Abstract
Background. Monitoring and evaluation of cancer screening programmes require accurate data on invitations, visits, test results, diagnoses and management. The purpose of this study was to evaluate the completeness and accuracy of histological diagnoses (cervical precancerous lesions and cancer) in the Finnish cervical cancer screening register by comparing data with the cancer register and the administrative hospital discharge register. Material and methods. Screening data covering all 16 353 screening episodes that resulted in a referral for colposcopy over the period of 1998–2007 were individually linked with hospital discharge and cancer register data using the unique personal identifier. Agreement between registers, as well as sensitivity, coverage and positive predictive values (PPV) for the screening register and the hospital discharge register diagnosis, were estimated. Invasive cases in the cancer register and pooled cases of precancerous lesions were used as reference case populations. Results. The sensitivity of the screening register for cervical cancer was 69%, the coverage 100% and the PPV 77%. Corresponding values for the hospital discharge register were 81%, 100% and 83%, respectively. Sensitivity of the screening register for cervical intraepithelial neoplasia grade 2 or worse (CIN2+) against the pooled case population was 89% and coverage 99%. Corresponding values for the hospital discharge register were 78% and 93%. Kappa-values for pair-wise agreement between the three registers ranged between 0.73 and 0.79, often the lesion grade was lower in the screening register than in the other two registers. Conclusions. The data in the screening register has high coverage and is thus useful for statistical and evaluation purposes. However, in order to improve the accuracy of diagnostic information, there are grounds to consider data retrieval through systematic linkage to other health care registers.
Screening is an effective strategy for cervical cancer prevention. A successful screening programme relies on a high level of organisation and continual monitoring of performance and evaluation of outcome. Reliable monitoring and quality assurance in turn require complete and accurate register data on invitations, visits, confirmed diagnosis and treatment, along with linkages of the screening data with other health-care registers [Citation1]. During the 1960s and early 1970s, and again since the early 1990s in Finland, an individual-level national screening register database has been maintained by the Mass Screening Registry (MSR), a part of the Finnish Cancer Registry (FCR) [Citation2,Citation3]. The register is based on data recorded by the screening laboratories, using standardised screening notifications and related data specifications.
Despite a long history of screening registration in the MSR, data on the screen-related histological diagnosis may be recorded using variable procedures and information quality. Data on cervical precancerous lesions and cancers are also recorded in the hospital discharge register and in the national cancer register. All these registers contain individual-level data and can be linked using the personal identifier available for all residents in Finland. The purpose of this study was to evaluate the completeness and accuracy of histological diagnoses (cervical precancerous lesions and cancer) in the screening register by comparing data with the cancer register and the administrative hospital discharge register.
Material and methods
The cervical cancer screening programme
Cervical cancer screening was introduced in 1963 in Finland and the programme expanded to national coverage by 1970. Screening is organised by municipalities as required by law [Citation4], and detailed directives for the implementation of screening are issued by the National Institute of Health and Welfare (THL, formerly STAKES). The collection of screening data is also legally mandated and enables the MSR to produce statistics on screening and to monitor programme performance. Municipalities, with few exceptions, invite women aged between 30 and 60 to five-yearly screening tests. By birth year cohort, the eligible women are drawn from the population register which provides name, current mailing address and the personal identifier. Invitations are sent regardless of screening or health history. The screening test in use is conventional cytology except for municipalities that participate in a randomised trial comparing primary high risk human papillomavirus (HPV) DNA testing to conventional cytology [Citation5–7]. Women with clearly positive test results, i.e. those referred for colposcopy, are actively followed up and data on histological confirmation and primary treatment are collected and electronically registered by the screening laboratory. Once complete, the screening data including personal identifier, baseline anamnestic information, screening test results and histological confirmation results are transferred to the MSR.
The Screening Register
The coordination, evaluation and further development of the screening programme are responsibilities of the Mass Screening Registry, founded in 1968. Complete individual-level data on the organised screening programme are currently available in the screening register from 1991 onwards in electronic format. Annually about 250 000 invitations and nearly 200 000 screening episodes are registered. Around 1600 referrals for colposcopy are made based on the screening test results, and 650 precancerous lesions or cancers are detected yearly. Smears or tests outside the programme are not yet centrally registered, though this is recommended by the current national guidelines. The MSR publishes yearly statistics covering key process indicators of the screening programme [Citation8], and evaluates the screening process and its effects [Citation9,Citation10].
