Abstract
Background. The costs for loco-regional treatment of peritoneal carcinomatosis from gastric cancer are not well investigated. The aims of this study were to evaluate the costs and clinical outcome of systemic chemotherapy followed by cytoreductive surgery and intraperitoneal chemotherapy compared to systemic chemotherapy only in patients with peritoneal carcinomatosis from gastric cancer. Material and methods. Ten patients were scheduled for systemic chemotherapy followed by loco-regional treatment. A reference group of 10 matched control patients treated with systemic chemotherapy only were used and both groups were evaluated with respect to clinical outcome and cost. Results. The mean overall cost in the loco-regional group was $145 700 (range $49 900–$487 800) and $59 300 (range $23 000–$94 800) for the control group. The mean overall survival for the loco-regional group was 17.4 months (range 6.0–34.3), and 11.1 months (range 0.1–24.2) for the systemic chemotherapy only group. The gain in life-years was 0.52 and in quality-adjusted life-years 0.49, leading to incremental cost per life-year and quality-adjusted life-years gained of $166 716 and $175 164, for loco-regional group compared to systemic chemotherapy. Discussion. Treatment of peritoneal carcinomatosis from gastric cancer is costly irrespective of treatment modality. If the survival benefit from adding loco-regional treatment to systemic chemotherapy indicated from this comparison is true, the incremental cost is considered high.
Peritoneal metastasis from gastric cancer (GC) implies poor prognosis, with survival of three to four months, and treatment remains a challenging problem [Citation1,Citation2]. After resection of the primary tumour, the median overall survival in patients with peritoneal carcinomatosis (PC) from GC is 9–10 months and there is no survival at five years [Citation1,Citation3]. Randomised studies indicate that palliative systemic chemotherapy extends median survival by three to nine months compared to best supportive care [Citation4–6].
Cytoreductive surgery (CRS) followed by intraperitoneal chemotherapy, for example as hyperthermic intraperitoneal chemotherapy (HIPEC) and/or early postoperative intraperitoneal chemotherapy (EPIC), have been studied in patients with GC and PC and produce a median survival ranging from 8.0 to 11.5 months [Citation7–9].
There are only a few studies on costs and cost-effectiveness of CRS and HIPEC in patients with PC, mainly from colorectal cancer [Citation10–14], and there are no studies of CRS and HIPEC in patients with PC from GC only. Since this treatment is resource consuming and health care resources limited, it is essential to analyse its potential cost-effectiveness.
The aims of this study were to evaluate the costs and clinical effectiveness of CRS + HIPEC + EPIC added to primary chemotherapy in patients with PC from GC compared with palliative systemic chemotherapy alone.
Patients and methods
Between January 2005 and July 2007, 10 consecutive patients with PC from GC were scheduled for neoadjuvant chemotherapy followed by CRS + HIPEC + EPIC at Uppsala University Hospital, Uppsala, Sweden. Demographic- and basic clinical patient data are summarised in and .
Table I. Demographic- and basic clinical data for patients in CRS + HIPEC + EPIC group.
Table II. Demographic- and basic clinical data for patients in systemic chemotherapy treatment group.
The costs and clinical outcome of the treatment for these patients were compared with 10 patients, matched according to age, gender, performance status, tumour extent, American Society of Anaesthesiologists (ASA) classification grade ≤2, and treated at the same time period with systemic chemotherapy only. The regional ethics committee approved the study and informed consent was obtained from each patient. The eligibility requirements for treatment for both groups were histological confirmed diagnosis of primary GC; histological and/or radiological confirmed PC diagnosis; no distant metastases; adequate renal-, haematopoietic- and liver functions; and WHO performance status (WHO) of ≤ 2. Analyses of all patients included were done with intention to treat.
For grading of therapy-related adverse events, the National Cancer Institute common toxicity criteria (NCI-CTC) version 3.0 was used [Citation15].
