Abstract
Treatment options for patients with advanced or metastatic esophageal squamous cell carcinoma (ESCC) are improving. Current guidelines recommend first-line pembrolizumab plus chemotherapy for patients with unresectable or metastatic ESCC, which has led to improvements in survival outcomes. Antiangiogenic therapy combined with immune checkpoint inhibitors can act synergistically to convert the immunosuppressive tumor microenvironment to an immune supportive microenvironment, thus enhancing antitumor immune responses. In preclinical models, the antiangiogenic agent lenvatinib combined with an anti-PD-1 agent showed synergistic antitumor activity. We describe the design and rationale for the randomized, open-label, phase III LEAP-014 study of lenvatinib in combination with pembrolizumab plus chemotherapy in patients with advanced or metastatic ESCC. Overall survival and progression-free survival are the dual primary end points.
Clinical Trial Registration: NCT04949256 (ClinicalTrials.gov)
Esophageal cancer
For the approximately 50% of patients with esophageal cancer who are diagnosed with unresectable or metastatic disease, current guidelines recommend first-line pembrolizumab in combination with a platinum-based chemotherapy or a fluoropyrimidine.
Given the limited survival benefit observed with the currently recommended first-line therapy of pembrolizumab plus chemotherapy, there is a considerable need for alternative strategies for patients with advanced, metastatic esophageal cancer.
Study rationale
PD-L1 and PD-L2 are expressed by tumor cells in approximately 20–40% of esophageal cancers and are associated with an unfavorable prognosis.
Pembrolizumab has shown antitumor activity in patients with advanced esophageal cancer, and the addition of pembrolizumab to standard-of-care chemotherapy may translate to increased effectiveness and improved patient outcomes.
Preclinical data demonstrated the immune-modulating effects of lenvatinib and synergistic antitumor activity of lenvatinib when combined with an anti-PD-1 agent.
Lenvatinib plus pembrolizumab was recently approved for first-line treatment of adult patients with advanced renal cell carcinoma.
In the phase II EPOC1706 trial, lenvatinib plus pembrolizumab showed promising antitumor activity and an acceptable safety profile in patients with advanced gastric cancer in both the first- and second-line setting.
LEAP-014 study design & eligibility criteria
LEAP-014 is a randomized, active-controlled, parallel-group, multisite, open-label, two-part, phase III study designed to evaluate the safety and efficacy of lenvatinib in combination with pembrolizumab plus chemotherapy compared with pembrolizumab plus chemotherapy in patients with esophageal squamous cell carcinoma.
Primary end points
The primary end point is safety and tolerability for part 1 (safety run-in).
The dual primary end points are overall survival and progression-free survival for part 2 (main study).
Key eligibility
Adults with histologically or cytologically confirmed diagnosis of previously untreated metastatic squamous cell carcinoma of the esophagus.
Conclusion
LEAP-014 study will further define the role of combination therapy with lenvatinib, pembrolizumab and chemotherapy in the first-line setting for patients with metastatic esophageal cancer, for whom there remains an unmet medical need for better treatment options that are tolerable.
In 2020, esophageal cancer was the seventh most commonly diagnosed cancer and the sixth most common cause of cancer-related mortality worldwide (604,000 new cases and 544,000 deaths) [Citation1]. Esophageal squamous cell carcinoma (ESCC) is a major histologic subtype of esophageal cancer, particularly in less developed countries; however, incidence varies geographically [Citation2]. The prognosis for esophageal cancer is poor, with 5-year overall survival (OS) rates of 20% for all stages and 5% for metastatic disease [Citation3]. Furthermore, approximately 50% of ESCC cases are diagnosed as stage III to IV disease [Citation4], highlighting the need for additional treatment strategies to improve survival in the advanced or metastatic setting.
First-line systemic therapy for locally advanced or metastatic ESCC has been platinum-based chemotherapy in combination with a fluoropyrimidine for several years [Citation5,Citation6]; however, based on results from recent clinical studies, immune checkpoint inhibitors plus chemotherapy represent a new standard of care [Citation6]. This shift in the treatment guidelines is based on results from the global, phase III KEYNOTE-590 study, which evaluated first-line pembrolizumab plus chemotherapy in 749 patients with unresectable advanced or metastatic esophageal cancer, most of whom had ESCC (n = 548; 73%) [Citation7]. In KEYNOTE-590, pembrolizumab plus chemotherapy was associated with significant and clinically meaningful improvements in OS (median: 12.6 vs 9.8 months; hazard ratio [HR]: 0.72; 95% CI: 0.60–0.88; p = 0.0006) and progression-free survival (PFS; median: 6.3 vs 5.8 months; HR: 0.65; 95% CI: 0.54–0.78; p < 0.0001) compared with chemotherapy among patients with ESCC [Citation7]. The findings of KEYNOTE-590 are also supported by promising results from other studies evaluating immunotherapy plus chemotherapy, including the ESCORT-1st study for camrelizumab plus chemotherapy [Citation8], the CheckMate-648 study for nivolumab plus chemotherapy as well as for nivolumab plus ipilimumab [Citation9], the JUPITER-06 study for toripalimab plus chemotherapy [Citation10], the ORIENT-15 study for sintilimab plus chemotherapy [Citation11], and the RATIONALE-306 study for tislelizumab plus chemotherapy [Citation12]. However, despite the improvements in clinical outcomes observed with immune checkpoint inhibitor therapies, response rates (27.7–75.5%) and OS (12.6–17.0 months) remain suboptimal [Citation7-11]. This highlights the need for alternative strategies with additional active agents in the first-line setting of advanced, metastatic esophageal cancer.
