dagher G. Quality matters: international standards for biobanking. Cell Prolif. 2022;55(8):e13282.
- Malek A, Bersinger NA. Human placental stem cells: biomedical potential and clinical relevance. J. Stem Cells 2011;6(2):75–92.
- Pogozhykh O, Prokopyuk V, Figueiredo C et al. Placenta and placental derivatives in regenerative therapies: experimental studies, history, and prospects. Stem Cells Int. 2018;2018:4837930.
- Pérez ML, Castells-Sala C, López-Chicón P et al. Fast protocol for the processing of split-thickness skin into decellularized human dermal matrix. Tissue Cell 2021;72:101572.
- Moore MA, Samsell B, Wallis G et al. Decellularization of human dermis using non-denaturing anionic detergent and endonuclease: a review. Cell Tissue Bank. 2015;16(2):249–259.
- Hahn HM, Lee DH, Lee IJ. Ready-to-use micronized human acellular dermal matrix to accelerate wound healing in diabetic foot ulcers: a prospective randomized pilot study. Adv. Skin Wound Care 2021;34(5):1–6.
- Mineta S, Endo S, Ueno T. Optimization of decellularization methods using human small intestinal submucosa for scaffold generation in regenerative medicine. Int. J. Exp. Pathol. 2023;104(6):313–320.
- Mirsadraee S, Wilcox HE, Korossis SA et al. Development and characterization of an acellular human pericardial matrix for tissue engineering. Tissue Eng. 2006;12(4):763–773.
- Mirsadraee S, Wilcox HE, Watterson KG et al. Biocompatibility of acellular human pericardium. J. Surg. Res. 2007;143(2):407–414.
- Kim BS, Das S, Jang J et al. Decellularized extracellular matrix-based bioinks for engineering tissue- and organ-specific microenvironments. Chem. Rev. 2020;120(19):10608–10661.
- Boulan L, Léopold P. What determines organ size during development and regeneration? Development 2021;148(1):dev196063.
- Buzi G, Lander AD, Khammash M. Cell lineage branching as a strategy for proliferative control. BMC Biol. 2015;13(1):13.
- Lu Y, Shao A, Shan Y et al. A standardized quantitative method for detecting remnant alpha-Gal antigen in animal tissues or animal tissue-derived biomaterials and its application. Scient. Reports 2018;8(1):15424.
- Joziasse DH, Oriol R. Xenotransplantation: the importance of the Galα1,3Gal epitope in hyperacute vascular rejection. Biochim. Biophys. Acta (BBA) 1999;1455(2):403–418.
- Sandrin MS, McKenzie IF. Gal alpha (1,3)Gal, the major xenoantigen(s) recognised in pigs by human natural antibodies. Immunol. Rev. 1994;141:169–190.
- Boneva RS, Folks TM, Chapman LE. Infectious disease issues in xenotransplantation. Clin. Microbiol. Rev. 2001;14(1):1–14.
- Ibrahim M, Ayyoubi HS, Alkhairi LA et al. Fish skin grafts versus alternative wound dressings in wound care: a systematic review of the literature. Cureus 2023;15(3):e36348.
- Magnusson S, Baldursson BT, Kjartansson H et al. Regenerative and antibacterial properties of acellular fish skin grafts and human amnion/chorion membrane: implications for tissue preservation in combat casualty care. Milit. Med. 2017;182(Suppl. 1):383–388.
- Coppola D, Lauritano C, Palma Esposito F et al. Fish waste: from problem to valuable resource. Mar. Drugs 2021;19(2):116.
- Leng L, Ma J, Sun X et al. Comprehensive proteomic atlas of skin biomatrix scaffolds reveals a supportive microenvironment for epidermal development. J. Tissue Eng. 2020;11:2041731420972310.
- Jin RH, Cui YC, Chen HJ et al. Three-dimensional bioprinting of a full-thickness functional skin model using acellular dermal matrix and gelatin methacrylamide bioink. Acta Biomater. 2021;131:248–261.
- Kawakatsu M, Urata Y, Goto S et al. Placental extract protects bone marrow-derived stem/progenitor cells against radiation injury through anti-inflammatory activity. J. Radiat. Res. 2013;54(2):268–276.