CRS + HIPEC + EPIC group
The first 10 patients (five female and five male) from an ongoing prospective phase II-study at Uppsala University, with a mean age of 59 years (39–72) were included in the study and treated for three (range 2–4.5) months with systemic chemotherapy. Four weeks after last chemotherapy patients without clinical and radiological signs of tumour progression underwent laparotomy, aimed at performing CRS + HIPEC followed by EPIC for five days. Patients with clinical and radiological signs of tumour progression during the systemic chemotherapy did not undergo the local treatment but continued with palliative systemic chemotherapy. Data on patient characteristics are summarised in and .
Neoadjuvant chemotherapy. The intention was to treat the patients with combination chemotherapy for three months. The choice of chemotherapy was individualised, but all patients received optimal drug combinations suitable for good performance patients with metastatic gastric cancer.
Eight patients were treated with an intravenous (i.v.) infusion of irinotecan (180 mg/m2 body surface area day 1) with either the bolus 5-fluorouracil (FLIRI, 5-FU, 500 mg/m2 days 1 and 2) leucovorin (LV) (60 mg/m2 days 1 and 2) q 2 weeks [Citation16] or with a modified deGramont schedule (FOLFIRI, bolus 5-FU 400 mg/m2, LV 200 mg/m2 2 h infusion day 1 followed by 2400 mg/m2 during 44 h) q 2 weeks. One of those patients was also treated with docetaxel (45 mg/m2 day 1) with 5-FU/Lv. Two patients were treated with EOX (epirubicin 50 mg/m2 day 1, oxaliplatin 130 mg/m2 day 1 and capecitabine 1250 mg/m2/d) q 3 weeks. Treatment details are summarised in .
Table III. Surgical procedures, tumour burden, completeness of surgical resection, preoperative and perioperative chemotherapy treatment for 10 patients in the CRS + HIPEC + EPIC group.
Routine clinical controls and blood sampling were done every treatment cycle. In order to rule out patients with progressive disease and distance metastasis, abdominal and thoracic computer tomography (CT)-scan evaluations were performed prior to surgery.
Surgical treatment. CRS was performed as described by Sugarbaker [Citation17]. Briefly, depending on disease extent, one or more of the following surgical procedures was carried: total or subtotal gastroectomy with D2 lymphadenectomy; greater omentectomy; ± cholecystectomy and dissection of duodenal-hepatic ligament; ± splenectomy; ± parietal peritonectomy; ± right and left upper quadrant peritonectomy; ± colon and small bowel resection; ± pelvic peritonectomy ± rectosigmoid resection ± hysterectomy. Immediately postoperatively, tumour load and completeness of cytoreduction for PC were recorded with the Peritoneal Cancer Index (PCI) [Citation18] and Completeness of Cytoreduction score (CC) [Citation19]. The PCI (range 1–39) consists of lesion size scores in 13 different regions of the abdomen: 0 = no tumour seen, 1 = tumour up to 0.5 cm, 2 = tumour up to 5 cm and 3 = tumour .>5 cm. The CC score is based upon the size of tumour left after cytoreduction: CC0 = no peritoneal seeding visible, CC1 = nodules up to 2.5 mm, CC2 = nodules up to 2.5 cm and CC3 = nodules > 2.5 cm.
