Abstract
Aim: To assess time to improvement in Quality of Life in Neurological Disorders (Neuro-QoL) domains for patients treated with natalizumab versus ocrelizumab. Methods: Patients enrolled in the MS PATHS network who initiated treatment with either natalizumab or ocrelizumab rated the Neuro-QoL domains of physical function, symptoms, emotional health, cognitive function and social ability. Results: Time to clinically meaningful improvement was significantly shorter with natalizumab versus ocrelizumab for cognitive function (event time ratio [95% CI]: 0.37 [0.24–0.57]; p < 0.001), sleep disturbance (0.45 [0.28–0.72]; p = 0.001), social role participation (0.37 [0.21–0.66]; p = 0.001) and social role satisfaction (0.5 [0.31–0.8]; p = 0.004). Conclusion: Natalizumab had shorter time to clinically meaningful improvement in cognitive, sleep, and social role Neuro-QoL domains versus ocrelizumab.
Plain language summary
Knowledge of treatment-related benefits associated with medication choices, including improvement of quality of life (QoL), are strong influential factors for patients to start and continue their therapies. Little is known about patient-reported time to onset of functional improvement upon the initiation of medications for multiple sclerosis (MS). The Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS) network, a repository of collaborative international data on routine MS management, includes patient-reported information on the health-related QoL using the Quality of Life in Neurological Disorders (Neuro-QoL) measure. This study included data from 883 eligible patients enrolled in MS PATHS, with the aim of assessing and comparing the time to improvement in physical, mental and social health for patients treated with natalizumab versus ocrelizumab using Neuro-QoL. Natalizumab and ocrelizumab are both high-efficacy treatment options for relapsing forms of MS. The results demonstrated that, compared with ocrelizumab, natalizumab treatment led to faster effect on mental and social health, as well as quicker improvements in physical functioning in the arms and hands. Overall, it took shorter time for natalizumab-treated patients to achieve better QoL compared with ocrelizumab. These findings highlight the importance of QoL in disease management and provide a patient perspective for healthcare providers when making decisions about high-efficacy treatments for their patients with MS.
Tweetable abstract
Natalizumab had shorter time to clinically meaningful improvement in the Neuro-QoL domains, Cognitive Function, Sleep Disturbance, Social Role Participation and Social Role Satisfaction versus ocrelizumab.
Understanding patient-reported changes in physical, psychological and social health after initiating disease-modifying therapies (DMTs) ought to play a larger role in clinical decision-making to foster adherence and to identify the optimal treatment for patients with multiple sclerosis (MS).
Improved quality of life (QoL) associated with MS DMTs can influence patient treatment adherence.
Natalizumab and ocrelizumab are among the most widely used high-efficacy DMTs approved to treat relapsing forms of MS. Clinical trials and real-world studies showed that natalizumab and ocrelizumab have effects on patient health-related QoL (HRQoL).
Managing MS can be challenging, as it is a complex and unpredictable disease with varying speed of onset of treatment efficacy and the long-term sustained effect of DMTs. These factors influence how quickly patients can expect to experience improved QoL.
Little is known about patient-reported time to onset of functional improvement upon the initiation of MS DMTs.
This real-world study using MS PATHS data demonstrated that natalizumab can shorten the time to clinically meaningful improvement in the Neuro-QoL domains of cognition, positive affect and well-being and satisfaction with social roles and activities compared with ocrelizumab.
These results expand on prior studies of HRQoL with natalizumab relating to improvements in cognition and social engagement associated with high-efficacy treatment.
The findings from this study may inform decision making for patients with MS and healthcare providers regarding QoL-related benefits associated with high-efficacy DMTs.
The neurotherapeutic landscape for multiple sclerosis (MS) is rapidly expanding. To identify the optimal treatment for patients with MS, understanding patient-reported changes in physical, psychological and social health after initiation of disease-modifying therapies (DMTs) ought to play a larger role in clinical decision-making to foster adherence [Citation1]. Natalizumab and ocrelizumab are among the most widely used high-efficacy DMTs approved to treat relapsing forms of MS [Citation2,Citation3]. Clinical trials and real-world evidence showed that natalizumab improved patient health-related quality of life (HRQoL) [Citation4–6]. In contrast to ocrelizumab (and other DMTs), patients treated with natalizumab have reported a ‘feel-good experience’, characterized by a generalized sense of improved health and well-being [Citation7–9]. Further, long-term natalizumab treatment in patients with early relapsing–remitting MS demonstrated benefits in confirmed disability improvement, cognitive processing speed and patient-reported outcomes (PROs), including physical and psychological quality of life (QoL) and patients' abilities to work and perform regular activities [Citation6].
The Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS) learning health system is a collaborative international network of healthcare institutions that gathered real-world data on routine MS management [Citation10]. A previous analysis used data from MS PATHS from patients with ≥1 Quality of Life in Neurological Disorders (Neuro-QoL) assessment in the prior year to assess patient-reported HRQoL outcomes in natalizumab- or ocrelizumab-treated patients. Patients who reported primary progressive MS and those who had prior exposure to ocrelizumab or natalizumab were excluded [Citation9]. In that study, neuroperformance outcomes were measured using Neuro-QoL [Citation11,Citation12], which contains 12 domains for physical, psychological and social health. The results showed statistically significant improvements in nine of 12 Neuro-QoL domains with natalizumab versus four of 12 domains with ocrelizumab [Citation9]. Although changes in PROs could be influenced by expectation bias associated with initiating a new therapy [Citation13,Citation14], the observed improvements associated with natalizumab in the prior study were unlikely driven by expectation bias because the magnitude of those improvements exceeded those associated with ocrelizumab [Citation9].
It has been reported that self-perception of a good QoL was an independent factor associated with treatment adherence in patients with chronic conditions [Citation15,Citation16]. Providing comprehensive medication information, including health outcomes of self-management and treatment regimen benefits for the patient, was identified as a facilitator for medication adherence [Citation17,Citation18]. Patients' desire to return to a ‘normal life’ is a strong motivation for DMT adherence [Citation19], in anticipation of experiencing the treatment's onset of action in a timely manner. Little is known about the temporal aspects of QoL measures and specific patient-reported neuroperformance improvement upon the initiation of MS DMTs. The current study aimed to assess time to improvement in each Neuro-QoL domain for patients treated with natalizumab versus ocrelizumab.
Patients & methods
Patients
Patient consent for the use of protected health information in accordance with national and local regulations was obtained using a format approved by the local institutional review board or ethics committee prior to enrolling in the MS PATHS system [Citation10].
Patients enrolled in the MS PATHS network as of 24 January 2023 (data cutoff for the current analysis) who initiated treatment with either natalizumab or ocrelizumab while enrolled in the MS PATHS network and had ≥1 Neuro-QoL assessment in the year prior to initiation were eligible. Baseline was defined as the last Neuro-QoL assessment within 1 year prior to the treatment initiation date. Patients were excluded for any of the following: self-report of primary progressive MS, prior treatment with natalizumab or ocrelizumab, missing values in the baseline covariates, no follow-up Neuro-QoL assessments after baseline and/or a baseline Neuro-QoL score <5 points below the maximum for positively worded domains or <5 points above the minimum for negatively worded domains (i.e., in this study when only looking at improvement, patients without an opportunity to improve ≥5 points in Neuro-QoL scores due to floor and ceiling effects). Patients who initiated natalizumab prior to the availability of ocrelizumab (28 March 2017) were also excluded.
Outcomes
T-scores for 12 Neuro-QoL domains were obtained at routine visits through the MS PATHS network as described previously [Citation9]. In brief, patients rated their feelings or functioning within the last 7 days on a 5-point scale. The domains included physical function (upper and lower extremity function) and symptoms (sleep disturbance and fatigue), emotional health (anxiety, depression, positive affect and well-being, emotional and behavioral dyscontrol and stigma), cognitive function and social ability (participation in and satisfaction with social roles and activities) (Supplementary Table 1). Neuro-QoL does not include domains that assess impaired sphincter function, impaired or double vision, or speech or swallowing difficulty.
Baseline was defined as the last Neuro-QoL measurement ≤1 year prior to initiating treatment with natalizumab or ocrelizumab. Events were defined as a clinically meaningful improvement in T-score when patients were on treatment before stopping or switching to another treatment. Clinically meaningful improvement in T-score was defined as a change of ≥5 points from baseline, previously identified as the threshold of minimally important differences in patient-reported QoL in chronic diseases [Citation20]. Since the Neuro-QoL measurements were only collected at routine visits, yet clinically meaningful improvement can occur at any time between two visits, interval-censored data were present in this study. Under this circumstance, event time was defined as the interval between the last visit before the improvement and the visit when the ≥5-point improvement was measured. For patients without clinically meaningful improvement in T-score, the censoring time was defined as the last visit during follow-up. Time to first clinically meaningful Neuro-QoL improvement in propensity score (PS)-matched natalizumab and ocrelizumab patients were compared.