- Bak DH, Na J, Im SI et al. Antioxidant effect of human placenta hydrolysate against oxidative stress on muscle atrophy. J. Cell. Physiol. 2019;234(2):1643–1658.
- Hong JW, Lee WJ, Hahn SB et al. The effect of human placenta extract in a wound healing model. Ann. Plast. Surg. 2010;65(1):96–100.
- Igarashi K, Sugimoto K, Hirano E. Placental extract suppresses the formation of fibrotic deposits by tumor necrosis factor alpha and transforming growth factor beta-induced epithelial-mesenchymal transition in ARPE-19 cells. BMC Res. Notes. 2021;14(1):407.
- Hao Y, Ma DH, Hwang DG et al. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 2000;19(3):348–352.
- Talmi YP, Sigler L, Inge E et al. Antibacterial properties of human amniotic membranes. Placenta 1991;12(3):285–288.
- Tan Q-W, Tang S-L, Zhang Y et al. Hydrogel from acellular porcine adipose tissue accelerates wound healing by inducing intradermal adipocyte regeneration. J. Invest. Dermatol. 2019;139(2):455–463.
- Pati F, Ha DH, Jang J et al. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials 2015;62:164–175.
- Cao G, Huang Y, Li K et al. Small intestinal submucosa: superiority, limitations and solutions, and its potential to address bottlenecks in tissue repair. J. Mat. Chem. B. 2019;7(33):5038–5055.
- McDevitt CA, Wildey GM, Cutrone RM. Transforming growth factor-beta1 in a sterilized tissue derived from the pig small intestine submucosa. J. Biomed. Mater. Res. A 2003;67(2):637–640.
- Magden GK, Vural C, Bayrak BY et al. Composite sponges from sheep decellularized small intestinal submucosa for treatment of diabetic wounds. J. Biomater. Appl. 2021;36(1):113–127.
- Shi L, Ramsay S, Ermis R et al. In vitro and in vivo studies on matrix metalloproteinases interacting with small intestine submucosa wound matrix. Int. Wound J. 2012;9(1):44–53.
- Capella-Monsonís H, Tilbury MA, Wall JG et al. Porcine mesothelium matrix as a biomaterial for wound healing applications. Mater. Today Bio. 2020;7:100057.
- Witz CA, Montoya-Rodriguez IA, Cho S et al. Composition of the extracellular matrix of the peritoneum. J. Soc. Gynecol. Investig. 2001;8(5):299–304.
- Hoganson DM, Owens GE, O’Doherty EM et al. Preserved extracellular matrix components and retained biological activity in decellularized porcine mesothelium. Biomaterials 2010;31(27):6934–6940.
- Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials 2011;32(12):3233–3243.
- Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials 2006;27(19):3675–3683.
- Prasertsung I, Kanokpanont S, Bunaprasert T et al. Development of acellular dermis from porcine skin using periodic pressurized technique. J. Biomed. Mater. Res. B Appl. Biomater. 2008;85(1):210–219.
- Reing JE, Brown BN, Daly KA et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials 2010;31(33):8626–8633.
- Chen RN, Ho HO, Tsai YT et al. Process development of an acellular dermal matrix (ADM) for biomedical applications. Biomaterials 2004;25(13):2679–2686.
- Funamoto S, Nam K, Kimura T et al. The use of high-hydrostatic pressure treatment to decellularize blood vessels. Biomaterials 2010;31(13):3590–3595.
- Brown BN, Valentin JE, Stewart-Akers AM et al. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 2009;30(8):1482–1491.
- Pati F, Jang J, Ha D-H et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014;5(1):3935.
- Jeong W, Kim MK, Kang HW. Effect of detergent type on the performance of liver decellularized extracellular matrix-based bio-inks. J Tissue Eng. 2021;12:2041731421997091.
- Courtman DW, Pereira CA, Kashef V et al. Development of a pericardial acellular matrix biomaterial: biochemical and mechanical effects of cell extraction. J. Biomed. Mater. Res. 1994;28(6):655–666.
