To the Editor,
The granular cell tumour (GCT) was described for the first time in 1926 by Abrikossoff [Citation1], and was originally considered as a muscle tumour. Recent immunohistochemical studies suggest a more likely neural origin [Citation2], characterised by strong expression of S-100 protein and neuron-specific enolase (NSE) [Citation3]. The vast majority (85 to 90%) of these tumours are usually solitary, benign and located in head and neck subcutaneous tissues [Citation4]. However, a few multifocal cases have been reported [Citation5] as well as metastatic cases [Citation6]. We report herein a case of a right cheek Abrikossoff tumour, with iterative local relapses and subsequent multifocal lesions and histologically-proven metastases.
A 64-year-old man was seen for a second advice. His medical history was marked by a right colic flexure intra-epithelial degenerated polyp discovered in 2003, treated by hemicolectomy. In 2005, he developed a lesion of the internal side of the right cheek which was subsequently resected. The pathological diagnosis was a 17 mm Abrikossoff tumour (). The pathological features were classical, with the presence of wide and granular eosinophilic large cells proliferation, without mitosis activity. These cells were Periodic Acid Schiff (P.A.S.) positive and intensively immunoreactive for Protein S 100. In June 2006, a second local relapse occurred, involving the inner side of the ramus mandibular up to the tonsil with a bone infiltration. The pathologist noted that some cells became irregular. In September 2007, a third local relapse occurred involving the right coronoid process and the inferior and superior facial vascular pedicle. The pathologist noted the appearance of perineural neuroplastic invasion and the presence of one metastatic lymph node, characterising a malignant GCT (). In January 2008, a fourth local relapse occurred; its treatment required a right transmandibulary buccopharyngectomy with fibular free flap for mandibular reconstruction and bilateral neck dissection. The diagnosis was “malignant GCT with lymph node involvement”.
Figure 1. Benign form of Abrikossoff tumour: roundly granular and eosinophilic cytoplasm cells without atypia or mitosis activity (hemalun-eosin-safran colouration, growth × 200).
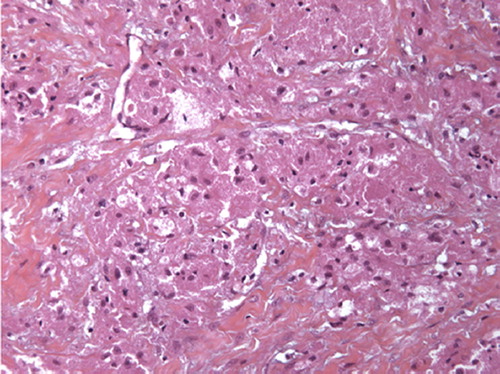
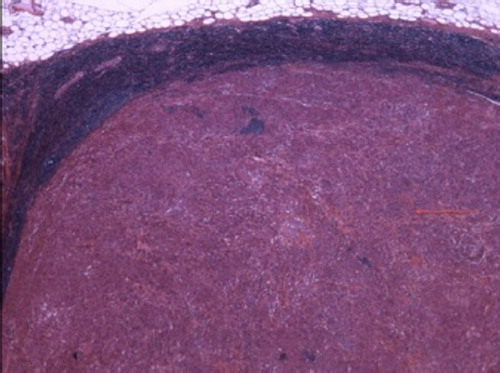
Figure 2. → Lymph node metastatic localisation (above: normal tissue of lymph node) (hemalun-eosin-safran colouration, growth × 200).
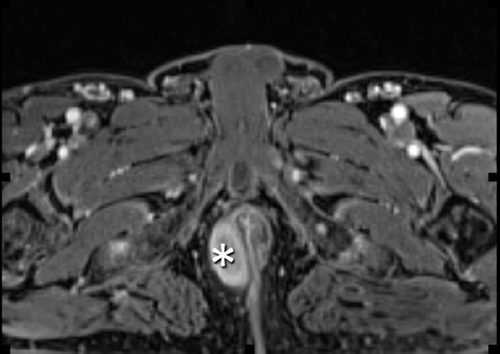
In late 2009, the patient presented right para-anal () and right sub-scapular lymph nodes. Because of his past history of degenerated polyp, a new colonoscopy was performed. A new polyp was found beyond the anal verge and local excision showed a T1 adenocarcinoma. Rectal ultrasound showed lymph nodes next to the implantation of the pedicular polyps.
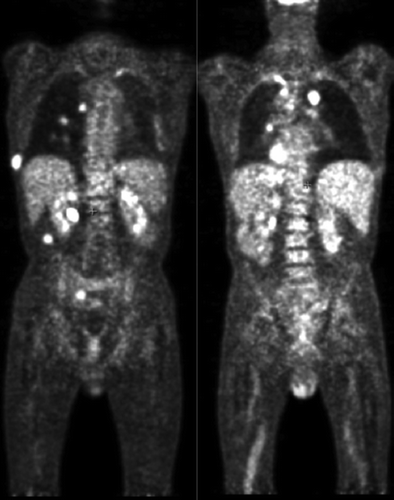
A 18-Fluoro-deoxy-glucose positron emission tomography coupled with a thoracic, abdominal and pelvic CT-scan showed numerous hypermetabolic spots: sub-renal latero-aortic lymph nodes, multiple nodular lung, bilateral pleural effusion, a right pleural supra-diaphragmatic mass, bilateral axillary subcutaneous lesions, one retro-peritoneal nodule, a right subcutaneous mass in the pre-sacral area, one prostatic lesion and the anal lesion () which was clinically found.