Background & rationale
PD-1 is a negative costimulatory receptor mainly expressed on activated T cells and is critical for terminating immune responses and preventing autoimmunity [Citation13,Citation14]. Tumor cells frequently use the PD-1 pathway to evade immune surveillance by upregulating the expression of its ligands, programmed death ligand 1 (PD-L1) and PD-L2 [Citation13,Citation15]. The binding of PD-1 to its ligands inhibits effector T-cell function, suppressing T-cell-mediated antitumor responses [Citation13,Citation16]. PD-L1 or PD-L2 expression in tumor cells has been detected in 15–80% of esophageal cancers [Citation14,Citation17-19] and is typically associated with an unfavorable prognosis [Citation14,Citation20,Citation21]. However, recent studies have suggested that PD-L1 expression can be used as a biomarker to predict response to immunotherapy [Citation14,Citation20,Citation22].
Pembrolizumab is a high-affinity, highly selective, humanized immunoglobulin G4-κ monoclonal antibody that binds to PD-1, directly blocking the interaction with its ligands [Citation23]. Evidence supporting the efficacy and safety of pembrolizumab in previously treated patients with PD-L1-positive esophageal cancer has been shown in the KEYNOTE-028 [Citation24], KEYNOTE-180 [Citation25] and KEYNOTE-181 [Citation22] studies.
In patients with PD-L1-positive esophageal cancer in the KEYNOTE-028 study, the majority of whom had ESCC (ESCC in 78% of patients and esophageal adenocarcinoma [EAC] in 22%), objective response rate (ORR) was 30% (28% in patients with ESCC), and median PFS and OS were 1.8 and 7.0 months, respectively [Citation24]. A total of 17% of patients experienced grade 3 treatment-related adverse events (AEs). No patient experienced grade 4 or 5 treatment-related AEs. Generally, similar results were observed in patients with advanced or metastatic EAC, ESCC or Siewert type 1 gastroesophageal junction (GEJ) adenocarcinoma who had experienced disease progression after receiving at least two prior lines of therapy in the KEYNOTE-180 study [Citation25] and in patients who had experienced disease progression after one prior therapy in the KEYNOTE-181 study [Citation22], particularly in patients whose tumors expressed PD-L1 combined positive score (CPS) ≥10.
Angiogenesis is an essential process for local tumor growth and invasion. The tumor microenvironment contains an imbalance of pro- and antiangiogenic factors to facilitate the recruitment of blood vessels [Citation26], which not only supply oxygen and nutrients and remove waste, but also sustain a favorable niche for tumor cells and serve as a conduit for their metastatic dissemination [Citation27]. VEGF and its family of receptors (VEGFRs 1-3) play a major role in tumor angiogenesis [Citation28] and have been shown to be overexpressed in most types of solid cancer [Citation29], including ESCC [Citation30]. VEGF signaling modulates the tumor microenvironment and plays an important role in T-cell activation, which is associated with increased expression of several immune checkpoint-inhibiting molecules (e.g., PD-1, CTLA-4, TIM-3 and LAG-3) that can suppress immune functions. Evidence also suggests that FGF and its receptor are also involved in angiogenesis [Citation31]. Furthermore, increased VEGF tumor expression can increase intratumoral regulatory T cells (Tregs), and inhibition of VEGF signaling decreased intratumoral Tregs in preclinical mouse models [Citation32-34]. As a result, several therapies targeting angiogenesis have been developed, such as monoclonal antibodies (e.g., bevacizumab and ramucirumab) and receptor tyrosine kinase inhibitors (e.g., sunitinib, sorafenib and pazopanib). Preclinical evidence supports synergy for combination therapy with an antiangiogenic agent and an immune checkpoint inhibitor. In preclinical models of breast cancer, pancreatic neuroendocrine tumor, and melanoma, blockade of VEGFA and angiopoietin-2 by a bispecific antibody (vanucizumab; A2V) promoted vascular regression, tumor necrosis, antigen presentation by intratumoral phagocytes, and accumulation of interferon γ-expressing cytotoxic T cells. Tumor control was also improved with the addition of an anti-PD-1 agent [Citation35]. A synergistic effect was also observed with the combination of anti-VEGFR2 and anti-PD-1 monoclonal antibodies in a murine colon cancer model, resulting in inhibition of tumor neovascularization due to VEGFR2 blockade and enhanced T-cell tumor infiltration and local immune activation due to PD-1 blockade. Finally, the combination of sunitinib with an anti-PD-1 antibody in a murine model of colorectal cancer expressing high levels of VEGFA resulted in a synergistic antitumor effect that was stronger than treatment with sunitinib alone. No effect was observed with anti-PD-1 treatment alone [Citation36].
Lenvatinib is a potent multiple kinase receptor inhibitor of VEGFRs 1-3 that also inhibits other kinases implicated in pathogenic angiogenesis, including FGF receptors 1-4, platelet-derived growth factor receptor A, the KIT receptor tyrosine kinase, and rearranged during transfection receptor [Citation37]. Lenvatinib was initially approved for the treatment of patients with locally recurrent or metastatic, progressive, radioactive iodine-refractory differentiated thyroid cancer [Citation38]. Subsequently, lenvatinib was approved for first-line treatment of patients with unresectable hepatocellular carcinoma (HCC), which was based on results of the REFLECT trial that demonstrated noninferiority between lenvatinib and sorafenib for OS (median: 13.6 vs 12.3 months; HR: 0.92; 95% CI: 0.79–1.06) in patients with unresectable or metastatic HCC [Citation39]. The immune-modulating effect of lenvatinib was initially demonstrated in preclinical models. In a syngeneic mouse tumor model, lenvatinib increased tumor-infiltrating CD8-positive T cells expressing granzyme B and interferon γ and plasmacytoid dendritic cells, and reduced tumor-associated macrophages [Citation40]. Furthermore, evidence of enhanced antitumor effects with lenvatinib plus an anti-PD-1 monoclonal antibody was observed in these preclinical models, with combination therapy providing greater antitumor activity than either agent as monotherapy [Citation40,Citation41]. Together, these data indicate that the immune-modulating effect of lenvatinib contributes to its antitumor activity and is complementary to that of immune checkpoint inhibition, thus enhancing antitumor responses when lenvatinib is administered in combination therapy with an anti-PD-1 agent. Furthermore, treatment with lenvatinib may overcome treatment resistance through inhibition of alternative pro-angiogenic pathways, such as FGF signaling [Citation42].