Statistical analyses
Baseline characteristics were reported using descriptive statistics, and absolute standardized mean differences (SMDs) between patients treated with natalizumab and ocrelizumab were calculated.
For each Neuro-QoL domain, patients treated with natalizumab were PS matched 1:3 to patients treated with ocrelizumab to control for confounding with adjustment of the following baseline covariates: age, sex, race (White vs non-White), years of education, MS duration, Patient Determined Disease Steps (PDDS) score, number of patient-reported relapses since prior visit (0, 1, 2, 3+), Processing Speed Test (PST) score, average Manual Dexterity Test (MDT) score, use of prior DMTs (binary), use of co-medications (binary) and the corresponding baseline Neuro-QoL assessment. The 1:3 PS matching technique was conducted to match 1 natalizumab patient to multiple ocrelizumab patients. In this manner, the overall study cohort was maximized, while maintaining good covariate balance overall (Supplementary Figure 1).
The following analyses were performed based on the 1:3 PS-matched cohort. Kaplan-Meier (KM) curves were used to estimate the probability of improvement during the follow-up period for patients treated with natalizumab and ocrelizumab within each Neuro-QoL domain. This represents a nonparametric comparison directly between the two treatment groups based on observed data without making any model assumptions. A smooth accelerated failure time (AFT) model was used to estimate the event time ratio for interval censoring data (natalizumab vs ocrelizumab). This model was employed to analyze the data for two reasons. First, it accommodates the analysis of complex interval-censored data without the need to specify an event time distribution. Second, it offers an intuitive interpretation of the result on the time scale directly. For example, if the event time ratio is 0.5, we can interpret that natalizumab accelerates the time to improvement or shortens the time to improvement by 50% in comparison with ocrelizumab. Additional PS methodologies, such as inverse probability of treatment weighting (IPTW), could not be utilized in this specialized analysis. Event time ratios and 95% confidence intervals (CIs) were calculated for each Neuro-QoL domain. Statistical significance was defined as p < 0.05. For sensitivity analysis, 100 imputation datasets were generated to impute the missing values in the baseline characteristics.
Results
Patients
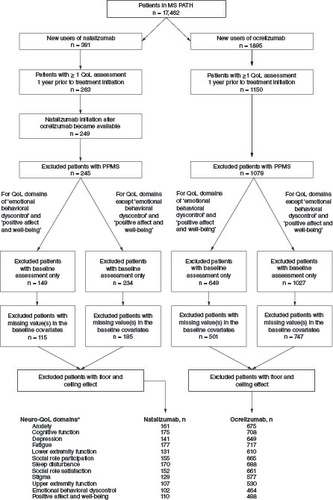
A total of 17,462 patients were enrolled in MS PATHS by the data cutoff date. Based on the inclusion criteria, different numbers of patients were identified as eligible for the various Neuro-QoL domains. Across the 12 domains, 102–177 patients who initiated natalizumab and 464–717 patients who initiated ocrelizumab were included in the analysis ().
Figure 1. Patient selection.
Data cutoff 24 January 2023. Numbers of patients for the various Neuro-QoL domains were different.
NOTE: As a result of reconfiguration of the Multiple Sclerosis Performance Test (MSPT) in April 2019, two of the Neuro-QoL domains-(1) positive affect and well-being and (2) emotional and behavioral dyscontrol – were removed. Hence, there are fewer subjects with available measurements in (1) and (2) compared with other domains.
MS PATHS: Multiple Sclerosis Partners Advancing Technology and Health Solutions; Neuro-QoL: Quality of Life in Neurological Disorders; PPMS: Primary progressive multiple sclerosis; QoL: Quality of life.