HIPEC and EPIC. HIPEC was administrated according to the Coliseum technique [Citation20] and was combined with EPIC for five days. For HIPEC five patients received cisplatin at a dose of 50 mg/m2 combined with doxorubicin 15 mg/m2. The carrier solution used for these drugs was low calcium peritoneal dialyse solution PD4 [Dianeal 13.6 mg/ml (Baxter, USA)]. The duration of the treatment was 90 min. Two patients received oxaliplatin 460 mg/m2 + concomitant i.v. 5-FU 500 mg/m2 and i.v. LV 60 mg/m2 during 30 min, based on previous findings by Elias et al. [Citation21]. The carrier solution for oxaliplatin was 50 mg/ml glucose i.v solution. Before perfusion, the patients were cooled to 35°C with a cooling blanket (Allon®). The intra-abdominal temperature during perfusion ranged from 42°C to 44°C. Four intra-abdominal drains were left in place after surgery and EPIC was given daily during the first five postoperative days. Six patients received EPIC, five with 5-FU (550 mg/m2 daily) and i.v. LV (60 mg/m2 daily) and one with paclitaxel (20 mg/m2 daily). One patient did not receive EPIC-treatment due to chemotherapy-related toxicity. Six patients received postoperative systemic chemotherapy, three in the adjuvant setting and three in the palliative setting.
Systemic chemotherapy group
Ten patients (three female and seven male, mean age 62 years, range 40–76), were selected as matched control patients from a randomised clinical trial (GATAC) [Citation22]. The selection was made without any knowledge of treatment response or survival. Data on patient characteristics are summarised in .
According to the GATAC protocol, patients were randomised between two groups. In group A, patients were treated with four two-week cycles of docetaxel at a dose of 45 mg/m², 5-FU 400 mg/m2, LV 200 mg/m2 day 1 and 5-FU 2400 mg/m2 44 h infusion days 1 + 2, followed by four two-week cycles of irinotecan 180 mg/m2 day 1 + 5-FU/LV as above days 1 and 2. In group B, patients were treated as above but started with the irinotecan containing regimen. Before randomisation, and after every fourth cycle, an abdominal CT-scan was preformed. The treatment was stopped if there was a tumour-progression, unacceptable side effects, or withdrawal of informed consent.
Six patients received the entire treatment and four received reduced treatment as detailed in . Four patients received chemotherapy subsequent to the study treatment. One received irinotecan + 5-FU and LV + radiotherapy, one received irinotecan + 5-FU and LV, one received FLOX (5-FU/LV with oxaliplatin 85 mg/m2) and one received EOX (see above).
Table IV. Treatment characteristics of the 10 patients in the systemic chemotherapy treatment group.
Costs
A detailed collection of resource use was conducted by a retrospective review of medical records. Resource use during assessment (pre-treatment) period, treatment period, and post-treatment period were collected and registered separately. Resource use was collected from the date of diagnosis (GC with PC) until death. All information on hospitalisations, diagnostics/other investigations, any type of treatments, and consultations were collected ().
Table V. Costs collected in the study.
Unit costs for resources were based on data from Uppsala University Hospital, Uppsala, Sweden, and the Swedish National Pharmacy 2008 pricelists. All costs are presented in US Dollars (US$) at 2008 values with an average exchange rate between Swedish krona (SEK) and US$ for the period 1 January 2008 until 31 December 2008 [Citation23] (1 US$ = 6.94 SEK). No discounting of costs or effects was made, as survival times for these patients were too short to make discounting relevant.
Survival
Overall survival was calculated for all patients from the date of first preoperative chemotherapy-cycle for the CRS + HIPEC + EPIC group, or from the date of inclusion in GATAC for the chemotherapy only group. The longest living patient died in May 2009.
Quality-adjusted survival
An impact of a disease can be measured in quality-adjusted life-years (QALY), which includes both the quantity and quality of life. QALY is calculated as survived years multiplied with health utility weights (HUW). One year with perfect health is QALY 1.0 whereas one year with 50% health is QALY 0.5.
