Abstract
Poorly differentiated neuroendocrine carcinomas (PDECs) represent highly malignant tumors with an immense tendency to metastasize and with a poor prognosis. The treatment consists of palliative chemotherapy and corresponds to the treatment of extensive stage small cell lung cancer. Material and methods. We present the patient characteristics and treatment results of 31 consecutive, chemonaïve patients with PDECs treated with carboplatin, etoposide, and vincristine. Results. The response rate was 52%, the disease control rate 77%, and the median overall survival 15.3 months. The one-year survival rate was 55%, and the two-year survival rate was 19%. The median progression free survival (PFS) time was 6.6 months. Survival rates did not correlate with the Ki-67 proliferation index. The treatment was well tolerated. Conclusion. Treatment results with carboplatin, etoposide, and vincristine in chemonaïve patients with PDECs are comparable to those in patients with SCLC. The prognosis is however poor.
Within the spectrum of neuroendocrine (NE) tumors, poorly differentiated NE carcinomas (PDECs) with a high proliferation rate account for around 15%. It is important to distinguish PDECs from the well differentiated NE carcinomas and NE tumors, since the clinical course and treatments of these three entities are very different. Many PDECs arise from the lungs, gastrointestinal tract, and pancreas. However, patients often present with disseminated disease with no apparent primary tumor location, i.e. cancer of unknown primary location (CUP). Patients with PDECs rarely have symptoms or signs of excessive hormonal production [Citation1,Citation2].
The immunohistochemical work-up will often show positive reaction for synaptophysin, but not necessarily for chromogranin A. By definition, the proliferation index (PI) exceeds 20%, expressed by the Ki-67 index [Citation3,Citation4].
In contrast to well-differentiated NE carcinomas and tumors, the aggressiveness of PDECs is similar to that of small cell lung cancer (SCLC), resulting in a median survival of approximately six months without treatment [Citation5,Citation6]. Most patients have metastatic disease at the time of diagnosis and are unsuitable for surgical treatment with curative intent [Citation7,Citation8]. Since the early 1990s the palliative treatment with chemotherapy has mimicked the treatment for disseminated SCLC and thus largely remained unchanged [Citation1,Citation2,Citation4,Citation9–12].
We present a retrospective analysis of patient characteristics and treatment efficacy in previously untreated patients with advanced stage PDEC who received treatment with carboplatin, etoposide and vincristine during a 2.5 year period at the Department of Oncology, European NET Centre of Excellence, Rigshospitalet, Copenhagen, Denmark.
Material and methods
Patients
Successive patients referred for treatment with the standard chemotherapy regimen for PDEC from May 2007 to December 2009 were identified. Patients with SCLC were excluded, as were patients with prior antineoplastic treatment. All patients had measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.0), WHO performance status 0–2, and adequate haematological, renal, and hepatic function. Patient files, laboratory results, pathology reports, and radiological examinations were available for exploration. No patients were lost to follow-up.
Immunohistochemistry
The Ki-67 index was determined immunohistochemically by applying a monoclonal mouse anti-human Ki-67 antigen (DAKO Clone MIB-1, Dako Denmark A/S, Glostrup, Denmark), Code M7240. Twenty hot spot areas (i.e. 20 areas within the tumor with a high count of immunoreactive tumor nuclei) were estimated and the mean percentage of Ki-67 cells calculated. The Ki-67 index was calculated by an experienced NET pathologist with no knowledge of patient related prognostic information.
Polyclonal Rabbit Anti-Human Chromogranin A (Dako Denmark A/S, Glostrup, Denmark) Code A 0430 was used for the demonstration of chromogranin in the tumor tissue. Synaptophysin was demonstrated using Monoclonal Mouse Anti Human Synaptophysin (Clone Snp 88, BioGenex Laboratories Inc., Fremont, USA), Code MU363. Monoclonal Mouse Anti-Human CD56 (Clone 1B6, NovoCastra, Trichem, Skanderborg, Denmark), Code NCL-CD56-1B6.
Treatment
Patients received carboplatin AUC 5 IV day 1 + etoposide 100 mg/m2 PO day 1–3, and vincristine 1.3 mg/m2 (maximum 2.0 mg) IV day 1 in the outpatient clinic. Treatment was repeated every three weeks until progression or intolerable toxicity up to a maximum of six courses. Dose modifications were made according to standard procedures for the three drugs. Standard prophylactic antiemetic therapy consisted of oral prednisolone, ondansetron, and metoclopramide. Prior to the first treatment, renal function was measured by 51Cr-EDTA clearance. Biochemical parameters were measured on day 1 and 14 in every course. Response evaluation (computed tomography) was carried out approximately every eight weeks and plus whenever required due to changes in clinical and radiological status. Follow-up visits took place every six to nine weeks.
Statistical analysis
Survival statistics was performed using Kaplan-Meier curves and log rank tests using the Graph Pad Prism™ v. 5 software (La Jolla, USA). A p-value below 0.05 was considered significant.