- Bodnar E, Olsen EG, Florio R et al. Damage of porcine aortic valve tissue caused by the surfactant sodiumdodecylsulphate. Thorac. Cardiovasc. Surg. 1986;34(2):82–85.
- Wu J, Ding Q, Dutta A et al. An injectable extracellular matrix derived hydrogel for meniscus repair and regeneration. Acta Biomater. 2015;16:49–59.
- Rieder E, Kasimir MT, Silberhumer G et al. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J. Thorac. Cardiovasc. Surg. 2004;127(2):399–405.
- Tudorache I, Cebotari S, Sturz G et al. Tissue engineering of heart valves: biomechanical and morphological properties of decellularized heart valves. J. Heart Valve Dis. 2007;16(5):567–573; discussion 574.
- Zhou J, Fritze O, Schleicher M et al. Impact of heart valve decellularization on 3-D ultrastructure, immunogenicity and thrombogenicity. Biomaterials 2010;31(9):2549–2554.
- Ozeki M, Narita Y, Kagami H et al. Evaluation of decellularized esophagus as a scaffold for cultured esophageal epithelial cells. J. Biomed. Mater. Res. A 2006;79(4):771–778.
- Kim MK, Jeong W, Lee SM et al. Decellularized extracellular matrix-based bio-ink with enhanced 3D printability and mechanical properties. Biofabrication 2020;12(2):025003.
- Grauss RW, Hazekamp MG, van Vliet S et al. Decellularization of rat aortic valve allografts reduces leaflet destruction and extracellular matrix remodeling. J. Thorac. Cardiovasc. Surg. 2003;126(6):2003–2010.
- Cartmell JS, Dunn MG. Effect of chemical treatments on tendon cellularity and mechanical properties. J. Biomed. Mater. Res. 2000;49(1):134–140.
- Roosens A, Somers P, De Somer F et al. Impact of detergent-based decellularization methods on porcine tissues for heart valve engineering. Ann. Biomed. Eng. 2016;44(9):2827–2839.
- Lee SJ, Lee JH, Park J et al. Fabrication of 3D printing scaffold with porcine skin decellularized bio-ink for soft tissue engineering. Materials 2020;13(16):9.
- Han W, Singh NK, Kim JJ et al. Directed differential behaviors of multipotent adult stem cells from decellularized tissue/organ extracellular matrix bioinks. Biomaterials 2019;224:119496.
- Liu P, Li Q, Yang Q et al. Three-dimensional cell printing of gingival fibroblast/acellular dermal matrix/gelatin–sodium alginate scaffolds and their biocompatibility evaluation in vitro [10.1039/D0RA02082F]. RSC Adv. 2020;10(27):15926–15935.
- Jang KS, Park SJ, Choi JJ et al. Therapeutic efficacy of artificial skin produced by 3D bioprinting. Materials (Basel) 2021;14(18):5177.
- Zhang D, Fu Q, Fu H et al. 3D-bioprinted human lipoaspirate-derived cell-laden skin constructs for healing of full-thickness skin defects. Int. J. Bioprint. 2023;9(4):718.
- Yu HW, Kim BS, Lee JY et al. Tissue printing for engineering transplantable human parathyroid patch to improve parathyroid engraftment, integration, and hormone secretionin vivo. Biofabrication 2021;13(3):035033.
- Nam H, Jeong H-J, Jo Y et al. Multi-layered free-form 3D cell-printed tubular construct with decellularized inner and outer esophageal tissue-derived bioinks. Scient. Rep. 2020;10(1):7255.
- Kim W, Kim GH. An intestinal model with a finger-like villus structure fabricated using a bioprinting process and collagen/SIS-based cell-laden bioink. Theranostics 2020;10(6):2495–2508.
- Dickman CTD, Russo V, Thain K et al. Functional characterization of 3D contractile smooth muscle tissues generated using a unique microfluidic 3D bioprinting technology. FASEB J. 2020;34(1):1652–1664.
- Lee H, Chae S, Kim JY et al. Cell-printed 3D liver-on-a-chip possessing a liver microenvironment and biliary system. Biofabrication 2019;11(2):025001.