Thus, in order to estimate QALYs in the two groups, values for health utility of the patients must be estimated. Since there are no data available on health utility in these patients, HUW was estimated and derived for the two groups based on the WHO performance status or Karnofsky Performance Score (KPS). KPS directly converts to HUW by a linear scale where HUW = KPS/100. WHO performance status was converted to KPS with a conversion system based on a modification of the recommendation by Buccheris et al. [Citation24], and the WHO International Classification of Functioning, Disability and Health. Thus, WHO 0 was converted to HUW 0.980; WHO 0-1 to 0.955; WHO 1 to 0.855; WHO 1-2 to 0.755; WHO 2 to 0.630; WHO 2-3 to 0.505; and, WHO 3 to 0.275. If data were missing, HUW was based on an estimated utility weight, for example HUW 0.3/120 for every 10th of a month when a patient was hospitalised; 0.40/120 for every 10th of a month in hospice; 0.45/120 for every 10th of a month when assistance by medical staff at home was indicated; 0.50/120 for every 10th of a month with parenteral nutrition; 0.60/120 for every 10th of a month if too tired for outpatient care; 0.70/120 for every 10th of a month first month postoperatively, if iatrogenic addicted to morphine, in periods with many side effects, fever or fatigue; 0.80/120 for every 10th of a month second month postoperatively, if pain or last month alive; 0.90/120 for every 10th of a month three months postoperatively, if diarrhoea or vomiting and 1.00/120 for every 10th of a month without residual tumour and more than three months postoperatively or information in journal like “feel very good”.
The cost per life-year gained was defined as the difference in costs for the two groups divided by the difference in life-years for the two groups and cost per QALY gained as the difference in costs for the two groups divided by the difference in QALY for the two groups.
Statistical methods
Survival was calculated by the Kaplan-Meier method. Confidence interval around mean costs was estimated with Bootstrap resampling method. The computer software package STATISTICA AXA version 8.0 (StatSoft, Tulsa, USA) was used for statistical evaluation of survival data, and Stata 9 (StataCorpLP, College Station, TX, USA) for bootstrap analyses.
Results
Treatment outcome
CRS + HIPEC + EPIC group. Seven of the 10 patients scheduled for CRS + HIPEC + EPIC actually received it; CC0 was achieved in six patients and CC1 in one patient. Three patients did not undergo CRS + HIPEC. In one patient due to tumour progression during the systemic chemotherapy and in two patients due to extensive tumour growth on the entire small intestine serosa, observed at laparotomy.
Five patients had AE grade III–IV related to chemotherapy, five patients had AE grade III–IV related to surgery, one patient had more unspecific AE grade III–IV, and one patient died within 90 days of surgery. The three patients not undergoing CRS + HIPEC + EPIC had no AE grade III–IV.
The mean hospitalisation period was 57 days (range 13–215). The mean number of outpatient physician visits was 12 (range 8–15). Mean overall survival from start of systemic chemotherapy was 17.4 months (range 6.0–34.3), and median overall survival was 14.3 months (). For the seven patients receiving the entire treatment, the mean overall survival was 20.5 months (range 6.0–34.3), and median overall survival was 15.3 months. Mean follow-up time was 18.9 months and median follow-up 16.1 months.
Figure 1. Overall survival in the CRS + HIPEC + EPIC group and systemic chemotherapy treated group. CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy; EPIC, early postoperative intraperitoneal chemotherapy; Systemic chemo, systemic chemotherapy treated group.
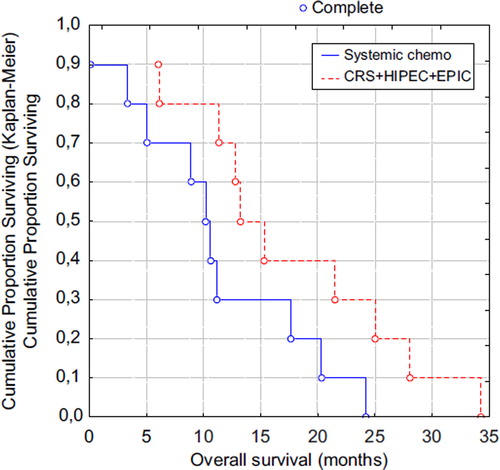
Systemic chemotherapy group. Mean overall survival from the date of inclusion was 11.1 months (range 0.1–24.2), and median overall survival was 10.4 months (). One patient died within 90 days of treatment onset. Five patients had AE grade III–IV. Mean hospitalisation period was 26 days (range 0–46) and mean number of outpatient physician visits was 15 (range 3–28). The mean follow-up time was 13.1 months and the median follow-up time was 12.0 months. The overall survival for this subgroup in the GATAC study was in line with all patients in GATAC [Citation22].