Before PS matching, eligible patients exhibited differences in baseline covariates. & Supplementary Table 2 show the baseline characteristics of patients who were eligible for the Neuro-QoL Cognitive Function domain before PS matching. Notably, the female to male ratio in this study was higher than the typical epidemiological distribution of MS (e.g., 3:1 female to male). In the MS PATHS database, the overall patient demographic is approximately 75% female, which has been reported in other studies using large databases, such as MSBase [Citation10,Citation21]. Specifically, for the NTZ cohort in our study and in other investigations, the prevalence of female patients with MS is slightly higher at 83% [Citation9]; these percentages were before PS matching. After PS matching, both groups included approximately 81% female patients. In general, patients who initiated ocrelizumab were older, had longer duration of MS, had higher PDDS scores, had lower PST scores, and were more likely to have received prior DMTs than patients who initiated natalizumab. Before matching, the time from baseline Neuro-QoL assessment to initiation of treatment (index date) was similar among patients treated with natalizumab or ocrelizumab, whereas the follow-up time showed that patients who initiated natalizumab stopped or switched to another treatment earlier than those who initiated ocrelizumab. For instance, in patients who were evaluable for the Neuro-QoL Cognitive Function domain, the mean (SD) interval between baseline assessment and index date was 155.8 (80.7) days among patients treated with natalizumab and 171.3 (84.8) days for those treated with ocrelizumab. The mean follow-up time for natalizumab and ocrelizumab-treated patients was 689.0 (535.3) and 784.7 (521.5) days, respectively ().
Table 1. Baseline characteristics of eligible MS PATHS patients treated with natalizumab or ocrelizumab who were evaluable for Neuro-QoL Cognitive Function domain before propensity score matching.
Table 2. On-study follow-up and assessments in MS PATHS patients treated with natalizumab or ocrelizumab who were evaluable for Neuro-QoL cognitive function domain.
After PS matching (1:3), depending on the individual Neuro-QoL domain, there were 86–144 natalizumab-treated patients and 258–432 ocrelizumab-treated patients (). Baseline characteristics after PS matching were well balanced between treatment groups, indicating satisfactory confounding adjustment across the measured covariates included in the PS model. Within each Neuro-QoL domain, patient baseline characteristics were similar after PS matching (SMD <0.1 is generally considered a small and acceptable group-wise difference). (for example) shows the generally well-balanced baseline characteristics after PS matching for patients evaluable for the Cognitive Function and Social Role Satisfactions Neuro-QoL domains (other domains not shown, but all were similarly well-balanced). Supplementary Figure 1 shows examples of PS distribution before and after PS matching.
Figure 2. Event time ratios in Neuro-QoL domains in 1:3 PS-matched patients before and after multiple imputation.
Results of accelerated failure time analyses.
CI: Confidence interval; Neuro-QoL: Quality of Life in Neurological Disorders; PS: Propensity score; PSM: PS matched.
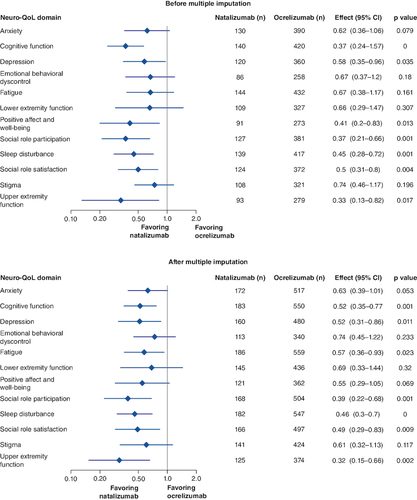
Table 3. Baseline covariates of PS-matched (1:3) natalizumab and ocrelizumab-treated patients for the Cognitive Function and Satisfaction with Social Roles and Activities Neuro-QoL domains.
Time to improvement in Neuro-QoL domains in PS-matched (1:3) natalizumab- & ocrelizumab-treated patients
Time to clinically meaningful improvement was significantly shorter with natalizumab than with ocrelizumab for the cognitive function (event time ratio [95% CI]: 0.37 [0.24–0.57]; p < 0.001), depression (0.58 [0.35–0.96]; p < 0.05), positive affect and well-being (0.41 [0.2–0.83; p < 0.05), social role participation (0.37 [0.21–0.66]; p = 0.001), sleep disturbance (0.45 [0.28–0.72]; p = 0.001), upper extremity function (0.33 [0.13–0.82]; p < 0.05) and social role satisfaction (0.5 [0.31–0.8]; p = 0.004) Neuro-QoL domains ().
Patients treated with natalizumab also tended to experience a shorter time to improvement for anxiety (0.62 [0.36–1.06]; p = 0.079), though the difference did not reach statistical significance. There were no statistical differences between treatment groups among the remaining five Neuro-QoL domains. When multiple imputation was conducted to account for missing data, the results were similar to those prior to imputation; however, the positive affect and well-being Neuro-QoL domain was no longer statistically significant after multiple imputation ().