- Duan Y, Huang W, Zhan B et al. Bioink derived from human placenta supporting angiogenesis. Biomed. Mater. 2022;17(5):055009.
- Badileanu A, Mora-Navarro C, Gracioso Martins AM et al. Fast automated approach for the derivation of acellular extracellular matrix scaffolds from porcine soft tissues. ACS Biomat. Sci. Engin. 2020;6(7):4200–4213.
- Fan Y, Lüchow M, Badria A et al. Placenta powder-infused thiol-ene PEG hydrogels as potential tissue engineering scaffolds. Biomacromolecules 2023;24(4):1617–1626.
- Bi H, Ye K, Jin S. Proteomic analysis of decellularized pancreatic matrix identifies collagen V as a critical regulator for islet organogenesis from human pluripotent stem cells. Biomaterials 2020;233:119673.
- Hubbell J. Matrix-bound growth factors in tissue repair. Swiss Med. Weekly 2006;136(25–26):387–391.
- Gurtovenko AA, Anwar J. Modulating the structure and properties of cell membranes: the molecular mechanism of action of dimethyl sulfoxide. J. Phys. Chem. B 2007;111(35):10453–10460.
- Ghorbani F, Ekhtiari M, Moeini Chaghervand B et al. Detection of the residual concentration of sodium dodecyl sulfate in the decellularized whole rabbit kidney extracellular matrix. Cell Tissue Bank. 2022;23(1):119–128.
- White LJ, Taylor AJ, Faulk DM et al. The impact of detergents on the tissue decellularization process: a ToF-SIMS study. Acta Biomater. 2017;50:207–219.
- Friedrich EE, Lanier ST, Niknam-Bienia S et al. Residual sodium dodecyl sulfate in decellularized muscle matrices leads to fibroblast activation in vitro and foreign body response in vivo. J. Tissue Eng. Regen. Med. 2018;12(3):e1704–e1715.
- Guler S, Aydin HM, Lü LX et al. Improvement of decellularization efficiency of porcine aorta using dimethyl sulfoxide as a penetration enhancer. Artif. Organs 2018;42(2):219–230.
- Kawazoye S, Tian SF, Toda S et al. The mechanism of interaction of sodium dodecyl sulfate with elastic fibers. J. Biochem. 1995;117(6):1254–1260.
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem. Biophys. Res. Commun. 1965;21(4):290–296.
- Girardeau-Hubert S, Lynch B, Zuttion F et al. Impact of microstructure on cell behavior and tissue mechanics in collagen and dermal decellularized extra-cellular matrices. Acta Biomat. 2022;143:100–114.
- Hamada S, Dubois V, Koenig A et al. Allograft recognition by recipient’s natural killer cells: molecular mechanisms and role in transplant rejection. Hla 2021;98(3):191–199.
- Lu T, Yang B, Wang R et al. Xenotransplantation: current status in preclinical research. Front. Immunol. 2019;10:3060.
- Chen Y, Mutukuri TT, Wilson NE et al. Pharmaceutical protein solids: drying technology, solid-state characterization and stability. Adv. Drug Deliv. Rev. 2021;172:211–233.
- Sheridan WS, Duffy GP, Murphy BP. Optimum parameters for freeze-drying decellularized arterial scaffolds. Tissue Eng. Part C Methods 2013;19(12):981–990.
- Zambon A, Vetralla M, Urbani L et al. Dry acellular oesophageal matrix prepared by supercritical carbon dioxide. J. Supercrit. Fluids 2016;115:33–41.
- Giobbe GG, Zambon A, Vetralla M et al. Preservation over time of dried acellular esophageal matrix. Biomed. Phys. Engin. Express 2018;4(6):065021.
- Tobin JJ, Walsh G. Medical Product Regulatory Affairs: Pharmaceuticals, Diagnostics, Medical Devices. Germany: Wiley; 2023.
- Mohapatra S. Sterilization and disinfection. Essent. Neuroanesth. 2017;929–944.
- Heseltine P. Book Review: Disinfection, Sterilization, and Preservation, 5th ed. SS Block, ed.; Philadelphia: Lippincott Williams & Wilkins, 2001; 1504 pages. Infect. Contr. Hospital Epidemiol. 2002;23(2):109.