The Cancer Register
The Finnish Cancer Registry (FCR) was founded in 1952 by the Cancer Society of Finland in collaboration with the National Board of Health. The functions of the FCR include collection, quality control and refinement of cancer data for the production of cancer statistics and for research. Hospitals, physicians and pathological and haematological laboratories are requested to report all cancer cases that come to their attention to the FCR. In addition, information on all death certificates in which cancer is mentioned are transferred from the files of Statistics Finland each year. Data fields cover diagnostic details (diagnostic coding currently follows the 3rd edition of the international classification of diseases for oncology, ICD-O-3), information on primary treatment, and cause and date of death where applicable. In addition to malignant neoplasms some premalignant conditions of the cervix uteri (ICD-O-3 topography codes C53.0–C53.9), are mandatory to report to the cancer registry [Citation11]. These include dysplasia gravis, which is not a separate entity in ICD-O-3 and is therefore registered with a local code, CIN3 with ICD-O-3 morphology code 8077/2, squamous cell carcinoma in situ (8070/2), carcinoma in situ NOS (8010/2) and adenocarcinoma in situ, AIS (8140/2). Registration of carcinoma in situ of the cervix uteri has taken place from the beginning of the register, dysplasia gravis and CIN3 in increasing amounts since 1988, but there has been clear under-registration of these diagnoses until the late 1990s [Citation8].
The Hospital Discharge Register
Hospital discharge data for inpatient episodes in all public hospitals have been collected since 1969 in the Finnish Hospital Discharge Register (HDR) maintained by THL. Data include dates of admission and discharge, treatments details as well as primary and subsidiary diagnoses coded according to the international classification of disease (ICD). ICD-10 has been in use since 1996. Data collection was expanded in 1994 to cover day-surgical outpatient procedures from all institutions providing health care, and the name of the register was changed to Care Registers for Social Welfare and Health Care (referred to as HDR in this paper). Starting from 1998 the scope was further expanded to include all outpatient visits in the public sector.
Linkage procedure
The MSR data, covering all screening episodes that resulted in a referral for colposcopy over the period of 1998–2007, were individually linked with HDR and FCR data using the personal identifier. In all, the screening data contained 16 353 referrals for colposcopy and involved 15 912 women. From the HDR, treatment episodes from 1998 to 2008 with malignant or premalignant diagnostic codes of the uterus, vagina or vulva, or a gynaecological procedure (including colposcopy, cervical biopsy, endocervical and endometrial curettage, cervical lesion ablation and conisation), were chosen, and the linkage amounted to 54 263 diagnostic and treatment episodes involving 12 832 individual women. From the FCR database, data on invasive or in situ cancers of the cervix, vagina, vulva and corpus uteri, as well as dysplasia gravis and CIN3 of the cervix diagnosed in 1998–2008 were linked to the screening data and contributed 2657 cases among 2644 women.
After linkage, screening events, treatment episodes and dates of diagnosis were chronologically ordered for each woman and screening episode. Next, the screen-detected diagnosis was determined for each screening episode and register. The HDR- and FCR-derived screen-detected diagnoses were defined as the highest grade cervical neoplasia within one year after a positive screening test result resulting in referral for colposcopy. Lesions diagnosed as dysplasia gravis, carcinoma in situ or CIN3 were grouped together as CIN3/AIS. Any FCR diagnosis was considered most-accurate-attainable because of the extensive verification in the cancer registration processes. The original patient records were consulted in 10 cases where the MSR or HDR screen-detected diagnosis was invasive cancer, but where no corresponding FCR records were found in the linked material. In these cases the relevant notifications to the FCR were also consulted. As a result, the FCR was updated with two additional cases of invasive cervical cancer and the primary site of one case of unspecified female genital organ cancer was changed to cervix. Of the remaining seven cases, three were adenocarcinomas of extrauterine origin (large intestine and ovary), two were low grade CIN-lesions and two were cases of cancer previously diagnosed and treated abroad and therefore not included in the FCR records as incident cancers.
For the estimation of record completeness on invasive cancer, diagnoses of the MSR and the HDR were compared against the updated FCR. For record completeness on CIN3/AIS or worse (CIN3+), the registers were compared against a pooled population consisting of cases where at least one of the three registers suggested CIN3+. Pooled case populations of CIN2+ and CIN1+ were similarly constructed.