QALYs
The average HUW was higher for patients in the CRS + HIPEC + EPIC group than for patients in the chemotherapy only group. As patients in the CRS + HIPEC + EPIC group generally spent more days in hospital than patients in the systemic chemotherapy alone group, the total difference in HUW for the two groups was small. Mean HUW in the CRS + HIPEC + EPIC group was calculated to 0.84, and mean HUW in the systemic chemotherapy group to 0.82.
Since start of treatment the mean number of QALYs in the CRS + HIPEC + EPIC group was 1.268 and 0.774 from the date of inclusion in the systemic chemotherapy only group, i.e. a difference of 0.52 in life-years and 0.49 QALYs ().
Table VI. Cost per life-years gained and per QALY gained.
Costs and cost-effectiveness
The mean cost per patient in the CRS + HIPEC + EPIC group was $145 700 (95% CI $91 500–$245 000) and $59 300 (95% CI $45 500–$73 800) for the systemic chemotherapy only group. The distributions of the costs are presented in . The CRS + HIPEC + EPIC group had much higher treatment and post-treatment costs. The main drivers of costs during the assessment (pre-treatment) period are diagnostic examinations like gastroscopies, CTs and laparatomies/laparascopies. During treatment period, the neoadjuvant chemotherapy ($6300, 4% of total costs), the surgical procedure ($29 300, 20% of total costs) and associated intensive care ($24 100, 17% of total costs) were the most important costs sources in the CRS + HIPEC + EPIC group. In the systemic chemotherapy only group, the chemotherapy ($8700, 15% of total costs) and associated visits ($5500, 9% of total costs) were the most important cost sources. In the post-treatment care, palliative chemotherapy and hospitalisations were key sources of costs, in particular in the surgical ward in the CRS + HIPEC + EPIC group. presents the main drivers of the cost during treatment period.
Figure 2. Mean costs for the CRS + HIPEC + EPIC group and systemic chemotherapy treated group. CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy; EPIC, early postoperative intraperitoneal chemotherapy.
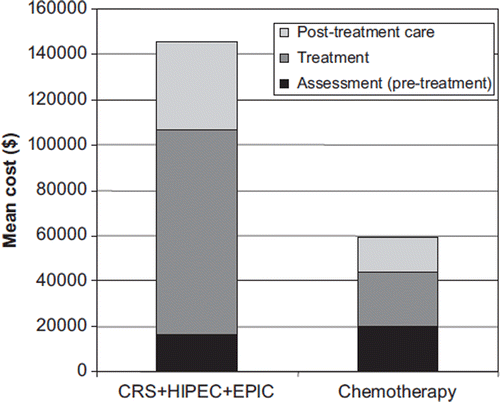
Table VII. Main drivers of the cost during the treatment period.
presents the difference in cost, life years, and QALYs, and the associated incremental cost. The cost per life year gained from CRS + HIPEC + EPIC compared to systemic chemotherapy only was estimated to $166 716 and the cost per QALY gained was estimated to $175 164.
The mean survival for the seven patients that were actually treated with CRS + HIPEC + EPIC was 20.5 month and mean cost $182 082 with a cost per life year gained of $157 396 and cost per QALY gained $166 681. Thus, the estimated costs and cost-effectiveness were essentially unaffected by a ‘per protocol’ analysis. The mean survival for the three patients that were not treated with CRS + HIPEC + EPIC was 13.4 month and means cost $60 901.