Figure 4. 18-Fluoro-deoxy-glucose positron emission tomography: multiple hypermetabolic hotspots (on those slides: bilateral nodular lung, pleural sus-diaphragmatic node, retro-peritoneal nodule under the right renal loge, pleura).
The most accessible lesions were biopsied in order to determine their nature (GCT vs. rectal adenocarcinoma). Bronchial biopsies were not contributive but typical features of GCT were found in clinically guided biopsies on the para-anal and sub-scapular lesions. Moreover, the pleural effusion cytology revealed atypical cells compatible with an Abrikossoff tumour with the wide and granular cytoplasm, and large Protein S 100 positive cells.
As the patient had no clear symptoms and as no efficient therapy was available, a watch-full surveillance was decided. Six month after the diagnosis of metastases, the patient was still in good health condition without evidence of progressive disease.
About 10–15% of GCT are multiple. The distinctive criteria for diagnosis of multifocal forms and metastatic malignant forms of GCT remain debated. Classically, for a clinical point of view, multifocal benign forms arise in soft tissue (subcutis and submucosa) and are slowly growing. On the contrary, metastatic malignant forms are locally aggressive and associated with visceral metastasis through hematogeneous spreading [Citation5].
Benign tumours exhibit usually polygonal or slightly spindled large cells proliferation, disposed in nests and with wide, granular and eosinophilic cytoplasm. The granules are large, and the nuclei are round, oval and often central, hyperchromatic, regular without atypies or mitosis. They are intensively P.A.S. Protein S 100 and NSE positive. Some authors have described some conflicting criteria for malignancy. According to Jardines et al., all multifocal CGT displaying the same histological pattern have to be considered as malignant [Citation7]. Brooks et al. have defined malignant forms as 1) a primary tumour with a diagnosis of GCT, 2) high mitosis activity, presence of wide nuclei and/or necrosis, 3) presence of metastases [Citation8]. In the absence of metastases, pleiomorphism associated with both high index activity and necrosis is mandatory to confirm malignancy. Moreover, a 6-criteria definition had been proposed by Fanburg-Smith et al.: 1) tumour necrosis, 2) spindle cells, 3) vesicular nuclei with preeminent nucleoli, 4) increased mitotic activity (>2/10 HPF), 5) high nucleocytoplasmic ratio and 6) pleiomorphism. Tumours with three or more of these criteria are considered malignant [Citation9].
Our case report illustrates the possible coexistence of both multifocal benign and metastatic malignant forms. The aggressive and relapsing primary tumour (progressively exhibiting necrosis, high mitotic rate, spindle cells in the iterative local relapses) associated with visceral metastasis have to be regarded as malignant GCT. The para-anal and sub-scapular soft tissue lesions exhibit diagnostic criteria for benign multifocal lesions: low mitotic rate, absence of necrosis and absence of spindle cells. Nevertheless, as previously mentioned these criteria remain debatable.
Surgery is the mainstay of treatment of GCT as it allows local tumour control. Adjuvant radiotherapy is an option for relapsing disease. There is no proven benefit from adjuvant chemotherapy [Citation10,Citation11]. There is no consensual treatment for metastatic GCT. In the present case, the disease is indolent and asymptomatic, so we have proposed a watchful surveillance.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abrikossoff AL. Uber Myome, ausgehend von der quergestreiften willkurlichen Muskulatur. Virchows Arch Pathol Anat 1926;260:215–33. German.
- Fine SW, Li M. Expression of calretinin and the alpha-subunit of inhibin in granular cell tumors. Am J Clin Pathol 2003;119: 259–64.
- Mukai M. Immunohistochemical localization of S-100 protein and peripheral nerve myelin proteins (P2 protein, P0 protein) in granular cell tumors. Am J Pathol 1983; 112:139–46.
- Sposto MR, Navarro CM, de Andrade CR. Granular cell tumour (Abrikossoff's tumour): Case series. Oral Oncol Extra 2006;42:194–7.
- Janoušková G, Campr V, Konkolòvá R, Zemanova R, Hoch J, Hercogova J. Multiple granular cell tumour. J Eur Acad Dermatol Venereol 2004;18:347–9.
- Khansur T, Balducci L, Tavassoli M. Granular cell tumor. Clinical spectrum of the benign and malignant entity. Cancer 1987;60:220–2.
- Jardines L, Cheung L, LiVolsi V, Hendrickson S, Brooks JJ. Malignant granular cell tumors: Report of a case and review of the literature. Surgery 1994;116:49–54.
- Brooks JJ, Williams CJ, Krikorian JG, Green M, Raghavan D. Textbook of uncommon cancer. Sussex, England: John Wiley & Sons; 1988.
- Fanburg-Smith JC, Meis-Kindblom JM, Fante R, Kindblom LG. Malignant granular cell tumor of soft tissue: Diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol 1998;22:779.
- Rosenthal SA, III ATT, Livolsi VA. Adjuvant radiotherapy for recurrent granular cell tumor. Cancer 1990;65: 897–900.
- Simsir A, Osborne BM, Greenebaum E. Malignant granular cell tumor: A case report and review of the recent literature. Human Pathol 1996;27:853–8.