Statistical methods
Completeness of records was described using sensitivity and coverage of register diagnosis compared with the diagnoses in the FCR (for invasive cancer) or a pooled diagnosis population (for CIN1+, CIN2+ and CIN3+, respectively). Coverage refers to the proportion of cases with any records in the MSR or HDR in the above mentioned case populations. Sensitivity refers to the proportion of cases with a specific diagnosis in the MSR or HDR in these case populations. Accuracy was measured by the positive predictive value (PPV) calculated as the proportion of cases confirmed by the FCR out of those with a recorded invasive cancer diagnosis in the MSR or HDR, and also by pair-wise agreement of register diagnoses as by the kappa statistic and the Wilcoxon signed-ranks test. Time trends for validity measures were fitted using ordinary least squares linear regression with 95% confidence levels. Confidence intervals for all proportion measures were calculated with the Agresti-Coull method, suitable for large sample binomial proportions [Citation12]. Cohen's kappa coefficient is a statistical measure of inter-rater agreement or inter-annotator agreement for qualitative (categorical) items [Citation13]. Kappa was generated with linear weighting to account for the ordered nature of the categories describing lesion severity. The other/missing category in the contingency tables can be interpreted as consisting mainly of lower grade lesions and/or non-neoplastic and normal findings with respect to the cervix, actual deficiency of coverage contributing far less. The 95% confidence intervals for the kappa statistic were estimated from the standard error. The Wilcoxon matched-pairs signed-ranks test was used for the analysis of grade distribution differences of screen-detected lesions [Citation14]. STATA/MP 11.0 software (StataCorp LP, College Station, TX) was used for all statistical calculations.
Results
In 1998–2007, 16 353 (0.9%) of 1.9 million smears in the cervical cancer screening programme resulted in referral for colposcopy. The histological diagnosis in the MSR was CIN1 in 2031 cases, CIN2 in 2278 cases, and CIN3/AIS in 2152 cases. Invasive cervical cancer (ICC) was found in 185 cases and in 9707 cases there was no CIN or cervical cancer recorded.
shows the screen-detected CIN3/AIS and ICC diagnoses from the three registers under comparison. Of 185 ICC cases in the MSR (first row total), 142 were confirmed by the FCR as incident cancer cases. This translates to a register PPV estimate for the MSR invasive cancer diagnosis of 77% (95% CI, 70–82%). According to the FCR, the remainder consisted of 25 other carcinomas (22 corpus carcinomas, one vaginal carcinoma and two other extra-uterine adenocarcinomas), 14 CIN3/AIS, two prevalent CIN3+ cases, one CIN2 and one suspected cervical adenocarcinoma that was confirmed by pathology over one year later. For the HDR invasive cancer diagnosis, the corresponding PPV was 83% (95% CI, 77–88%). Of the 2152 CIN3/AIS cases in the MSR (second row total), 1712 (80%) were confirmed by the FCR. The linearly weighted kappa statistic for agreement across the three categories of ICC, CIN3/AIS and other, as outcome for the 16 353 positive screening tests was 0.79 (95% CI, 0.78–0.81) between MSR and FCR, 0.74 (95% CI, 0.73–0.75) between MSR and HDR, and 0.76 (95% CI, 0.75–0.78) between HDR and FCR.
Table I. Screen-detected cases of ICC and CIN3/AIS in three health care registers.
Diagnoses of all grades were compared between the MSR and the HDR (). In 64% of screen-detected CIN1+ cases in the MSR, the same specific diagnosis was found in the HDR. An additional 10% were matched by cervical lesions of a different malignancy grade in the HDR. In 502 women (3.1% of those referred for colpocopy), a neoplastic lesion of the cervix (CIN1+) was recorded in the HDR but not in the MSR. The linearly weighted kappa coefficient for overall agreement over the five categories shown in was 0.73 (95 % CI, 0.72–0.75).
Table II. Screen-detected diagnoses from the MSR and the HDR in 1998–2007 (n = 16 353).
Out of altogether 207 cases of ICC in the FCR, MSR recognised 142 as ICC (69%), 43 as CIN3/AIS (21%), 13 as CIN1/2 (6%), two as other cancers (1%), and a non-neoplastic finding with respect to the cervix had been reported for seven (3%) (). HDR recognised, respectively, 167 as ICC (81%), 37 as CIN3/AIS (18%), and three (1%) as non-neoplastic with respect to the cervix. The coverage of records, irrespective of specific diagnosis, for confirmed ICC cases was thus 100% (98–100%) for both these registers. The sensitivity estimates for ICC at the CIN1+ cut-off was 96% (92–98%) for the MSR and 99% (96–100%) for the HDR.