Results
From May 2007 to December 2009, we identified 40 patients from the NET database with PDECs who had received treatment with carboplatin, etoposide, and vincristine. Nine patients were excluded from analysis: eight patients had received other first line treatment and one patient received the treatment as adjuvant after radical surgery. Hence, 31 patients were eligible for analysis, 13 females and 18 males. The median age was 63 years (range 33–82 years). Performance status: 0/1/2 = 10/16/5 (). All patients had disseminated disease. In 24 patients (77%) two or more organ systems were involved. Nine patients (34%) were classified as having carcinoma of unknown primary (CUP). All of these patients had a PET-CT and an extensive immunohistochemical work-up done to try to identify the primary tumor site. Histologically, 29 patients (94%) had PDEC; two patients (7%) had PDEC combined with adenocarcinoma and transitional carcinoma, respectively. Immunohistochemical staining for synaptophysin was positive in 30/31 (97%). Chromogranin A was positive in 16/31 (52%), partly positive in 9/31 (29%), negative in 5/31 (16%) and not performed in 1/31 (3%). In the single patient with negative chromogranin A and negative synaptophysin the diagnosis of PDEC was made on the basis of morphology and a positive immunohistochemical staining for CD56 combined with the clinical presentation of the patient. The Ki-67 proliferation index ranged from 20% to 100%; the index reaching 50% or higher in 24 (77%). Octreotide scintigraphy was positive in four of 22 (18%) scanned patients: three had octreotide uptake exceeding the liver (level 3), one had lower uptake (level 2).
Table I. Characteristics of 31 PDEC patients.
Patients received a median of five courses (range 1–8) of treatment. Three patients (10%) achieved complete response (CR), 13 (42%) partial response (PR), eight (26%) no change (NC), and seven (23%) progressive disease (PD). The overall response rate (CR + PR) was 52%, and the disease control rate (CR + PR + NC) 77%. The median overall survival time (OS) was 15.3 months (), the one-year survival rate 55%, and the two-year survival rate 19%. The median progression free survival (PFS) time was 6.6 months.
Figure 1. Overall survival (left panel) and progression-free survival (right panel) for 31 patients with PDEC receiving 1st line treatment with carboplatin, etoposide, and vincristine.
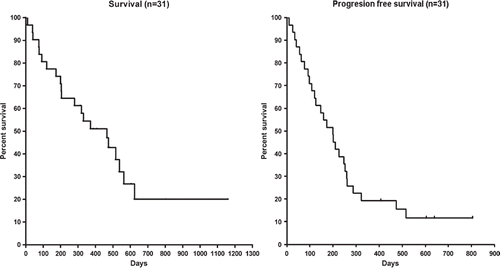
Subgroup analysis showed, that patients receiving first line chemotherapy only (n = 17) had median OS of 10.5 months versus 17.6 months among those patients receiving second or third line therapy (n = 14). This difference was not statistically significant (χ2 = 0.68, p = 0.41). The PFS in these subgroups was 5.7 versus 6.6 months, respectively (χ2 = 2.29, p = 0.13). For the subgroups CUP (n = 9) vs. Non-CUP (n = 22), the OS was 15.3 months vs. 12.2 months (χ2 = 0.50, p = 0.49), and the PFS was 5.2 months vs. 6.6 months (χ2 = 0.08, p = 0.77).
The distribution of the Ki-67 index did not correlate with OS. There was no statistical significant difference in median survival between patients with CgA-positive tumors (n = 16) and those with negative/partly positive tumors (n = 15) (χ2 = 2.41, p = 0.13).
No patients were diagnosed with brain metastases before or during treatment or at follow-up. One patient received prophylactic cranial irradiation after chemotherapy.
There were no treatment related deaths. Eight patients (26%) experienced grade 4 febrile neutropenia and were admitted for standard empirical antibiotic treatment. Seven patients (23%) had grade 3 neutropenia and two patients (6%) had grade 3 thrombocytopenia. None of the patients had neuropathy, and no patients developed renal toxicity. Emesis was well controlled on the standard prophylactic anti-emetic therapy.
Discussion
Patients with advanced PDECs have a poor prognosis. In the analysis from the Surveillance, Epidemiology, and End Results (SEER) of epidemiology and prognostic factors for over 35 000 NE tumors the median survival for 4054 patients with PDECs was 10 months [Citation13]. However, overall patients with distant metastases had a median survival of only five months and a one-year survival rate of 25%. In our present material, patients with distant metastases achieved a median survival of 15.3 months and a one-year survival of 55%, which is indicative of a relatively marked survival benefit from the treatment. However, data may not be comparable. The number of patients with grade 3–4 haematological toxicity was in line with what to expect with this combinatory regimen and the patients disease burden.