Sensitivity analyses
If all patients in the CRS + HIPEC + EPIC group had got the intended treatment, based on the costs for the seven patients that were actually treated with CRS + HIPEC + EPIC, the mean cost per patient in the CRS + HIPEC + EPIC group should have been $173 100. This is 19% higher than the observed mean cost per patient in the CRS + HIPEC + EPIC group.
If the patients in the CRS + HIPEC + EPIC group should have had 50% less (or more) complications, the reduction (or increase) in the mean cost per patient in the CRS + HIPEC + EPIC group should have been $12 400 (8% of the mean cost per patient). The calculation is based on the principle, that 50% change of complications accordingly makes a 50% change of the main drivers of the cost for complications (radiological examinations and time in ICU and at ward).
Discussion
PC from GC is an extremely aggressive tumour-disease and with best supportive care, the disease is often fatal within a few months [Citation1,Citation2]. With current knowledge, this patient group is mostly treated with chemotherapy [Citation5,Citation25,Citation26] and sometimes in combination with radiotherapy [Citation27]. A growing treatment option for PC is CRS + HIPEC + EPIC, with promising results if from colorectal cancer [Citation28] and pseudomyxoma [Citation19]. However, there is still a lack of data on the benefit of CRS + HIPEC + EPIC in patients with PC from GC and there is ongoing debate whether this treatment should be used in routine care [Citation3].
Results from cost and cost-effectiveness analyses are important in deciding the allocation of health-care resources. Such analyses are therefore increasingly used in health-care decision making and the concept cost-effectiveness thresholds (i.e. a maximum accepted cost per unit of health gain) are proposed and used in resource-allocation decisions [Citation29].
This is to our knowledge the first health-economic study on CRS + HIPEC + EPIC treatment in patients with PC from GC only. The number of patients possible to include was small and non-randomised comparisons are inherently hazardous. However, the controls were matched and treated during the same time period within the same geographical area as the CRS + HIPEC + EPIC group.
The results indicate a prolonged survival for the combined treatment approach compared to systemic chemotherapy only, but at considerably higher costs and with a very high incremental cost per QALY gained. This leads to an incremental cost per additional life-year gained above what is usually considered cost-effective in Sweden and many other countries [Citation29]. A treatment like the one assessed here that adds a cost of about $86 000 per patient would probably need to also add at least one additional life-year (i.e. have an incremental cost per QALY gained below about $70 000–$80 000), to be considered reasonably cost-effective compared to other health care interventions available.
One patient in the CRS + HIPEC + EPIC group had a very high treatment cost, $487 756 due to complications with re-operations including a prolonged postoperative period at the intensive care unit. Although there now and then will always be patients with costs much higher than average, it is difficult to draw a definite conclusion to which extent this could occur from a small number of patients as in this present study. It could therefore be interesting to also assess the outcome after exclusion of such patients, if assumed to occur with a very low frequency in a larger patient population or at similar frequency in the two groups. With the patient who had the very high treatment cost excluded, the difference between groups CRS + HIPEC + EPIC vs systemic chemotherapy would be $48 411, gain in survival 0.56 years, the cost per life-years gained $86 938, and cost per QALY gained $86 292 which still is a fairly high incremental cost, but more in accordance with currently accepted cost-effectiveness thresholds in Sweden [Citation29].
The mean cost of treatment with CRS + HIPEC + EPIC in patients with PC has previously been estimated in a prospective study by Chua et al. [Citation14], in a retrospective study by Baratti et al. [Citation10], in a retrospective study by Bonastre et al. [Citation12] and in a prospective study by Spiliotis et al. [Citation11]. Chua had an estimate of $58 378 per procedure over six financial years for 136 patients, Baratti had an estimate of $53 073 per patient for 376 patients, Bonastre had an estimate of $37 229 per patient, based on all procedures during a two-year period for 73 patients and Spiliotis a mean cost estimated from 24 patients of $23 700 per patient (range $12 800–$51 200). In the present study, the mean cost of treatment was considerably higher, maybe due to the longer follow-up time and a longer mean hospital stay (57 days), compared to 33, 24 and 28 days, respectively, in the Chua, Baratti and Bonastre study. In the Chua, Baratti and Spiliotis studies only costs from the primary hospital stay were included. Longer hospital stay in our study was probably due to higher morbidity rate. However, a learning curve is not the plausible reason for the morbidity rate, as dedicated surgeons have preformed CRS with good results in Uppsala [Citation30,Citation31] during the past two decades.