Table IIIa. MSR and HDR diagnoses for cancer cases confirmed by the FCR (n = 207).
From among the 2874 pooled CIN3+ cases that were recorded in any of the three registers, the corresponding numbers of CIN3+ and CIN1+ were 2337 (81%) and 2698 (94%) for the MSR, and 2152 (75%) and 2340 (81%) for the HDR (b). The FCR included 2286 (80%) of the pooled CIN3 + cases.
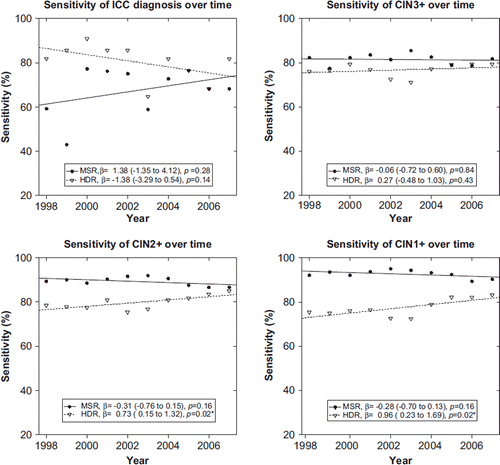
There were altogether 5176 cases of CIN2+ and 7183 cases of CIN1+ in the pooled case populations (c, d). The MSR contained 4615 (89%) of the CIN2+ diagnoses and 6646 (93%) of the CIN1+ diagnoses. The corresponding numbers for HDR were 4054 (78%) and 5447 (76%). Sensitivity estimates of all MSR diagnoses were stable over the study period (). There was a small improvement in the sensitivity of HDR CIN2+ and CIN1+ diagnoses, however.
Figure 1. Time trends of register sensitivity over the period 1998 to 2007. Regression coefficients (β) with 95% confidence intervals and associated p-values of the linear regression models are indicated. MSR, Mass Screening Register; HDR, Hospital Discharge Register.
The distributions of time intervals between the date of the screening sample and the date of the eventual screen-related histological diagnosis, was different in the three registers. For MSR records where the date of diagnosis was available (8479 of 16 353), the median time interval between the screening sample and final diagnosis was 3.1 months and intervals longer than six months were observed in 14% of cases. In the FCR, the median time interval was 2.0 months with only 8% of intervals longer than six months. In the HDR material, the median time interval was 4.5 months, with 33% of intervals longer than six months. By removing the time constraint of one year on screen-related diagnoses, an additional 563 (7.8%) of the pooled screen-related CIN1+ diagnoses were found in the HDR material. In the pooled CIN1+ population, the HDR also contained 155 treatment episodes with cervical lesion destruction or conisation procedures, without any corresponding CIN1+ diagnosis.
Analysis of the grade distribution of cervical lesions showed a trend for less severe diagnoses in the MSR compared to those from the HDR. Using only CIN3 and ICC diagnoses, there was also a trend for less severe grading in the MSR than in the FCR and a similar difference between the HDR and the FCR.
Discussion
The current study assessed for the first time the information quality of the histological diagnosis of cervical precancerous lesions and cancers within the national screening database, by comparison to the cancer register and the hospital discharge register. Data from before 1998 were not used due to low coverage of information on precancerous lesions in the cancer and hospital discharge registers. In extension, we wanted to assess the feasibility of using regular linkage to other health care registers to improve the quality of the screening register.
Correlation of information sources
Agreement of specific diagnoses of cervical lesions recorded in the screening register and the two other databases was fairly good. Some variation was expected as the registers are maintained for different purposes and information is reported through various channels.
Specifically, the definition of primary site and the selection of a particular health service visit providing the diagnosis were important sources of variation, but also incomplete records were an issue. In the HDR, coverage of any CIN1+ was good at 91% and the CIN1+ sensitivity estimate was 76%, but the HDR lacked about a third of the CIN1 lesions found in the MSR. These estimates match roughly with earlier estimates of data coverage and sensitivity of the HDR using cardiovascular events [Citation15–17]. From the data in this study, using the FCR and patient history reviews as the gold standard, a sensitivity of 81% was estimated for invasive cervical cancer in the HDR.