Based on this rationale, clinical trials designed to examine the combination of lenvatinib and pembrolizumab for the treatment of a variety of solid tumors are currently ongoing. Recent evidence from the single-arm phase Ib/II KEYNOTE-146 study (study 111) of lenvatinib plus pembrolizumab in patients with selected solid tumors has shown that this combination could be an effective and tolerable treatment option [Citation43], with promising antitumor activity reported in both treatment-naive (ORR per Response Evaluation Criteria in Solid Tumors [RECIST] for immune-based therapies [iRECIST], 72.7%) and previously treated (ORR per iRECIST, 55.8%) metastatic renal cell carcinoma (RCC) [Citation44], as well as previously treated advanced endometrial cancer (EC; ORR per iRECIST, 38.0%) [Citation45]. Lenvatinib plus pembrolizumab was also assessed in the phase Ib KEYNOTE-524 (study 116) trial, which reported promising antitumor activity and manageable toxicity in patients with treatment-naive unresectable HCC (ORR per modified RECIST, 46.0%) [Citation46], and in the phase II EPOC1706 study, which reported promising antitumor activity in patients with treatment-naive and previously treated advanced gastric cancer (ORR, 69%) [Citation47]. Furthermore, lenvatinib plus pembrolizumab was recently approved for the first-line treatment of adult patients with advanced RCC, based on positive results of the phase III CLEAR (KEYNOTE-581) study [Citation48], and as second-line therapy for patients with advanced EC that is not microsatellite instability-high or mismatch repair deficient based on results of the phase III KEYNOTE-775 study [Citation49,Citation50]. Last, a clinical trial program comprising phase II and III studies, the LEAP program, is evaluating the efficacy and safety of lenvatinib plus pembrolizumab with or without chemotherapy in several tumor types, including HCC, melanoma, breast cancer, ovarian cancer, gastric cancer, colorectal cancer, glioblastoma multiforme, biliary tract cancer, NSCLC, head and neck squamous cell carcinoma and UC [Citation51]. The development of the LEAP program was based on encouraging results from the KEYNOTE-146 and KEYNOTE-524 studies. Several LEAP studies are planned or ongoing at present [Citation51]. For example, the phase III LEAP-015 trial is ongoing and is designed to assess the efficacy and safety of first-line lenvatinib plus pembrolizumab plus chemotherapy followed by consolidation with lenvatinib plus pembrolizumab versus chemotherapy alone in unresectable or metastatic gastroesophageal adenocarcinoma [Citation52], and the phase II LEAP-004 study has shown promising antitumor activity with lenvatinib plus pembrolizumab in previously treated patients who received PD-1/L1 inhibitor-based therapy and had advanced melanoma (ORR per RECIST, 21.4%) [Citation53]. Taken together, cumulative findings from both the KEYNOTE and LEAP studies provide strong rationale and support for investigating lenvatinib plus pembrolizumab plus chemotherapy in the first-line treatment setting for patients with advanced and metastatic ESCC.
LEAP-014 study
We describe the design and rationale of the phase III LEAP-014 study, which will assess the efficacy and safety of lenvatinib in combination with pembrolizumab plus chemotherapy compared with pembrolizumab plus chemotherapy for the first-line treatment of metastatic ESCC (ClinicalTrials.gov identifier: NCT04949256).
Design
Study design
LEAP-014 is a randomized, active-controlled, parallel-group, multisite, open-label, phase III study of lenvatinib in combination with pembrolizumab plus chemotherapy compared with pembrolizumab plus chemotherapy in patients with ESCC. The study has no treatment crossover. There will be two parts to the study: a safety run-in stage (part 1) and the main study (part 2). After enrollment of the global portion of the study, the study may remain open to enrollment in China alone until the target number of patients from China has been enrolled to meet local regulatory requirements.
In part 1, six patients will be treated for induction with intravenous pembrolizumab 400 mg every 6 weeks (Q6W) for two cycles plus oral lenvatinib 8 mg daily plus a cisplatin (intravenous, 80 mg/m2) plus 5-fluorouracil (5-FU; intravenous, 4000 mg/m2 on days 1–5) chemotherapy regimen (FP) for four cycles, and approximately six patients in China, Hong Kong, Republic of Korea and Taiwan will be treated for induction with intravenous pembrolizumab 400 mg Q6W for two cycles plus oral lenvatinib 8 mg daily plus paclitaxel (intravenous, 175 mg/m2) plus cisplatin (intravenous, 75 mg/m2) (TP) for 4 cycles. All 12 patients will be treated for consolidation with pembrolizumab 400 mg Q6W for ≤16 doses plus lenvatinib 20 mg daily (A). Patients will be closely monitored for 21 days after the first dose of study intervention (i.e., the dose-limiting toxicity evaluation period). If ≥3 instances of dose-limiting toxicity occur during part 1 (i.e., FP or TP safety run-in), then enrollment in part 2 will be delayed to allow for examination of safety data and to consider design changes. These patients will continue to receive study intervention and be followed after treatment as applicable. Further, these patients will receive full study treatment, which includes induction, consolidation, follow-up and eligibility for a second course of treatment for patients in arm 1 of the main study (part 2).