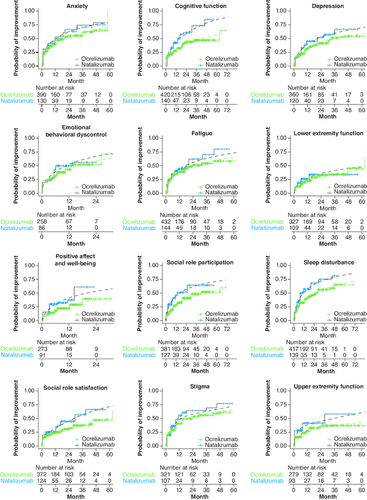
The estimated probability of a 5-point improvement over time was compared between the two treatment groups, based on the KM method and curves derived from the AFT model. The curves estimated by the AFT model closely overlay with the KM estimates across the Neuro-QoL domains, suggesting reasonable model fit to the observed data. Compared with patients treated with ocrelizumab, those treated with natalizumab showed a higher probability of improvement for >24 months in the Neuro-QoL domain positive affect and well-being. In the remainder of the domains, except for Emotional Behavioral Dyscontrol and Lower Extremity Function, the probability of improvement remined higher for 48–60 months ().
Figure 3. Probability of clinically meaningful improvement in Neuro-QoL domains by Kaplan-Meier and accelerated failure time analysis in 1:3 PS-matched patients (solid lines represent the Kaplan-Meier estimates and dashed lines represent estimates from the accelerated failure time model).
Neuro-QoL: Quality of Life in Neurological Disorders; PS: Propensity score.
Discussion
This PS-matched comparative effectiveness study of patients enrolled in MS PATHS demonstrated that natalizumab treatment results in a shorter time to clinically meaningful improvement in the Neuro-QoL domains of cognitive function, depression, positive affect and well-being, social role participation, sleep disturbance, social role satisfaction and upper extremity function compared with ocrelizumab treatment. Compared with ocrelizumab, natalizumab treatment led to a faster effect on mental and social health as assessed by Neuro-QoL. Along with quicker improvements in physical functioning in the arms and hands, it took a shorter time for natalizumab-treated patients to achieve overall better QoL compared with patients treated with ocrelizumab.
Important baseline characteristics were measured and included in the PS model to control for potential confounding in this study. The 1:3 matching technique (natalizumab:ocrelizumab) is representative of current MS PATHS treatment patterns using these DMTs. The distribution of baseline covariates following PS matching was, overall, comparable between the treatment groups, enabling a reasonable comparison of Neuro-QoL improvement during follow-up.
The precise mechanism by which natalizumab and ocrelizumab exert their therapeutic effects in MS is unknown. However, the different time kinetics of natalizumab versus ocrelizumab may be understood based on their respective mechanisms of action. Natalizumab inhibits the α4-mediated adhesion of leukocytes to their counter-receptors (i.e., VCAM-1), and prevents transmigration of leukocytes across the endothelium to the central nervous system. Ocrelizumab is presumed to bind to CD20, a cell surface antigen present on pre-B and mature B lymphocytes, and results in antibody-dependent cellular cytolysis and complement-mediated lysis [Citation2,Citation3].
Studies have investigated the different physiological effects natalizumab has on patients, including the ‘feel-good experience’ during treatment [Citation22,Citation23]. However, there is limited information on the patient-reported experience during ocrelizumab treatment, which is summarized in the present study. Information on patient-reported QoL while on treatment may help healthcare providers and patients in their clinical decision making. While the efficacy onset of natalizumab and ocrelizumab were comparable in clinical trials [Citation24,Citation25], statistically significant differences in the time to onset of improved QoL were demonstrated in this study.
Hersh et al. [Citation9] reported that at an average of 1 year after starting therapy, a difference was observed between natalizumab and ocrelizumab in the Neuro-QoL domains of cognition and satisfaction with social roles and activities in patients enrolled in MS PATHS. Similarly, in this study, the projections of the KM and AFT model-estimated improvement probabilities () demonstrated that the differences in effects between the two treatments remained over time. For most of the Neuro-QoL domains, the difference in the probability of achieving a clinically meaningful improvement lasted for over 60 months.