The cancer register is considered to be close to complete with regard to invasive cancers due to multiple notification sources [Citation18]. Only three missing (one of which was registered, but as an unspecified female genital organ cancer) invasive cervical cancer cases confirmed by hospital records were found in the current linkage covering 10 years and 207 cases, corresponding to a completeness estimate of up to 99% of the original records. For CIN3+, the corresponding estimate was 80% of the pooled, unverified, CIN3+ population. The lower completeness estimate for CIN3+ diagnoses was caused by a combination of over-reporting in the other two registers and, possibly, lingering under-reporting of these lesions by pathologists and clinicians to the cancer registry.
The coverage of the MSR was generally very high. The MSR coverage of the pooled CIN1+ cases was 99%, with a CIN1+ sensitivity estimate of 93%. Still, 502 (or 3.1% of total referrals) CIN1+ diagnoses that were denoted as other than CIN1+ or missing in the screening records were found in the HDR linkage. Some of these cases may represent over-reporting in the HDR. The accuracy of HDR diagnostic coding has previously been studied with regards to cardiovascular diseases and reported as a PPV of 86–90% [Citation16,Citation17]. In the current study and using the FCR and patient history review as gold standard, the PPV of the HDR for invasive cervical cancer was 83%. For lower grade cervical disease, PPVs could not be calculated because the pooled diagnoses were not verified from original records.
No significant trends in completeness or sensitivity estimates were found in the MSR over the study period, indicating stable quality over time. The CIN1 + and CIN2 + sensitivity estimates of the HDR increased during the study period, which probably reflects an improvement in registration coverage of outpatient treatment episodes.
The FCR is a source of verified cancers and severe precancerous lesions. Based on the information from the FCR, some 65 cases could be revised to invasive cancer in the MSR, the majority from CIN3, over 10 years of screening registration. Similarly, 366 cases could be revised to CIN3, the majority from CIN1-2. Often, the diagnosis in the screening records is the first available diagnosis, and the diagnosis does not always remain unchanged throughout the management process.
The screen-detected diagnosis dilemma
It can be difficult to unequivocally define a cervical lesion as being screen-detected. The distinction between the primary diagnostic episode and follow-up, relapse, or progression is not always easy to ascertain as multiple colposcopy visits, biopsies, and excisional procedures over the course of several months are common in the history of women with cervical lesions. Also, exact pathologic grading of the lesions may well vary between visits that can include the diagnostic colposcopy with biopsies, treatment colposcopy with excision of the lesion and the transitional zone, and follow-up colposcopy with biopsies. The criterion for choosing the diagnosis from several alternatives has varied and not been very well controlled. For these reasons, and in order to include all relevant diagnoses, we chose a diagnostic window of 365 days after a positive screening sample. The highest grade cervical lesion recorded during this time frame was considered to be the relevant screen-related diagnosis. This definition is also recommended by the European guidelines [Citation1]. It does not take into account that a woman, who has failed to comply with a colposcopy referral following an abnormal screening test, may independently seek medical attention based on symptoms within the year specified. However, this would not markedly bias our results as referral compliance in the Finnish programme is very high (> 99%) [Citation19].
The delay between screening sample and screen-detected diagnoses derived from the different registers was shorter in the FCR than in the HDR. In the HDR, a third of diagnoses were recorded after an interval of more than six months. It is possible that some of the lag is due to diagnoses from pathology reports being recorded in the HDR only in conjunction with the subsequent health care contact. In the FCR and the MSR, the date of the histological sample can be recorded as the date of diagnosis. In addition, in the FCR the date of the earlier diagnosis remains if a case of carcinoma in situ is later, but within 12 months, assessed to be invasive.
There was a trend for less severe screen-detected diagnoses derived from the MSR than in the HDR or the FCR. The systematic selection of the most severe diagnosis in the HDR during one year partly explains this difference. In addition, it seems that the MSR diagnosis also underestimated the grade of lesions due to recording policies, in particular, the use of only diagnostic (first) colposcopy biopsy results instead of longer term follow-up of diagnostic information. Consequently there is a need to revise the recording practices by adding regular register linkage procedures.