- Wilson AJ, Nayak S. Disinfection, sterilization and disposables. Anaesth. Inten. Care Med. 2013;14(10):423–427.
- Soares GC, Learmonth DA, Vallejo MC et al. Supercritical CO2 technology: the next standard sterilization technique? Mat. Sci. Engin. 2019;99:520–540.
- Harrell CR, Djonov V, Fellabaum C et al. Risks of using sterilization by gamma radiation: the other side of the coin. Int. J. Med. Sci. 2018;15(3):274–279.
- Mrázová H, Koller J, Kubišová K et al. Comparison of structural changes in skin and amnion tissue grafts for transplantation induced by gamma and electron beam irradiation for sterilization. Cell Tissue Bank. 2016;17(2):255–260.
- von Versen-Hoeynck F, Steinfeld AP, Becker J et al. Sterilization and preservation influence the biophysical properties of human amnion grafts. Biologicals 2008;36(4):248–255.
- Rosario DJ, Reilly GC, Ali Salah E et al. Decellularization and sterilization of porcine urinary bladder matrix for tissue engineering in the lower urinary tract. Regen. Med. 2008;3(2):145–156.
- Pekkarinen T, Hietala O, Lindholm TS et al. Influence of ethylene oxide sterilization on the activity of native reindeer bone morphogenetic protein. Int. Orthop. 2004;28(2):97–101.
- Sloff M, Janke HP, de Jonge PKJD et al. The impact of γ-irradiation and EtO degassing on tissue remodeling of collagen-based hybrid tubular templates. ACS Biomat. Sci. Engin. 2018;4(9):3282–3290.
- Dai Z, Ronholm J, Tian Y et al. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016;7:2041731416648810.
- Boiano JM, Steege AL. Ethylene oxide and hydrogen peroxide gas plasma sterilization: precautionary practices in U.S. hospitals. Zentralsterilisation (Wiesb) 2015;23(4):262–268.
- Gosztyla C, Ladd MR, Werts A et al. A comparison of sterilization techniques for production of decellularized intestine in mice. Tissue Eng. Part C Methods 2020;26(2):67–79.
- Russell N, Oliver RA, Walsh WR. The effect of sterilization methods on the osteoconductivity of allograft bone in a critical-sized bilateral tibial defect model in rabbits. Biomaterials 2013;34(33):8185–8194.
- Nichols A, Burns D, Christopher R. Studies on the sterilization of human bone and tendon musculoskeletal allograft tissue using supercritical carbon dioxide. J. Orthopaed. 2009;6(2):e9.
- Qiu QQ, Leamy P, Brittingham J et al. Inactivation of bacterial spores and viruses in biological material using supercritical carbon dioxide with sterilant. J. Biomed. Mater. Res. B Appl. Biomater. 2009;91(2):572–578.
- Shaw J, Au L, Hull B et al. eds. Supercritical Carbon Dioxide Sterilization Minimally Affects Human Allograft Skin Morphology and Biomechanics. ASME 2010 Summer Bioengineering Conference; 2010.
- Balestrini JL, Liu A, Gard AL et al. Sterilization of lung matrices by supercritical carbon dioxide. Tissue Eng. Part C Methods 2016;22(3):260–269.
- Hennessy RS, Jana S, Tefft BJ et al. Supercritical carbon dioxide-based sterilization of decellularized heart valves. JACC Basic Transl. Sci. 2017;2(1):71–84.
- Wehmeyer JL, Natesan S, Christy RJ. Development of a sterile amniotic membrane tissue graft using supercritical carbon dioxide. Tissue Eng. Part C Methods 2015;21(7):649–659.
- Won J-Y, Lee M-H, Kim M-J et al. A potential dermal substitute using decellularized dermis extracellular matrix derived bio-ink. Artif. Cells Nanomed. Biotechnol. 2019;47(1):644–649.
- Chen L, Li Z, Zheng Y et al. 3D-printed dermis-specific extracellular matrix mitigates scar contraction via inducing early angiogenesis and macrophage M2 polarization. Bioact. Mat. 2022;10:236–246.