Managing MS can be challenging, as it is a complex and unpredictable disease with physical, cognitive and emotional symptoms. Clinicians are interested in knowing the speed of onset of treatment efficacy and the long-term sustained effect of DMTs, but also in how quickly patients can expect to experience a better QoL. Long-term real-world data would provide information on whether such differences are durable as patients remain on treatment and whether there is a ‘catch-up’ in functional improvement in patients treated with other effective therapies. In certain cases, despite positive Neuro-QoL scores, patients may discontinue natalizumab treatment, mainly due to John Cunningham virus (JCV)-positive status, which increases the risk of progressive multifocal leukoencephalopathy (PML) [Citation26].
This retrospective observational study has limitations that are worth noting. First, there were three-times more patients with MS who were treated with ocrelizumab than with natalizumab in the current cohort. However, this proportion is consistent with MS DMT treatment patterns when considering that, historically, natalizumab was considered a second- or third-line therapy for MS mainly due to safety concerns relating to the potential risk of PML [Citation27,Citation28], while ocrelizumab utilization is broader – potentially leading to selection bias. The 1:3 PS matching approach (natalizumab:ocrelizumab) in the current study is representative of the treatment patterns among all patients enrolled in the MS PATHS learning health system and therefore not expected to be related to selective recruitment. Further, the excellent covariate balance achieved across treatment groups using PS methodology, despite different baseline sample sizes, reassures the study outcomes.
Second, we excluded patients with known primary progressive MS (i.e., within 1 year prior to the initiation of natalizumab or ocrelizumab, respectively), since natalizumab is not indicated for this disease course and otherwise would have created a biased cohort. However, MS type was missing in nearly half the population across both treatment groups, which means some patients with primary progressive MS may have potentially been included. This is a limitation of data collection in the MS PATHS database since MS type is a self-reported measure. Patients frequently miss reporting MS type during follow-up visits after the initial report when enrolling in the MS PATHS network. Reassuringly, in the overall population within the MS PATHS database, only 7.5% of patients had primary progressive MS [Citation10]. Since the overall percentage of patients with primary progressive MS is relatively low, we do not expect that the potential inclusion of this disease course in the current study would significantly impact the results.
Third, the frequency and timing of assessments were not prespecified, and the length of follow-up varied among patients, though was similar in average duration to the first Neuro-QoL assessment. Inherent to observational studies, these factors may have contributed to various forms of bias (e.g., recall, reporting and detection). In this study, relapses were patient-reported (therefore open to recall/reporting bias) and not confirmed by a clinician. Although PS matching controlled for measured baseline patient characteristics, hidden bias (e.g., unmeasured variables such as magnetic resonance imaging data) may have influenced the study outcomes. Further, approximately 40% of the ocrelizumab patients were excluded in the analysis because they remained unmatched. Therefore, our results are an estimation of the average effect in an ocrelizumab-treated population in which patients' characteristics resemble those of the natalizumab-treated cohort and do not represent the average effect in the overall population. While all proper procedures were followed for PS matching, the two nonmatched populations had differences in age, duration of MS, PDDS scores, PST scores and the number of prior DMTs – baseline characteristics that may limit the generalizability of the overall population.
Fourth, baseline variables within 1 year prior to natalizumab or ocrelizumab initiation were used in the PS-matching model, limiting our ability to assess functional decline prior to DMT initiation. This is because of the reduction in sample size and power resulting from the criterion of including only patients with ≥2 assessments in the year prior to the treatment initiation date; a decrease in functional performance was excluded in the model. The limited sample size across many domains focusing on patients who already had the first improvement precluded a conclusive analysis.
Fifth, there is no current consensus for the definition of a clinically meaningful change in Neuro-QoL scores. However, after reviewing the literature, the threshold of clinically meaningful improvement of ≥5 points is meant to reflect 0.5 SD. The minimally important difference for HRQoL instruments was determined to be 0.5 SD, independent of disease state or HRQoL instrument [Citation20]. In addition, differences in study design and schedule of evaluations could underlie differences in psychosocial outcomes achieved in studies of these two DMTs.
Finally, it is possible that fluctuations in the Neuro-QoL scores between meaningful and nonmeaningful values occurred after the first improvement measurement (i.e., influenced by life events that were independent of DMT initiation). Such fluctuation is a common limitation with patient self-reported outcome measures.
Further investigations are warranted to determine the mechanisms underlying the apparent delay in functional improvement in mental and social health and physical functioning in the arms and hands (as assessed by Neuro-QoL) in ocrelizumab-treated patients compared with natalizumab-treated patients.