Three CRS + HIPEC + EPIC studies on patients with GC and PC have been presented. One prospective [Citation7], one non-randomised with case control patients [Citation8], and one retrospective [Citation9]. Perioperative morbidity rate in the present study was higher for patients with extensive CRS (71%) than the 47%, 35% and 43% for comparable patients in the previously published studies. In our study, CRS was extensive due to advanced tumour growth, and the only reason for refraining from surgery was widespread PC in the small bowel. This could explain the extended duration of surgery (mean 9.1 h including HIPEC-time compared with 5.2 h excluding HIPEC-time for Glehen [Citation7], and probably increased morbidity. Moreover, higher morbidity with the use of platinum-based HIPEC could be expected, and this could be one reason for higher morbidity in our cohort, as both Glehen and Hall used Mitomycin C [Citation7,Citation8,Citation30]. Another reason for higher costs in this study was the use of neo-adjuvant treatment and EPIC, and it is possible this extra chemotherapy raised the morbidity-rate in this patient-group and the perioperative chemotherapy directly affected the costs. In the present study, all major costs during the entire follow-up period were calculated at microcosting level whereas this was not the case in the comparable studies.
There are a number of limitations with the present study. Thus, the comparison was based on small numbers of patients not being randomised to their treatments and there was also no QoL data collected. There was also another selection in the CRS + HIPEC + EPIC group, since the most severe cases, with tumour progression during the neo- adjuvant treatment and extensive tumour growth on the entire small intestine serosa, did not proceed to the surgical intervention.
In conclusion, the results of this study indicate that treatment of PC from GC is costly, irrespective of treatment modality. If the slightly prolonged survival from the CRS + HIPEC + EPIC treatment compared to systemic chemotherapy only is correct, the incremental cost per QALY gained has to be considered too high in most public health-care systems. However, for new surgical procedures it is likely that the intervention may be optimised over time, with more experience and knowledge and better patient selection leading to improved cost-effectiveness.
Acknowledgements
This work was supported by the Uppsala University Hospital (sådd-ALF), Sweden. The authors gratefully acknowledge Pehr Lind at Karolinska Institute, Stockholm, Sweden, for permitting access to patient's data from GATAC participants in Stockholm.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, . Peritoneal carcinomatosis from non- gynecologic malignancies: Results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88:358–63.
- Meijer S, De Bakker OJ, Hoitsma HF. Palliative resection in gastric cancer. J Surg Oncol 1983;23:77–80.
- Bozzetti F, Yu W, Baratti D, Kusamura S, Deraco M. Locoregional treatment of peritoneal carcinomatosis from gastric cancer. J Surg Oncol 2008;98:273–6.
- Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: A systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006;24:2903–9.
- Glimelius B, Ekstrom K, Hoffman K, Graf W, Sjoden PO, Haglund U, . Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 1997;8:163–8.
- Janunger KG, Hafstrom L, Glimelius B. Chemotherapy in gastric cancer: A review and updated meta-analysis. Eur J Surg Acta Chir 2002;168:597–608.
- Glehen O, Schreiber V, Cotte E, Sayag-Beaujard AC, Osinsky D, Freyer G, . Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch Surg 2004;139:20–6.
- Hall JJ, Loggie BW, Shen P, Beamer S, Douglas Case L, McQuellon R, . Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J Gastrointest Surg 2004;8:454–63.