- Zhou F, Hong Y, Liang R et al. Rapid printing of bio-inspired 3D tissue constructs for skin regeneration. Biomaterials 2020;258:120287.
- Bernal PN, Delrot P, Loterie D et al. Volumetric bioprinting of complex living-tissue constructs within s. Advan. Mat. 2019;31(42):1904209.
- Bernal PN, Bouwmeester M, Madrid-Wolff J et al. Volumetric bioprinting of organoids and optically tuned hydrogels to build liver-like metabolic biofactories. Advan. Mat. 2022;34(15):2110054.
- Li W, Wang M, Ma H et al. Stereolithography apparatus and digital light processing-based 3D bioprinting for tissue fabrication. iScience 2023;26(2):106039.
- Li J, Chen M, Fan X et al. Recent advances in bioprinting techniques: approaches, applications and future prospects. J. Translat. Med. 2016;14(1):271.
- Li X, Liu B, Pei B et al. Inkjet bioprinting of biomaterials. Chem. Rev. 2020;120(19):10793–10833.
- Boularaoui S, Al Hussein G, Khan KA et al. An overview of extrusion-based bioprinting with a focus on induced shear stress and its effect on cell viability. Bioprinting 2020;20:e00093.
- Zhao F, Cheng J, Sun M et al. Digestion degree is a key factor to regulate the printability of pure tendon decellularized extracellular matrix bio-ink in extrusion-based 3D cell printing. Biofabrication 2020;12(4):045011.
- Hinton TJ, Jallerat Q, Palchesko RN et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Advan. 2015;1(9):e1500758.
- Ouyang L, Highley CB, Sun W et al. A generalizable strategy for the 3D bioprinting of hydrogels from nonviscous photo-crosslinkable Inks. Advan. Mater. 2017;29(8):1604983.
- Pereira RF, Lourenco BN, Bartolo PJ et al. Bioprinting a multifunctional bioink to engineer clickable 3D cellular niches with tunable matrix microenvironmental cues. Adv. Healthc. Mater. 2021;10(2):e2001176.
- Fu H, Zhang D, Zeng J et al. Application of 3D-printed tissue-engineered skin substitute using innovative biomaterial loaded with human adipose-derived stem cells in wound healing. Int. J. Bioprint. 2023;9(2):674.
- Muir VG, Qazi TH, Weintraub S et al. Sticking together: injectable granular hydrogels with increased functionality via dynamic covalent inter-particle crosslinking. Small 2022;18(36):2201115.
- Dorj B, Won JE, Kim JH et al. Robocasting nanocomposite scaffolds of poly(caprolactone)/hydroxyapatite incorporating modified carbon nanotubes for hard tissue reconstruction. J. Biomed. Mater. Res. A 2013;101(6):1670–1681.
- Moura D, Pereira RF, Gonçalves IC. Recent advances on bioprinting of hydrogels containing carbon materials. Mater. Today Chem. 2022;23:100617.
- Zhang X, Chen X, Hong H et al. Decellularized extracellular matrix scaffolds: recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022;10:15–31.
- Solarte David VA, Güiza-Argüello VR, Arango-Rodríguez ML et al. Decellularized tissues for wound healing: towards closing the gap between scaffold design and effective extracellular matrix remodeling [review]. Front. Bioeng. Biotechnol. 2022;10:821852.
- Liu H, Gong Y, Zhang K et al. Recent advances in decellularized matrix-derived materials for bioink and 3D bioprinting. Gels. 2023;9(3):195.
- Kim BS, Ahn M, Cho W-W et al. Engineering of diseased human skin equivalent using 3D cell printing for representing pathophysiological hallmarks of type 2 diabetes in vitro. Biomaterials 2021;272:120776.
- Kim BS, Gao G, Kim JY et al. 3D cell printing of perfusable vascularized human skin equivalent composed of epidermis, dermis, and hypodermis for better structural recapitulation of native skin. Adv. Healthc. Mater. 2019;8(7):1801019.
- Fang Y, Han Y, Wang S et al. Three-dimensional printing bilayer membranous nanofiber scaffold for inhibiting scar hyperplasia of skin. Biomater. Adv. 2022;138:212951.