Figure 1. LEAP-014 study design.
(A) Part 1. (B) Part 2.
†TP chemotherapy may only be administered to patients in China, Hong Kong, Republic of Korea, and Taiwan. A maximum of 10% of patients enrolled in the study are permitted to receive TP chemotherapy.
CPS: Combined positive score; ESCC: Esophageal squamous cell carcinoma; FP: Cisplatin plus 5-fluorouracil; iv.: Intravenous; mFOLFOX6: Oxaliplatin plus 5-fluorouracil plus leucovorin; OS: Overall survival; PD-L1: Programmed death ligand 1; PFS: Progression-free survival; PO: Orally; Q2W: Every 2 weeks; Q3W: Every 3 weeks; Q6W: Every 6 weeks; QD: Once daily; ROW: Rest of world; TP: Paclitaxel plus cisplatin.
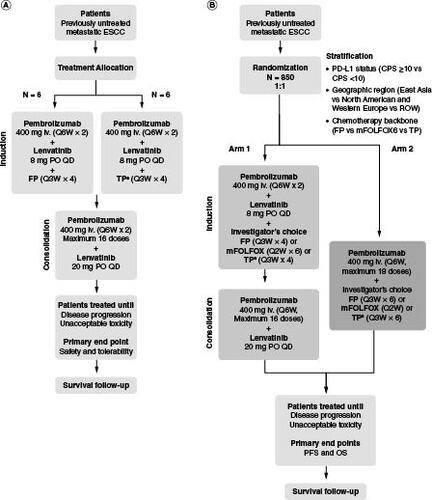
In part 2, approximately 850 patients (not including those participating in part 1) will be randomly assigned 1:1 to an induction phase with pembrolizumab plus lenvatinib plus chemotherapy (FP or mFOLFOX6 [intravenous oxaliplatin 85 mg/m2 plus bolus intravenous 5-FU 400 mg/m2 plus continuous intravenous 5-FU 2400 mg/m2 plus either intravenous leucovorin 400 mg/m2 or intravenous levoleucovorin 200 mg/m2 every 2 weeks for six cycles], or TP [in China, Hong Kong, Republic of Korea and Taiwan only]) followed by consolidation with pembrolizumab plus lenvatinib (arm 1) or pembrolizumab plus chemotherapy (FP or mFOLFOX6 or TP [in China, Hong Kong, Republic of Korea, and Taiwan only]; arm 2; B). The choice of chemotherapy backbone will be decided by the investigator. The duration of chemotherapy will be limited to 12 weeks in combination with lenvatinib and pembrolizumab (induction phase). After completing chemotherapy, patients without disease progression (as assessed by blinded independent centralized review [BICR] using RECIST, version 1.1 [RECIST v1.1]) will continue pembrolizumab therapy for up to 2 years (consolidation phase; not exceeding 16 cycles of treatment). Patients who tolerate treatment with lenvatinib 8 mg/day throughout the induction phase will have their dose increased to 20 mg/day during the consolidation phase and will continue treatment until disease progression or unacceptable toxicity.
Use of an initial chemotherapy induction treatment phase in the current study is supported by findings from the phase III KEYNOTE-062 trial of first-line pembrolizumab or pembrolizumab plus chemotherapy versus chemotherapy alone in patients with advanced gastric cancer, which revealed early inferior OS Kaplan–Meier curves for pembrolizumab monotherapy versus that of chemotherapy alone during the first 6–9 months, followed by convergence of both OS curves after this period [Citation54]. In contrast, initial inferior survival was not observed with pembrolizumab plus chemotherapy versus chemotherapy alone [Citation54], suggesting that early treatment with chemotherapy may be beneficial. Furthermore, the role of continuing chemotherapy in combination with pembrolizumab was not clear given that no significant difference in median OS was observed between pembrolizumab plus chemotherapy and chemotherapy alone [Citation54]. These observations from KEYNOTE-062 provide the rationale for an initial chemotherapy-induction strategy when investigating lenvatinib plus pembrolizumab, followed by the withdrawal of chemotherapy and continued treatment with lenvatinib plus pembrolizumab during a consolidation treatment phase, which could lead to an overall decrease in chemotherapy-related toxicity and increased efficacy relative to chemotherapy alone.
The rationale for the 8 mg dose of lenvatinib during the induction phase of this study was based on results of a phase I dose-finding study of lenvatinib plus chemotherapy (carboplatin plus paclitaxel) in Japanese patients with chemotherapy-naive NSCLC, which identified 4 mg twice daily as the maximum tolerated dose for lenvatinib that provided promising antitumor activity [Citation55]. Additionally, use of an 8 mg once-daily dose of lenvatinib plus pembrolizumab and platinum-based chemotherapy was also demonstrated in part 1 (the safety run-in) of the phase III LEAP-006 trial in patients with metastatic NSCLC, which showed manageable safety and tolerability [Citation56]. Furthermore, pharmacokinetic modeling of data from phase I lenvatinib studies indicated that the higher lenvatinib exposure from once-daily dosing correlated with improved antitumor responses versus twice-daily dosing, with no differences in safety and tolerability between the two dosing schedules [Citation57]. The rationale for the 20 mg starting dose of lenvatinib during the consolidation phase of the current study was also based on the results of previous studies, with lenvatinib (20 mg) plus pembrolizumab demonstrating promising antitumor activity in the phase Ib/II KEYNOTE-146 (selected advanced solid tumors) [Citation43-45], phase II EPOC1706 (advanced gastric cancer) [Citation47], phase III KEYNOTE-581/CLEAR (advanced RCC) [Citation58], and phase III KEYNOTE-775 (advanced EC) [Citation49] clinical studies.