- Yonemura Y, Kawamura T, Bandou E, Takahashi S, Sawa T, Matsuki N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg 2005;92:370–5.
- Baratti D, Scivales A, Balestra MR, Ponzi P, Di Stasi F, Kusamura S, . Cost analysis of the combined procedure of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). Eur J Surg Oncol 2010;36:463–9.
- Spiliotis J, Tentes AA, Vaxevanidou A, Korakianitis OS, Rogdakis A, Mirelis C, G, . Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal carcinomatosis. Preliminary results and cost from two centers in Greece. J Buon 2008;13:205–10.
- Bonastre J, Jan P, de Pouvourville G, Pocard M, Estphan G, Elias D. [Cost of an intraperitoneal chemohyperthermia (IPCH) related to cytoreductive surgery]. Ann Chir 2005; 130:553–61.
- Bonastre J, Chevalier J, Elias D, Classe JM, Ferron G, Guilloit JM, . Cost-effectiveness of intraperitoneal chemohyperthermia in the treatment of peritoneal carcinomatosis from colorectal cancer. Value Health 2008;11: 347–53.
- Chua TC, Martin S, Saxena A, Liauw W, Yan TD, Zhao J, . Evaluation of the cost-effectiveness of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (peritonectomy) at the St George Hospital peritoneal surface malignancy program. Ann Surg 2010;251:323–9.
- DTCD ND. Common terminology criteria for adverse events v3.0 (CTAE). In: Cancer Therapy Evaluation Program; 2006
- Glimelius B, Sorbye H, Balteskard L, Bystrom P, Pfeiffer P, Tveit K, . A randomized phase III multicenter trial comparing irinotecan in combination with the Nordic bolus 5-FU and folinic acid schedule or the bolus/infused de Gramont schedule (Lv5FU2) in patients with metastatic colorectal cancer. Ann Oncol 2008;19:909–14.
- Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995; 221:29–42.
- Vazquez Vde L, Sugarbaker PH. Cholecystectomy, lesser omentectomy, and stripping of the omental bursa: A peritonectomy procedure. J Surg Oncol 2003;84:45–9.
- Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol 1999;6:727–31.
- Sugarbaker PH, Averbach AM, Jacquet P, Stephens AD, Stuart OA. A simplified approach to hyperthermic intraoperative intraperitoneal chemotherapy (HIIC) using a self retaining retractor. Cancer Treat Res 1996;82:415–21.
- Elias D, Bonnay M, Puizillou JM, Antoun S, Demirdjian S, El OA, . Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: Pharmacokinetics and tissue distribution. Ann Oncol 2002; 13:267–72.
- Gubanski M, Johnsson A, Fernebro E, Kadar L, Karlberg I, Flygare P, . Randomized phase II study of sequential docetaxel and irinotecan with 5-fluorouracil/folinic acid (leucovorin) in patients with advanced gastric cancer: The GATAC trial. Gastric Cancer 2010;13:155–61.
- OANDA [Internet]. OANDA Foreign Exchange Information. 2009. Available from: http://wwwoanda.com.
- Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: A prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer 1996;32A:1135–41.
- Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 1995;71:587–91.
- Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 1993;72:37–41.
- Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, . Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. New Engl J Med 2001;345:725–30.
- Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, . Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737–43.
- Eichler HG, Kong SX, Gerth WC, Mavros P, Jonsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: How are cost-effectiveness thresholds expected to emerge? Value Health 2004;7: 518–28.
- van Leeuwen BL, Graf W, Pahlman L, Mahteme H. Swedish experience with peritonectomy and HIPEC. HIPEC in peritoneal carcinomatosis. Ann Surg Oncol 2008;15: 745–53.
- Hansson J, Graf W, Pahlman L, Nygren P, Mahteme H. Postoperative adverse events and long-term survival after cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol 2009;35:202–8.