Conclusions
There was considerable agreement between the three registers despite their different use and data collection methods. The data in the MSR has high coverage and is useful for statistical and evaluation purposes. However, there is a potential for regular update of diagnostic data. In order to use the most accurate information available, there are grounds to consider collecting data on diagnoses and management by systematic data retrieval through linkage procedures with other health care registers.
Acknowledgements
The authors thank Sanna Kuivalainen from the Mass Screening Registry for the retrieval of patient records, and Timo Hakulinen from the Cancer Registry and Jari Forsström from the National Institute of Health and Welfare for reviewing the manuscript especially regarding the respective registers. This study was supported by the European Union Seventh Framework Programme contract EUROCOURSE – Europe against Cancer: Optimisation of the Use of Registries for Scientific Excellence in research.
Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Arbyn M, Anttila A, Jordan J, Ronco G, Schenck U, Segnan N, . European guidelines for quality assurance in cervical cancer screening. 2nd ed. Luxembourg: Office for Official Publications of the European Communities; 2008.
- Hakama M, Rasanen-Virtanen U. Effect of a mass screening program on the risk of cervical cancer. Am J Epidemiol 1976;103:512–7.
- Anttila A, Nieminen P. Cervical cancer screening programme in Finland. Eur J Cancer 2000;36:2209–14.
- Government Decree on Screenings (1339/2006).
- Leinonen M, Nieminen P, Kotaniemi-Talonen L, Malila N, Tarkkanen J, Laurila P, . Age-specific evaluation of primary human papillomavirus screening vs. conventional cytology in a randomized setting. J Natl Cancer Inst 2009; 101:1612–23.
- Anttila A, Hakama M, Kotaniemi-Talonen L, Nieminen P. Alternative technologies in cervical cancer screening: A randomised evaluation trial. BMC Public Health 2006;6:252.
- Anttila A, Kotaniemi-Talonen L, Leinonen M, Hakama M, Laurila P, Tarkkanen J, . Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: Randomised study within organised screening programme. BMJ 2010;340:c1804.
- Finnish Cancer Registry. Cancer in Finland 2006 and 2007. Cancer Society of Finland Publication No. 76, Helsinki, 2009.
- Lonnberg S, Anttila A, Kotaniemi-Talonen L, Kujari H, Melkko J, Granroth G, . Low proportion of false-negative smears in the Finnish program for cervical cancer screening. Cancer Epidemiol Biomarkers Prev 2010;19:381–7.
- Anttila A, Pokhrel A, Kotaniemi-Talonen L, Hakama M, Malila N, Nieminen P. Cervical cancer patterns with automation-assisted and conventional cytological screening: A randomized study. Int J Cancer 2011;128:1204–12.
- Pukkala E. Finland – cancer registration. In: Pukkala E, Söderman B, Okeanov A, Storm H, Rahu M, Hakulinen T, . Cancer atlas of Northern Europe. Cancer Society of Finland Publication 62, Helsinki, 2001. 50–51.
- Brown L, Cai T, DasGupta A. Interval estimation for a binomial proportion. Stat Sci 2001;16:101–33.
- Gwet K. Inter-rater reliability: Dependency on trait prevalence and marginal homogeneity. Stat Methods Inter-Rater Reliability Assess 2002;2:1–9.
- Wilcoxon F. Individual comparisons by ranking methods. Biometrics 1945;1:80–3.
- Keskimäki I, Aro S. Accuracy of data on diagnoses, procedures and accidents in the Finnish Hospital Discharge Register. Int J Health Sci 1991;2:15–21.
- Tolonen H, Salomaa V, Torppa J, Sivenius J, Immonen-Raiha P, Lehtonen A. The validation of the Finnish Hospital Discharge Register and Causes of Death Register data on stroke diagnoses. Eur J Cardiovasc Prev Rehabil 2007;14:380–5.
- Pajunen P, Koukkunen H, Ketonen M, Jerkkola T, Immonen-Raiha P, Karja-Koskenkari P, . The validity of the Finnish Hospital Discharge Register and Causes of Death Register data on coronary heart disease. Eur J Cardiovasc Prev Rehabil 2005;12:132–7.
- Teppo L, Pukkala E, Lehtonen M. Data quality and quality control of a population-based cancer registry. Experience in Finland. Acta Oncol 1994;33:365–9.
- Ronco G, van Ballegooijen M, Becker N, Chil A, Fender M, Giubilato P, . Process performance of cervical screening programmes in Europe. Eur J Cancer 2009;45:2659–70.