Treatment based on platin and etoposide is widely recommended [Citation14]. The recommendation is based on relatively few, older studies, and no controlled, randomized studies have been published. In a pivotal study a response rate of 67% was demonstrated in 18 patients with anaplastic NE tumors. The median survival was 19 months. Well differentiated NE tumors did not respond [Citation1]. In another study, 41 patients with PDECs were treated with cisplatin and etoposide. The median survival was 15 months and the response rate 42% [Citation2]. The two studies are not fully comparable to the data presented here for several reasons: Neither studies used the 2004 WHO tumor classification [Citation4], patients were approximately 10 years younger (median age) than in our material, and in one of the studies only 80% had distant metastases. These factors may influence the results on the efficacy and survival data.
Our study showed, that the treatment outcome measured by survival data was unaffected by the Ki67 value within the range of 20–100%. This is in contrast to well-differentiated NE tumors where such an association has been demonstrated [Citation15]. This above-mentioned information may therefore add to the growing knowledge on the characteristics of PDECs. We found a statistically non-significant trend towards longer survival in patients receiving second and third line treatment versus those receiving first line only. This trend probably reflects a selection bias in the clinical setting, where only fit patients are offered additional therapy upon progression.
For historical reasons, we used a three-drug regimen based on carboplatin, etoposide, and vincristine (a former standard SCLC regimen), which is a feasible outpatient based treatment regimen with a well-described toxicity profile. In the palliative treatment of advanced cancers, e.g. advanced lung cancer, cisplatin is often substituted with carboplatin due to lower toxicity and more feasible administration. Whether the addition of vincristine to platin plus etoposide increases the efficacy of the regimen is not known. In SCLC, vincristine has actually been outfaced from most platin based combinatory chemotherapy regimens. The results from an ongoing Nordic PDEC registry study may be able to show differences in outcome depending on slightly different platin based chemotherapy regimens.
The response rates and survival data in PDEC are comparable with those of first-line treatment of extensive stage SCLC [Citation16].
In conclusion, previously untreated patients with disseminated PDECs achieve treatment results comparable to those of patients with extensive stage SCLC, which is indicative of a survival benefit, compared to no treatment. The prognosis is however still poor and new therapy options should be sought for.
Declaration of interest: There are no conflicts of interest to be declared.
References
- Moertel CG, Kvols LK, O’Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer 1991;68:227–32.
- Mitry E, Baudin E, Ducreux M, Sabourin JC, Rufie P, Aparicio T, . Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer 1999;81:1351–5.
- Oberg K, Astrup L, Eriksson B, Falkmer SE, Falkmer UG, Gustafsen J, . Guidelines for the management of gastroenteropancreatic neuroendocrine tumours (including bronchopulmonary and thymic neoplasms). Part I – general overview. Acta Oncol 2004;43:617–25.
- Janson ET, Sorbye H, Welin S, Federspiel B, Gronbaek H, Hellman P, . Nordic Guidelines 2010 for diagnosis and treatment of gastroenteropancreatic neuroendocrine tumours. Acta Oncol 2010;49:740–56.
- Johnson LA, Lavin P, Moertel CG, Weiland L, Dayal Y, Doos WG, . Carcinoids: The association of histologic growth pattern and survival. Cancer 1983;51:882–9.
- Rindi G, Bordi C, Rappel S, La RS, Stolte M, Solcia E. Gastric carcinoids and neuroendocrine carcinomas: Pathogenesis, pathology, and behavior. World J Surg 1996;20:168–72.
- Hainsworth JD, Johnson DH, Greco FA. Poorly differentiated neuroendocrine carcinoma of unknown primary site. A newly recognized clinicopathologic entity. Ann Intern Med 1988;109:364–71.
- Pelley RJ, Bukowski RM. Recent advances in diagnosis and therapy of neuroendocrine tumors of the gastrointestinal tract. Curr Opin Oncol 1997;9:68–74.
- Moertel CG, Johnson CM, McKusick MA, Martin JK, Jr., Nagorney DM, Kvols LK, . The management of patients with advanced carcinoid tumors and islet cell carcinomas. Ann Intern Med 1994;120:302–9.
- Nilsson O, Van CE, Delle FG, Yao JC, Pavel ME, McNicol AM, . Poorly differentiated carcinomas of the foregut (gastric, duodenal and pancreatic). Neuroendocrinology 2006;84:212–5.
- Ahlman H, Nilsson O, McNicol AM, Ruszniewski P, Niederle B, Ricke J, . Poorly-differentiated endocrine carcinomas of midgut and hindgut origin. Neuroendocrinology 2008;87:40–6.
- Oberg K. Chemotherapy and biotherapy in the treatment of neuroendocrine tumours. Ann Oncol 2001;12(Suppl 2):S111–4.
- Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, . One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063–72.
- National Comprehensive Cancer Network [Internet]. Fort Washington: NCCN; 2009. Clinical practice guidelines in oncology: Neuroendocrine tumors v.2.2009. Available from: http://www.nccn.org/professionals/physician_gls/PDF/neuroendocrine.pdf.
- Binderup T, Knigge U, Loft A, Federspiel B, Kjaer A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res 2010;16:978–85.
- Langer SW, Sorensen M. Treatment of small cell lung cancer. Hansen HH. London: Informa; 2009.