Conclusion
This real-world study using MS PATHS data demonstrated that natalizumab can shorten the time to clinically meaningful improvement in the Neuro-QoL domains of cognition, positive affect and well-being, and satisfaction with social roles and activities compared with ocrelizumab. Quicker improvements in QoL may impact adherence to DMTs and deserves further exploration. These results expand on prior studies of HRQoL with natalizumab, implicating improvements in cognition and social engagement associated with highly effective therapy. Further, these results may be informative for patients with MS and their healthcare providers, enhancing awareness of disease management as well as making decisions about high-efficacy treatments.
Author contributions
CM Hersh contributed to the conception of the study. M Pang did the formal analysis and handled data curation. RL Avila handled data curation and supervised the development of the manuscript. CM Hersh, M Pang, DM Miller, MP McGinley, M Hyland, T Ziemssen and RL Avila contributed to the methodology, interpretation of data, review and editing of the manuscript.
Financial disclosure
This study and its medical writing support was funded by Biogen (MA, USA). The authors had full editorial control of the manuscript and provided their final approval of all content. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
Medical writing support for this article was funded by Biogen.
Ethical conduct of research
In accordance with MS PATHS requirements for enrollment, patients with a confirmed diagnosis of MS, including clinically isolated syndrome, and the ability to understand the purpose and the risks of the project are eligible to enroll. In line with national and local subject privacy regulations, patients provide authorization for the use of protected health information. The authorization format was determined by the local institutional review board or ethics committee. The investigators and research coordinators are encouraged to invite all patients at each MS center to participate. Patients consented to sharing of pseudoanonymized data with the MS PATHS network investigators and sponsor, and it was approved by all site institutional review boards.
Data sharing statement
The datasets generated and analyzed during the current study are not publicly available. The authors fully support sharing whenever possible. Requests for deidentified data should be made to Biogen via established company data sharing policies and processes detailed at http://clinicalresearch.biogen.com/.
Supplementary Figure S1 and Tables S1-S2
Download MS Word (264.6 KB)Acknowledgments
We are grateful to I Koulinska for her contribution to the conceptualization and early development of this manuscript. S Li of Envision Pharma Group (Horsham, UK) wrote the first draft with input from authors and C Farrell from Excel Scientific Solutions copyedited and styled the manuscript per journal requirements.
Competing interests disclosure
CM Hersh has received speaking, consulting, and/or advisory board fees from Alexion, Biogen, Bristol Myers Squibb, EMD-Serono, Genentech, Genzyme, Novartis, and TG Therapeutics. She has received research support paid directly to her institution by Biogen, Genentech, National Institutes of Health, Novartis, and Patient-Centered Outcomes Research Institute. M Pang and RL Avila are employees and shareholders of Biogen. DM Miller served as consultant for Roche and has received royalties from the Cleveland Clinic for licensing MSPT-related technology. MP McGinley receives funding from the National Institutes of Health and Agency for Healthcare Research and Quality. She has also served on scientific advisory boards for Genentech and EMD Serrano and received research funding from Novartis, Biogen and Genentech. M Hyland received research support from Biogen and the Patient-Centered Outcomes Research Institute. T Ziemssen received grants and study funding as well as speaking consulting fees from Biogen, BMS, Hexal, Merck, Novartis, Roche, Sanofi, TEVA and Viatris. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
References
- Neter E, Glass-Marmor L, Haiien L, Miller A. Concordance between persons with multiple sclerosis and treating physician on medication effects and health status. Patient Prefer. Adherence 15, 939–943 (2021).
- Tysabri (natalizumab) [package insert]. Biogen Inc., Cambridge, MA, USA (2021).
- Ocrevus (ocrelizumab) [package insert]. Genentech, Inc., South San Francisco, CA, USA (2020).
- Perumal J, Fox RJ, Balabanov R et al. Outcomes of natalizumab treatment within 3 years of relapsing–remitting multiple sclerosis diagnosis: a prespecified 2-year interim analysis of STRIVE. BMC Neurol. 19(1), 116 (2019).
- Perumal J, Balabanov R, Su R et al. Natalizumab in early relapsing–remitting multiple sclerosis: a 4-year, open-label study. Adv. Ther. 38(7), 3724–3742 (2021).
- Perumal J, Balabanov R, Su R et al. Improvements in cognitive processing speed, disability, and patient-reported outcomes in patients with early relapsing–remitting multiple sclerosis treated with natalizumab: results of a 4-year, real-world, open-label study. CNS Drugs 36(9), 977–993 (2022).