Randomization will be stratified by PD-L1 status (CPS <10 vs CPS ≥10), region (East Asia vs North America and Western Europe vs rest of world) and intended chemotherapy backbone (FP vs mFOLFOX6 vs TP). This study will be open label, and therefore both the investigator and patient will know the interventions administered.
Eligibility criteria
Eligibility criteria are described in detail in . Briefly, male and female patients ≥18 years of age with histologically or cytologically confirmed metastatic ESCC were eligible for enrollment. All patients will be required to provide a fresh biopsy specimen or archival tissue to assess PD-L1 status at randomization. Patients with samples insufficient (in terms of quality or quantity) to assess PD-L1 status will not be enrolled. To avoid inclusion of patients who are not clinically stable at randomization, patients with cachexia will not be eligible for enrollment because cachexia is a sign of rapidly progressing disease; patients with this disease require nutritional support before they can be considered for the study. Patients who experienced a significant bleeding episode of the gastrointestinal tract within 12 weeks before randomization will be excluded from the study because of the potential risk of bleeding with lenvatinib treatment. Patients must be able to swallow the lenvatinib capsule to be eligible for enrollment. During the course of the study, if a patient is unable to swallow or has a feeding tube, lenvatinib capsules can be dissolved (without breaking or crushing) in a small glass of liquid such as water, milk, or apple juice.
Table 1. Eligibility criteria for the LEAP-014 study.
Outcome measures/end points
In part 1, the primary end point will be safety and tolerability. In part 2, the dual primary end points will be OS and PFS. OS is defined as the time from randomization to death due to any cause. PFS is defined as the time from randomization to the first documented disease progression per RECIST v1.1 as determined by BICR or death due to any cause (whichever occurs first).
The secondary end points in part 2 will include ORR (complete response [CR] or partial response [PR]) per RECIST v1.1 as determined by BICR, DOR (the time from the first documented evidence of CR or PR until disease progression or death due to any cause, whichever occurs first) per RECIST v1.1 as determined by BICR, efficacy in patients whose tumors are PD-L1 CPS ≥10, health-related quality of life (HRQoL; assessed using the EORTC Quality of Life Questionnaire Core 30 items [EORTC QLQ-C30] and EORTC Quality of Life Questionnaire esophageal module 18 items [EORTC QLQ-OES-18] scores), and safety and tolerability.
Exploratory end points in part 2 will include additional assessments of HRQoL (EuroQol 5 Dimensions, 5 Levels [EQ-5D-5L] scores), evaluation of the pharmacokinetics of lenvatinib when coadministered with pembrolizumab plus doublet platinum chemotherapy, and identification of molecular (genomic, metabolic and/or proteomic) biomarkers of response or resistance to treatment.
Study procedures
Tumor imaging and assessment of disease will be conducted using computed tomography (CT), or magnetic resonance imaging (MRI) if CT is contraindicated. If brain scans are performed, MRI scans are preferred, but CT scans are acceptable if MRI is contraindicated. Bone scans may also be performed to evaluate bone metastases. The type of imaging used for a patient will be consistent throughout the study. Initial tumor imaging will be performed during screening (≤28 days before randomization) and will include CT of the abdomen, pelvis and chest, which is thought to show the most common sites of disease or metastases for the majority of patients while still considering the radiation burden. It is expected that all known sites of disease will be consistently scanned to include the full disease burden for a patient; depending on the location of their tumors, patients may also undergo head and neck imaging at the investigator's discretion. To monitor disease status, imaging will be performed Q6W (or more often if clinically indicated) for ≤1 year. Patients who remain on treatment will then have scans performed Q9W until disease progression is identified by the investigator and verified by the BICR, the start of new anticancer treatment, withdrawal of consent or death (whichever occurs first). Patients are required to have their scans performed at week 12 assessed by the investigator before initiating the consolidation phase.
Safety will be monitored throughout the study and for 30 days after the end of treatment (90 days for serious AEs [SAEs] or 30 days if the patient initiates a new anticancer therapy), including AEs, AEs leading to discontinuation, SAEs, fatal AEs, physical examinations, vital signs, electrocardiograms, echocardiograms or multiple-gated acquisition scans, Eastern Cooperative Oncology Group performance status and laboratory values (including pregnancy). AEs will be graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
HRQoL, as assessed by the EORTC QLQ-C30, EORTC QLQ-OES-18 and EQ-5D-5L questionnaires, will be administered electronically in the order listed Q6W (on day 1 of every pembrolizumab cycle) until the completion of 2 years of study treatment or discontinuation of study interventions. HRQoL will also be assessed at treatment discontinuation and safety follow-up.
PD-L1 expression will be assessed in a central laboratory by use of a Good Manufacturing Practice immunohistochemistry assay (PD-L1 IHC 22C3 pharmDx Investigational Use Only diagnostic kit; Agilent). PD-L1 expression will be measured using CPS defined as the number of PD-L1-staining cells (tumor cells, lymphocytes and macrophages) divided by the total number of viable tumor cells, multiplied by 100.
Statistics
Primary efficacy analyses will be performed in the intention-to-treat population (all randomly assigned patients in part 2) and analyzed by randomized treatment group. Patients in part 1 will be excluded from all part 2 efficacy analyses. Safety will be assessed in the all-patients-as-treated population, defined as all randomly assigned patients in part 2 who received ≥1 dose of study treatment, and will be analyzed by treatment received; patients in parts 1 and 2 will be analyzed separately.
The primary hypotheses for OS and PFS will be evaluated by comparing lenvatinib plus pembrolizumab in combination with chemotherapy (FP/mFOLFOX6/TP) to pembrolizumab plus chemotherapy (FP/mFOLFOX6/TP) using a stratified log-rank test. The hazard ratio will be estimated using a stratified Cox proportional hazard regression model. Event rates over time will be estimated within each treatment group using the Kaplan–Meier method. The stratified Miettinen and Nurminen method, with strata weighted by sample size, will be used for the analysis of ORR. For key safety end points, analyses in which 95% CI will be provided for between-treatment differences in the percentage of patients with events will be performed using the Miettinen and Nurminen method.