- Hu Y, Wu B, Xiong Y et al. Cryogenic 3D printed hydrogel scaffolds loading exosomes accelerate diabetic wound healing. Chem. Engin. J. 2021;426:130634.
- Abaci A, Guvendiren M. Designing decellularized extracellular matrix-based bioinks for 3D bioprinting. Advan. Healthc. Mat. 2020;9(24):2000734.
- Liu Y, Hsu S-h. Synthesis and biomedical applications of self-healing hydrogels [mini review]. Front. Chem. 2018;6:449.
- Choi YJ, Park H, Ha DH et al. 3D bioprinting of in vitro models using hydrogel-based bioinks. Polymers (Basel) 2021;13(3):366.
- Chaudhuri O, Gu L, Klumpers D et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mat. 2016;15(3):326–334.
- Wang H, Heilshorn SC. Adaptable hydrogel networks with reversible linkages for tissue engineering. Advan. Mat. 2015;27(25):3717–3736.
- Hazur J, Detsch R, Karakaya E et al. Improving alginate printability for biofabrication: establishment of a universal and homogeneous pre-crosslinking technique. Biofabrication 2020;12(4):045004.
- Khetan S, Guvendiren M, Legant WR et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mat. 2013;12(5):458–465.
- Pereira RF, Barrias CC, Bártolo PJ et al. Cell-instructive pectin hydrogels crosslinked via thiol-norbornene photo-click chemistry for skin tissue engineering. Acta Biomaterialia 2018;66:282–293.
- Morgan FLC, Moroni L, Baker MB. Dynamic bioinks to advance bioprinting. Advan. Healthc. Mater. 2020;9(15):1901798.
- Shin M, Galarraga JH, Kwon MY et al. Gallol-derived ECM-mimetic adhesive bioinks exhibiting temporal shear-thinning and stabilization behavior. Acta Biomat. 2019;95:165–175.
- Morgan FLC, Fernández-Pérez J, Moroni L et al. Tuning hydrogels by mixing dynamic cross-linkers: enabling cell-instructive hydrogels and advanced bioinks. Advan. Healthc. Mater. 2022;11(1):2101576.
- Parenteau-Bareil R, Gauvin R, Berthod F. Collagen-based biomaterials for tissue engineering applications. Materials 2010;3(3):1863–1887.
- Behre A, Tashman JW, Dikyol C et al. 3D bioprinted patient-specific extracellular matrix scaffolds for soft tissue defects. Adv. Healthc. Mater. 2022;11(24):e2200866.
- Bin Y, Dongzhen Z, Xiaoli C et al. Modeling human hypertrophic scars with 3D preformed cellular aggregates bioprinting. Bioact. Mater. 2022;10:247–254.
- de Melo BAG, Jodat YA, Cruz EM et al. Strategies to use fibrinogen as bioink for 3D bioprinting fibrin-based soft and hard tissues. Acta Biomater. 2020;117:60–76.
- Jorgensen AM, Varkey M, Gorkun A et al. Bioprinted skin recapitulates normal collagen remodeling in full-thickness wounds. Tissue Eng. Part A 2020;26(9–10):512–526.
- Kang MS, Kwon M, Lee SH et al. 3D printing of skin equivalents with hair follicle structures and epidermal-papillary-dermal layers using gelatin/hyaluronic acid Hydrogels. Chem. Asian J. 2022;17(18):e202200620.
- Stichler S, Böck T, Paxton N et al. Double printing of hyaluronic acid/poly(glycidol) hybrid hydrogels with poly(ε-caprolactone) for MSC chondrogenesis. Biofabrication 2017;9(4):044108.
- Zhong Y, Ma H, Lu Y et al. Investigation on repairing diabetic foot ulcer based on 3D bio-printing Gel/dECM/Qcs composite scaffolds. Tissue Cell 2023;85:102213.
- Kang B, Park Y, Hwang DG et al. Facile bioprinting process for fabricating size-controllable functional microtissues using light-activated decellularized extracellular matrix-based bioinks. Advan. Mater. Technolog. 2022;7(1):2100947.