- Foley J, Berkovich R, Gudesblatt M. Natalizumab-treated patients with relapsing-remitting multiple sclerosis report better “feel-good” outcomes in key physical, emotional, and cognitive domains compared to other disease-modifying therapies. Int. J. MS Care 22(Suppl. 2), 87 (2020).
- Vartanian T, Berkovich R, Foley J et al. Characterizing the positive experience reported by natalizumab-treated patients with relapsing multiple sclerosis. European Charcot Foundation 2019 Annual Symposium. Baveno, Italy (2019).
- Hersh CM, Kieseier B, De Moor C et al. Impact of natalizumab on quality of life in a real-world cohort of patients with multiple sclerosis: results from MS PATHS. Mult. Scler J. Exp. Transl. Clin. 7(2), 20552173211004634 (2021).
- Mowry EM, Bermel R, Williams JR et al. Harnessing real-world data to inform decision-making: Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS). Front. Neurol. 11, 632 (2020).
- Cella D, Lai JS, Nowinski CJ et al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology 78(23), 1860–1867 (2012).
- Miller DM, Bethoux F, Victorson D et al. Validating Neuro-QoL short forms and targeted scales with people who have multiple sclerosis. Mult. Scler. 22(6), 830–841 (2016).
- Bechman K, Yates M, Norton S, Cope AP, Galloway JB. Placebo response in rheumatoid arthritis clinical trials. J. Rheumatol. 47(1), 28–34 (2020).
- D'amico E, Haase R, Ziemssen T. Review: patient-reported outcomes in multiple sclerosis care. Mult. Scler. Relat. Disord. 33, 61–66 (2019).
- Fernandez-Lazaro CI, Garcia-Gonzalez JM, Adams DP et al. Adherence to treatment and related factors among patients with chronic conditions in primary care: a cross-sectional study. BMC Fam. Pract. 20(1), 132 (2019).
- Jüngst C, Graber S, Simons S, Wedemeyer H, Lammert F. Medication adherence among patients with chronic diseases: a survey-based study in pharmacies. QJM 112(7), 505–512 (2019).
- Kvarnström K, Airaksinen M, Liira H. Barriers and facilitators to medication adherence: a qualitative study with general practitioners. BMJ Open 8(1), e015332 (2018).
- Kvarnström K, Westerholm A, Airaksinen M, Liira H. Factors contributing to medication adherence in patients with a chronic condition: a scoping review of qualitative research. Pharmaceutics 13(7), 1100 (2021).
- Shaw Y, Metes ID, Michaud K et al. Rheumatoid arthritis patients' motivations for accepting or resisting disease-modifying antirheumatic drug treatment regimens. Arthritis Care Res. (Hoboken) 70(4), 533–541 (2018).
- Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med. Care 41(5), 582–592 (2003).
- He A, Merkel B, Brown JWL et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 19(4), 307–316 (2020).
- Foley J, Berkovich R, Gudesblatt M et al. Characterizing the ‘feel-good experience’ in multiple sclerosis patients treated with natalizumab or other therapies. Neurodegener. Dis. Manag. 13(1), 23–34 (2023).
- Berkovich R, Foley J, Gudesblatt M et al. RRMS patients treated with natalizumab report better outcomes and treatment satisfaction than patients treated with ocrelizumab. Presented at: Consortium of Multiple Sclerosis Centers. National Harbor, MD, USA (June 1–4, 2022).
- Kappos L, O'Connor PW, Polman CH et al. Clinical effects of natalizumab on multiple sclerosis appear early in treatment course. J. Neurol. 260(5), 1388–1395 (2013).
- Barkhof F, Kappos L, Wolinsky JS et al. Onset of clinical and MRI efficacy of ocrelizumab in relapsing multiple sclerosis. Neurology 93(19), e1778–e1786 (2019).
- Clerico M, Artusi CA, Liberto AD et al. Natalizumab in multiple sclerosis: long-term management. Int. J. Mol. Sci. 18(5), 940 (2017).
- Brandstadter R, Katz Sand I. The use of natalizumab for multiple sclerosis. Neuropsychiatr. Dis. Treat. 13, 1691–1702 (2017).
- Outteryck O. Natalizumab in relapsing–remitting multiple sclerosis. Expert Rev. Neurother. 16(5), 471–481 (2016).