Two efficacy interim analyses are planned to assess OS, PFS and ORR in this study. The first interim safety analysis for reviewing safety data by the external data monitoring committee (eDMC) will be planned after at least 20 patients in arm 1 of the main study have completed the induction phase for lenvatinib in combination with pembrolizumab and FP/mFOLFOX6. A separate eDMC will be planned after at least ten patients in arm 1 of the main study have completed the induction phase for lenvatinib in combination with pembrolizumab and TP. The eDMC will then review safety data periodically in the study.
Conclusion
Findings from the KEYNOTE-590 study indicate that pembrolizumab in combination with chemotherapy provides promising antitumor activity and has an acceptable safety profile in patients with previously treated, advanced esophageal cancer. We describe the methodology for the phase III LEAP-014 study, which will assess the efficacy and safety of the antiangiogenic agent lenvatinib in combination with the immune checkpoint inhibitor pembrolizumab plus chemotherapy, followed by consolidation with lenvatinib plus pembrolizumab, compared with pembrolizumab plus chemotherapy for the first-line treatment of advanced or metastatic ESCC. The results from this study will help define the role of combination therapy in the first-line setting for patients with esophageal cancer who are ineligible for curative surgery, a patient population for whom treatment options are limited, and for whom there remains an unmet medical need for more efficacious and tolerable treatment options.
Author contributions
All authors contributed to the conception, design, or planning of the study. Acquisition of the data: Not applicable. Analysis of the data: Not applicable. Interpretation of the results: Not applicable. A Adenis, M Shah, T Doi and L Yu contributed to drafting of the manuscript. All authors critically reviewed the manuscript and approved the final version for submission.
Financial disclosure
J-M Sun reports no relevant affiliations or financial interests. A Adenis reports institutional grants or contracts from Bayer and Sanofi; payments or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events from Bristol Myers Squibb, Merck Sharp & Dohme LLC, and Novartis; support from Servier, Merck Sharp & Dohme LLC, Bristol Myers Squibb, and Pierre Fabre for attending meetings and/or travel; and participated on the data safety monitoring boards or advisory boards of Bristol Myers Squibb, Merck Sharp & Dohme LLC, Bayer, Astellas, and Pierre Fabre. P Enzinger reports consulting fees from ALX Oncology, Arcus Bioscience, Astellas, AstraZeneca, Blueprint Medicines, Bristol Myers Squibb, Chimeric Therapeutics, Celgene, Coherus, Daiichi Sankyo, Five Prime, Ideaya, Istari, Legend, Lilly, Loxo, Merck Sharp & Dohme LLC, Novartis, Ono Pharmaceutical, Servier, Taiho, Takeda, Turning Point Therapeutics, Xencor, and Zymeworks. M Shah reports institutional grants from Merck. K Kato reports grants or contracts from Merck & Co., Inc.; consulting fees from Ono Pharmaceutical, Bristol Myers Squibb, Beigene/Novartis, and AstraZeneca; and payments or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events from Ono Pharmaceutical and Bristol Myers Squibb; and participated on the data safety monitoring boards or advisory boards of Ono Pharmaceutical, Merck & Co., Inc., and Bristol Myers Squibb. J Bennouna reports payments or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events from Merck Sharp & Dohme LLC, Bristol Myers Squibb, Daiichi Sankyo, Sanofi-Aventis, Novartis, and Pfizer; support from AstraZeneca and Pfizer for attending meetings and/or travel; and participated on the data safety monitoring boards or advisory boards of Merck Sharp & Dohme LLC, Bristol Myers Squibb, and AstraZeneca. T Doi reports grants or contracts from Lilly, Merck Sharp & Dohme LLC, Daiichi Sankyo, Sumitomo Dainippon, Taiho, Novartis, Merck Biopharma, Janssen Pharma, Boehringer Ingelheim, Pfizer, Bristol Myers Squibb, AbbVie, Eisai, IQVIA, and Chugai Pharma; consulting fees from Sumitomo Dainippon, Taiho, Takeda, Chugai Pharma, AbbVie, Bayer, Rakuten Medical, Otsuka Pharma, KAKEN Pharma, KYOWA KIRIN, SHIONOGI, and PRA Health Science; payments or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events from Bristol Myers Squibb, Rakuten Medical, Ono Pharmaceutical, Daiichi Sankyo, and AstraZeneca; and participated on the data safety monitoring boards or advisory boards of Merck Sharp & Dohme LLC, Daiichi Sankyo, Amgen, Novartis, Boehringer Ingelheim, Janssen Pharma, AbbVie, Bayer, and Astellas Pharma. NN Hawk and L Yu report no relevant affiliations or financial interests. S Shah reports support for the present manuscript as an employee of Merck & Co., Inc.; and owns Merck stock or stock options. P Bhagia reports support for the present manuscript as an employee of Merck & Co., Inc.; and support from Merck & Co., Inc. for attending meetings and/or travel. L Shen reports consulting fees from Mingji Biopharmaceutical, Haichuang Pharmaceutical and Hernour Biomed; payments for speakers' bureaus from Hutchison Whampoa, Hengrui, ZaiLab, and CSTONE Pharmaceutical; and participated on the advisory boards of Merck Sharp & Dohme LLC, Bristol Myers Squibb, Boehringer Ingelheim, Sanofi, Roche and Servier. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Conflict of interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
Medical writing and editorial assistance were provided by HC Cappelli, PhD, CMPP, of ApotheCom (PA, USA). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Ethical disclosure
The authors attest that the study protocol was approved by the appropriate ethics committee or institutional review board at each participating center, and will be conducted in accordance with standards of Good Clinical Practice and the Declaration of Helsinki. All participants will provide written informed consent before enrollment.
Data sharing statement
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) is obligated to protect the rights and privacy of trial participants. To fulfill the company's obligation, MSD has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. As outlined on the MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php), a detailed research proposal that includes the background and rationale, objectives of the research, a scientific hypothesis, statistical analysis plan, and publication plan must be submitted through EngageZone along with the curricula vitae of all researchers, including the biostatistician. Completed applications will be promptly assessed for feasibility. If the request is considered feasible, a committee of MSD subject matter experts will assess the qualifications of the requestors and the scientific validity of the request. If the proposal is approved, the researcher must enter into a standard data-sharing agreement with MSD before anonymized data is provided, in line with data privacy legislation. There are circumstances that may prevent MSD from sharing the requested data, including country or region-specific regulations such as the European Union General Data Privacy Regulation. If the request is declined, it will be communicated to the investigator. MSD data-sharing metrics can be accessed at www.merck.com/clinicaltrials/pdf/MicrositeDataSharingMetrics_20190711.pdf.
Acknowledgments
The authors thank the patients and their families and caregivers and all investigators and site personnel for participating in this trial. We also thank Tajalli Upadhyaya (of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA) and CE Okpara (of Eisai Inc., Nutley, NJ, USA), for their contributions to this analysis.
Funding
Eisai Inc., Nutley, NJ, USA and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL et al. Global cancer statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021).
- Huang J, Koulaouzidis A, Marlicz W et al. Global burden, risk factors, and trends of esophageal cancer: an analysis of cancer registries from 48 countries. Cancers (Basel) 13(1), 141 (2021).
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J. Clin. 71(1), 7–33 (2021).
- Then EO, Lopez M, Saleem S et al. Esophageal cancer: an updated surveillance epidemiology and end results database analysis. World J. Oncol. 11(2), 55–64 (2020).
- Lordick F, Carneiro F, Cascinu S et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 33(10), 1005–1020 (2022).
- NCCN clinical practice guidelines in oncology. Esophageal and esophagogastric junction cancers version 2.2022. www.nccn.org/guidelines/guidelines-detail?category=1&id=1433 (04/21/2022).
- Sun JM, Shen L, Shah MA et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, Phase III study. Lancet 398(10302), 759–771 (2021).
- Luo H, Lu J, Bai Y et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA 326(10), 916–925 (2021).
- Doki Y, Ajani JA, Kato K et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N. Engl. J. Med. 386(5), 449–462 (2022).
- Wang ZX, Cui C, Yao J et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): a multi-center Phase III trial. Cancer Cell 40(3), 277–288 (2022).
- Shen L, Lu ZH, Wang JY et al. LBA52 Sintilimab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced or metastatic esophageal squamous cell cancer: first results of the Phase III ORIENT-15 study. Ann. Oncol. 32, S1330 (2021).
- Yoon H, Kato K, Raymond E et al. LBA-1 RATIONALE-306: randomized, global, placebo-controlled, double-blind Phase III study of tislelizumab plus chemotherapy versus chemotherapy as first-line treatment for advanced or metastatic esophageal squamous cell carcinoma (ESCC). Ann. Oncol. 33, S375 (2022).
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12(4), 252–264 (2012).
- Vrána D, Matzenauer M, Neoral Č et al. From tumor immunology to immunotherapy in gastric and esophageal cancer. Int. J. Mol. Sci. 20(1), 13 (2018).
- Raufi AG, Klempner SJ. Immunotherapy for advanced gastric and esophageal cancer: preclinical rationale and ongoing clinical investigations. J. Gastrointest Oncol. 6(5), 561–569 (2015).
- Cui C, Yu B, Jiang Q, Li X, Shi K, Yang Z. The roles of PD-1/PD-L1 and its signalling pathway in gastrointestinal tract cancers. Clin. Exp. Pharmacol. Physiol. 46(1), 3–10 (2019).
- Ohigashi Y, Sho M, Yamada Y et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin. Cancer Res. 11(8), 2947–2953 (2005).
- Svensson MC, Borg D, Zhang C et al. Expression of PD-L1 and PD-1 in chemoradiotherapy-naïve esophageal and gastric adenocarcinoma: relationship with mismatch repair status and survival. Front. Oncol. 9, 136 (2019).
- Wakita A, Motoyama S, Nanjo H et al. PD-L1 expression is a prognostic factor in patients with thoracic esophageal cancer treated without adjuvant chemotherapy. Anticancer Res. 37(3), 1433–1441 (2017).
- Jiang Y, Lo AWI, Wong A et al. Prognostic significance of tumor-infiltrating immune cells and PD-L1 expression in esophageal squamous cell carcinoma. Oncotarget 8(18), 30175–30189 (2017).
- Yagi T, Baba Y, Ishimoto T et al. PD-L1 expression, tumor-infiltrating lymphocytes, and clinical outcome in patients with surgically resected esophageal cancer. Ann. Surg. 269(3), 471–478 (2019).
- Kojima T, Shah MA, Muro K et al. Randomized Phase III keynote-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J. Clin. Oncol. 38, 4138–4148 (2020).
- KEYTRUDA® (pembrolizumab) injection, for intravenous use. Merck & Co., Inc., Rahway, NJ, USA (2023).
- Doi T, Piha-Paul SA, Jalal SI et al. Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J. Clin. Oncol. 36(1), 61–67 (2018).
- Shah MA, Kojima T, Hochhauser D et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the Phase II KEYNOTE-180 Study. JAMA Oncol. 5(4), 546–550 (2019).
- Zhu C, Ma X, Hu Y et al. Safety and efficacy profile of lenvatinib in cancer therapy: a systematic review and meta-analysis. Oncotarget 7(28), 44545–44557 (2016).
- Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol. 15(5), 325–340 (2018).
- Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell 176(6), 1248–1264 (2019).
- Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat. Rev. Cancer 13(12), 871–882 (2013).
- Gockel I, Moehler M, Frerichs K et al. Co-expression of receptor tyrosine kinases in esophageal adenocarcinoma and squamous cell cancer. Oncol. Rep. 20(4), 845–850 (2008).
- Touat M, Ileana E, Postel-Vinay S, André F, Soria JC. Targeting FGFR signaling in cancer. Clin. Cancer Res. 21(12), 2684–2694 (2015).
- Wada J, Suzuki H, Fuchino R et al. The contribution of vascular endothelial growth factor to the induction of regulatory T-cells in malignant effusions. Anticancer Res. 29(3), 881–888 (2009).
- Suzuki H, Onishi H, Wada J et al. VEGFR2 is selectively expressed by FOXP3high CD4+ Treg. Eur. J. Immunol. 40(1), 197–203 (2010).
- Terme M, Pernot S, Marcheteau E et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 73(2), 539–549 (2013).
- Schmittnaegel M, Rigamonti N, Kadioglu E et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci. Transl. Med. 9(385), eaak9670 (2017).
- Voron T, Colussi O, Marcheteau E et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T-cells in tumors. J. Exp. Med. 212(2), 139–148 (2015).
- Capozzi M, De Divitiis C, Ottaiano A et al. Lenvatinib, a molecule with versatile application: from preclinical evidence to future development in anti-cancer treatment. Cancer Manag. Res11. 3847–3860 (2019).
- Nair A, Lemery SJ, Yang J et al. FDA approval summary: lenvatinib for progressive, radio-iodine-refractory differentiated thyroid cancer. Clin. Cancer Res. 21(23), 5205–5208 (2015).
- Nair A, Reece K, Donoghue MB et al. FDA supplemental approval summary: lenvatinib for the treatment of unresectable hepatocellular carcinoma. Oncologist 26(3), e484–e491 (2021).
- Kato Y, Tabata K, Kimura T et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T-cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLOS ONE 14(2), e0212513 (2019).
- Kimura T, Kato Y, Ozawa Y et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 109(12), 3993–4002 (2018).
- Yamamoto Y, Matsui J, Matsushima T et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc. Cell 6, 18 (2014).
- Taylor MH, Lee CH, Makker V et al. Phase IB/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors. J. Clin. Oncol. 38(11), 1154–1163 (2020).
- Lee CH, Shah AY, Rasco D et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a Phase Ib/2 study. Lancet Oncol. 22(7), 946–958 (2021).
- Makker V, Taylor MH, Aghajanian C et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J. Clin. Oncol. 38(26), 2981–2992 (2020).
- Finn RS, Ikeda M, Zhu AX et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 38(26), 2960–2970 (2020).
- Kawazoe A, Fukuoka S, Nakamura Y et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, Phase II trial. Lancet Oncol. 21(8), 1057–1065 (2020).
- FDA approves lenvatinib plus pembrolizumab for advanced renal cell carcinoma. www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lenvatinib-plus-pembrolizumab-advanced-renal-cell-carcinoma (01/12/2022).
- Makker V, Colombo N, Casado Herráez A et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N. Engl. J. Med. 386(5), 437–448 (2022).
- FDA grants regular approval to pembrolizumab and lenvatinib for advanced endometrial carcinoma. www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-pembrolizumab-and-lenvatinib-advanced-endometrial-carcinoma (10/05/2022).
- Taylor MH, Schmidt EV, Dutcus C et al. The LEAP program: lenvatinib plus pembrolizumab for the treatment of advanced solid tumors. Future Oncol. 17(6), 637–648 (2021).
- Cohen DJ, Tabernero J, Van Cutsem E et al. A randomized Phase III study evaluating the efficacy and safety of first-line pembrolizumab plus lenvatinib plus chemotherapy versus chemotherapy in patients with advanced/metastatic gastroesophageal adenocarcinoma: LEAP-015. J. Clin. Oncol. 40(Suppl. 4), TPS369 (2022).
- Arance A, De La Cruz-Merino L, Petrella TM et al. Phase II LEAP-004 study of lenvatinib plus pembrolizumab for melanoma with confirmed progression on a programmed cell death protein-1 or programmed death ligand 1 inhibitor given as monotherapy or in combination. J. Clin. Oncol. 41(1), 75–85 (2022).
- Shitara K, Van Cutsem E, Bang YJ et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: The KEYNOTE-062 Phase III Randomized Clinical Trial. JAMA Oncol. 6(10), 1571–1580 (2020).
- Nishio M, Horai T, Horiike A et al. Phase I study of lenvatinib combined with carboplatin and paclitaxel in patients with non-small-cell lung cancer. Br. J. Cancer 109(3), 538–544 (2013).
- Nishio M, Peled N, Zer A et al. 1313P Phase III LEAP-006 safety run-in (part 1): 1L pembrolizumab (pembro) + chemotherapy (chemo) with lenvatinib (len) for metastatic NSCLC. Ann. Oncol. 31, S848–S849 (2020).
- Gupta A, Koetz B, Hanekom W, O'brien JP, Wanders J, Jansen M. Population pharmacokinetics (PK) and exposure/response relationships of the receptor tyrosine kinase inhibitor E7080 in phase I studies. Eur. J. Can. Suppl. 8(7), 143 (2010).
- Motzer R, Alekseev B, Rha SY et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N. Engl. J. Med. 384(14), 1289–1300 (2021).

